Reduction of Deuterium Level Supports Resistance of Neurons to Glucose Deprivation and Hypoxia: Study in Cultures of Neurons and on Animals
Abstract
:1. Introduction
2. Material and Methods
2.1. Obtaining Water with Different Deuterium Content
2.2. Investigation of the Effect of Reduced Deuterium Content at the Cellular Level
2.2.1. Chemicals and Supplements
2.2.2. Obtaining Neuronal-Glial Cell Cultures
2.2.3. Modeling Glucose Deprivation
2.2.4. Assessment of the Level of Neuronal Death
2.3. Study of the Effect of Reduced Deuterium Content in Rats
2.3.1. Animal Model
2.3.2. Simulation of Hypoxic Effects on Rats
- group 1 (NW, Control)—intact rats that received in the diet water with a deuterium concentration equal to the natural one (δ2H = −37‰) for six weeks, without hypoxic exposure;
- group 2 (DDW)—rats fed with DDW in the diet (δ2H = −679‰) for six weeks, without hypoxic exposure;
- group 3 (NW, Hypoxia)—rats fed with water with a deuterium concentration equal to the natural one (δ2H = −37‰) for six weeks, on the 43rd day of the experiment subjected to acute hypoxia;
- group 4 (DDW, Hypoxia)—rats that received DDW in the diet (δ2H = −679‰) for six weeks, on the 43rd day of the experiment subjected to acute hypoxia.
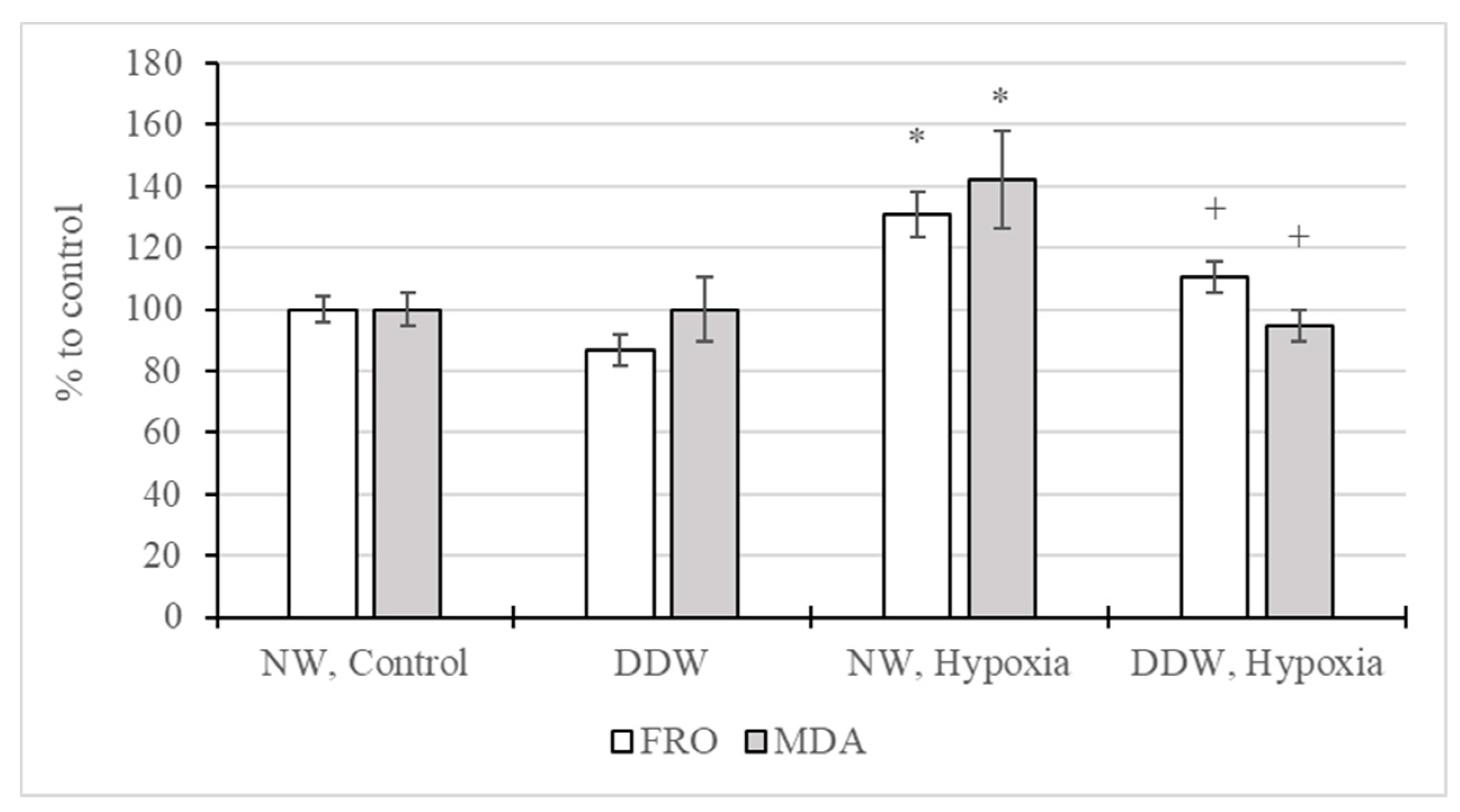
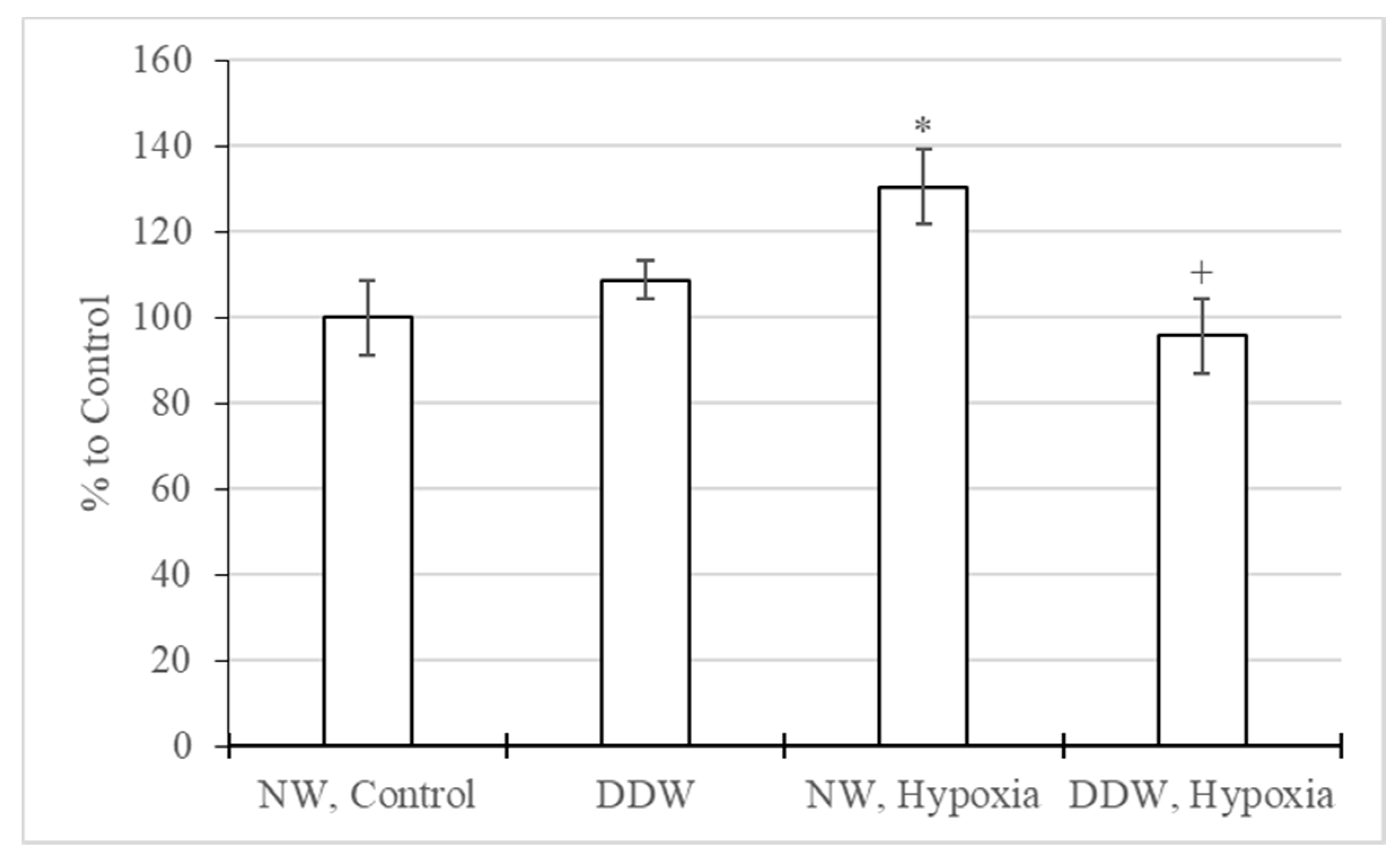
2.3.3. Study of Oxidative and Antioxidant Factors in the Rat Brain
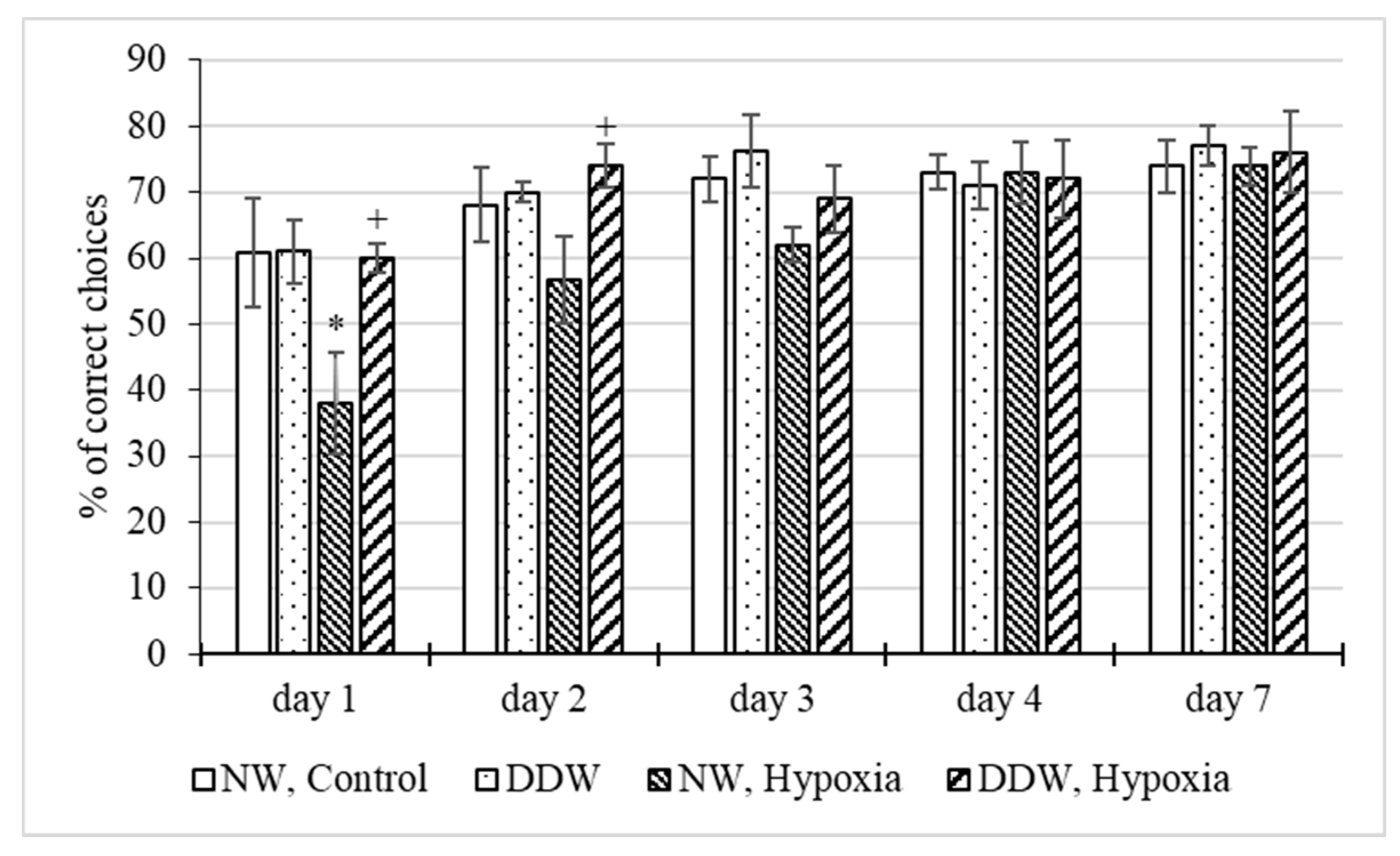
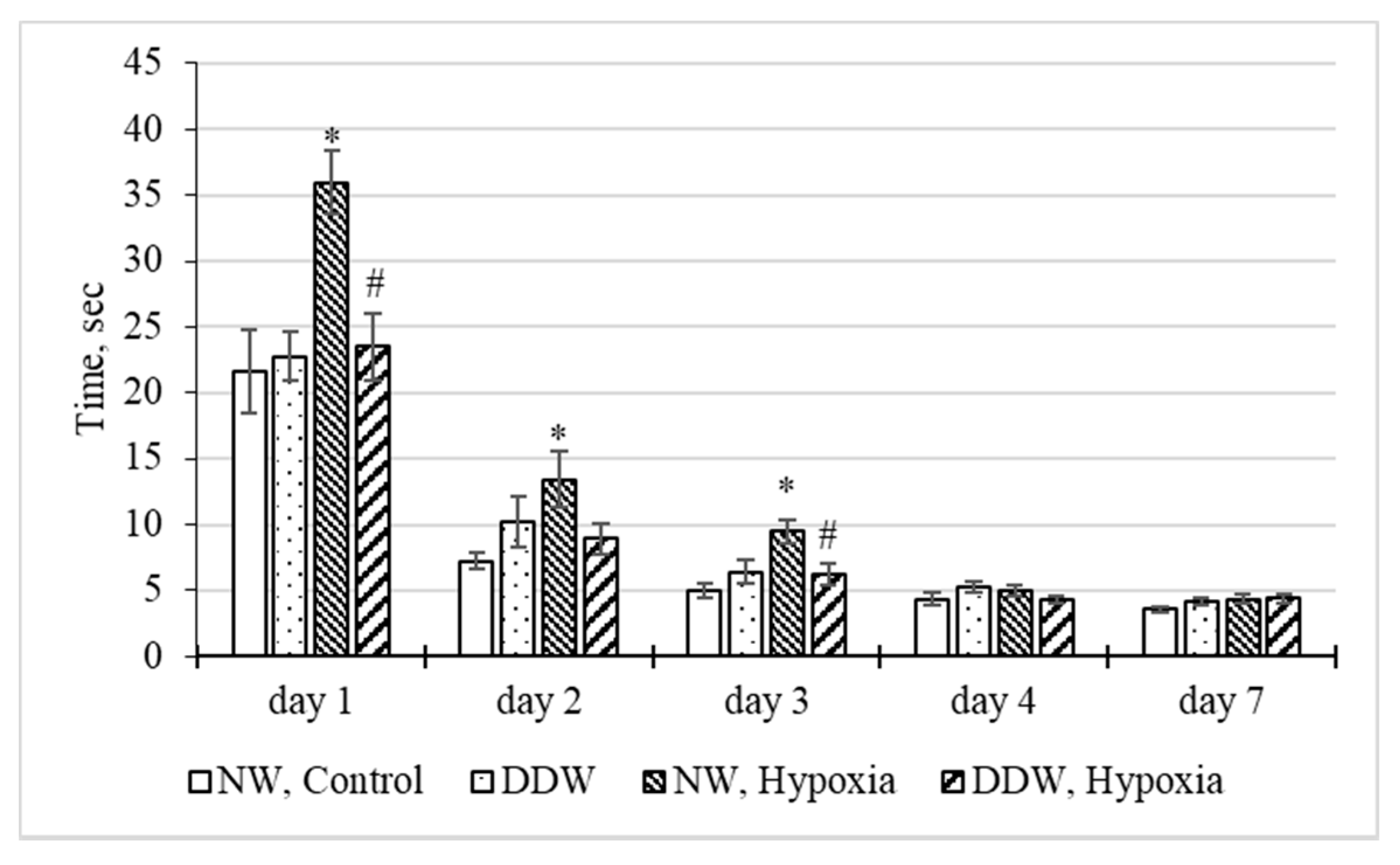
2.3.4. Study of the Behavior of Rats in the T-maze Test
2.3.5. Determination of Deuterium Content in the Brain of Rats
2.4. Statistical Analysis
3. Results
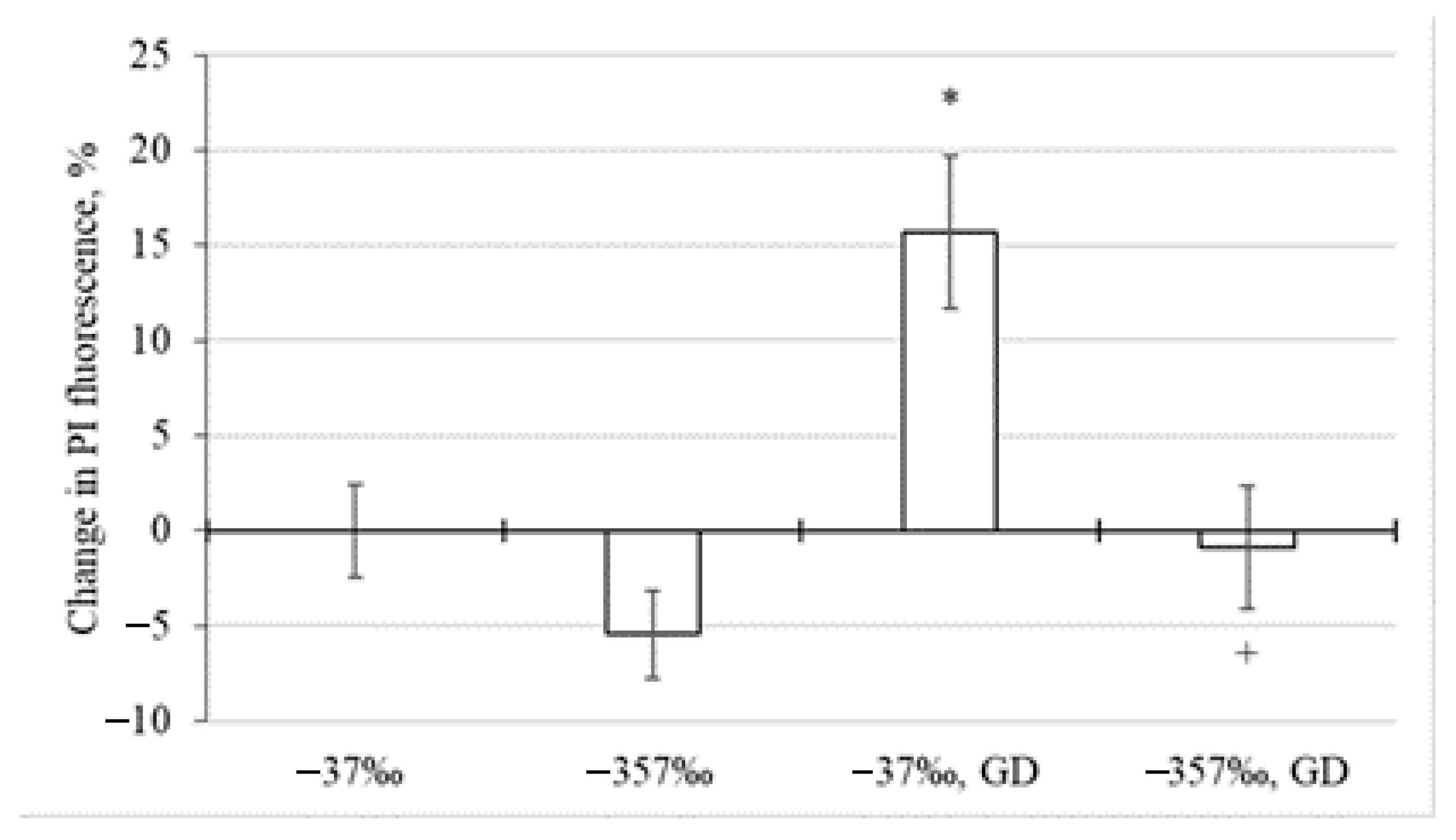
Influence of Environment with Different Deuterium Content on Neuronal Death during Glucose Deprivation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kselíková, V.; Vítová, M.; Bišová, K. Deuterium and its impact on living organisms. Folia Microbiol. 2019, 64, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Basov, A.; Fedulova, L.; Baryshev, M.; Dzhimak, S. Deuterium-depleted water influence on the isotope 2H/1H regulation in body and individual adaptation. Nutrients 2019, 11, 1903. [Google Scholar] [CrossRef] [Green Version]
- Basov, A.; Fedulova, L.; Vasilevskaya, E.; Dzhimak, S. Possible Mechanisms of Biological Effects Observed in Living Systems during 2H/1H Isotope Fractionation and Deuterium Interactions with Other Biogenic Isotopes. Molecules 2019, 24, 4101. [Google Scholar] [CrossRef] [Green Version]
- Somlyai, G.; Somlyai, I.; Fórizs, I.; Czuppon, G.; Papp, A.; Molnár, M. Effect of Systemic Subnormal Deuterium Level on Metabolic Syndrome Related and other Blood Parameters in Humans: A Preliminary Study. Molecules 2020, 25, 1376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halenova, T.; Zlatskiy, I.; Syroeshkin, A.; Maximova, T.; Pleteneva, T. Deuterium-Depleted Water as Adjuvant Therapeutic Agent for Treatment of Diet-Induced Obesity in Rats. Molecules 2020, 25, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elharram, A.; Czegledy, N.M.; Golod, M.; Milne, G.L.; Pollock, E.; Bennett, B.M.; Shchepinov, M.S. Deuterium-reinforced polyunsaturated fatty acids improve cognition in a mouse model of sporadic Alzheimer’s disease. FEBS J. 2017, 284, 4083–4095. [Google Scholar] [CrossRef] [PubMed]
- Fedulova, L.V.; Basov, A.A.; Vasilevskaya, E.R.; Dzhimak, S.S. Gender difference response of male and female immunodeficiency rats treated with tissue-specific biomolecules. Curr. Pharm. Biotechnol. 2019, 20, 245–253. [Google Scholar] [CrossRef]
- Andreyev, A.Y.; Tsui, H.S.; Milne, G.L.; Shmanai, V.V.; Bekish, A.V.; Fomich, M.A.; Pham, M.N.; Nong, Y.; Murphy, A.N.; Clarke, C.F.; et al. Isotope-reinforced polyunsaturated fatty acids protect mitochondria from oxidative stress. Free Radic. Biol. Med. 2015, 82, 63–72. [Google Scholar] [CrossRef]
- Yaglova, N.V.; Obernikhin, S.S.; Timokhina, E.P.; Yaglov, V.V. Response of Pituitary-Thyroid Axis to a Short-Term Shift in Deuterium Content in the Body. Bull. Exp. Biol. Med. 2021, 171, 262–264. [Google Scholar] [CrossRef]
- Basov, A.A.; Kozin, S.V.; Bikov, I.M.; Popov, K.A.; Moiseev, A.V.; Elkina, A.A.; Dzhimak, S.S. Changes in prooxidant-antioxidant system indices in the blood and brain of rats with modelled acute hypoxia which consumed a deuterium-depleted drinking diet. Biol. Bull. 2019, 46, 531–535. [Google Scholar] [CrossRef]
- Yaglova, N.V.; Obernikhin, S.S.; Timokhina, E.P.; Diatropova, M.A.; Diatropov, M.E.; Yaglov, V.V. Impact of lower deuterium intake to the organism on thermoregulation. Bull. Exp. Biol. Med. 2021, 171, 538–542. [Google Scholar] [CrossRef]
- Kozin, S.; Skrebitsky, V.; Kondratenko, R.; Kravtsov, A.; Butina, E.; Moiseev, A.; Malyshko, V.; Baryshev, M.; Elkina, A.; Dzhimak, S. Electrophysiological activity and survival rate of rats nervous tissue cells depends on D/H isotopic composition of medium. Molecules 2021, 26, 2036. [Google Scholar] [CrossRef] [PubMed]
- Beaudoin-Chabot, C.; Wang, L.; Smarun, A.V.; Vidovic, D.; Shchepinov, M.S.; Thibault, G. Deuterated polyunsaturated fatty acids reduce oxidative stress and extend the lifespan of C. elegans. Front. Physiol. 2019, 10, 641. [Google Scholar] [CrossRef] [Green Version]
- Basov, A.; Drobotenko, M.; Svidlov, A.; Gerasimenko, E.; Malyshko, V.; Elkina, A.; Baryshev, M.; Dzhimak, S. Inequality in the Frequency of the Open States Occurrence Depends on Single 2H/1H Replacement in DNA. Molecules 2020, 25, 3753. [Google Scholar] [CrossRef]
- Mladin, C.; Ciobica, A.; Lefter, R.; Popescu, A.; Bild, W. Deuterium-depleted water has stimulating effects on long-term memory in rats. NeurosciLetter 2014, 583, 154–158. [Google Scholar] [CrossRef]
- Mladin, C.; Ciobica, A.; Lefter, R.; Popescu, A.; Bild, W. Deuterium depletion induces anxiolytic-like effects in rats. Arch. Biol. Sci. 2014, 66, 947–953. [Google Scholar] [CrossRef] [Green Version]
- Strekalova, T.; Evans, M.; Chernopiatko, A.; Couch, Y.; Costa-Nunes, J.; Cespuglio, R.; Chesson, L.; Vignisse, J.; Steinbusch, H.W.; Anthony, D.C.; et al. Deuterium content of water increases depression susceptibility: The potential role of a serotonin-related mechanism. Behav. Brain Res. 2015, 277, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Podlesak, D.W.; Torregrossa, A.-M.; Ehleringer, J.R.; Dearing, M.D.; Passey, B.H.; Cerling, T.E. Turnover of oxygen and hydrogen isotopes in the body water, CO2, hair, and enamel of a small mammal. Geochim. Cosmochim. Acta 2008, 72, 19–35. [Google Scholar] [CrossRef]
- Frolov, V.Y.; Baryshev, M.G.; Dzhimak, S.S.; Lomakina, L.V.; Bolotin, S.N.; Petriev, I.S. A Method of Obtaining Water with a Reduced Deuterium. Content. Patent 2013101254, 10 October 2013. [Google Scholar]
- Petriev, I.; Pushankina, P.; Lutsenko, I.; Shostak, N.; Baryshev, M. Synthesis, Electrocatalytic and Gas Transport Characteristics of Pentagonally Structured Star-Shaped Nanocrystallites of Pd-Ag. Nanomaterials 2020, 10, 2081. [Google Scholar] [CrossRef] [PubMed]
- Stelmashook, E.V.; Isaev, N.K.; Plotnikov, E.Y.; Uzbekov, R.E.; Alieva, I.B.; Arbeille, B.; Zorov, D.B. Effect of transitory glucose deprivation on mitochondrial structure and functions in cultured cerebellar granule neurons. Neurosci. Lett. 2009, 461, 140–144. [Google Scholar] [CrossRef]
- Lau, A.C.; Cui, H.; Tymianski, M. The use of propidium iodide to assess excitotoxic neuronal death in primary mixed cortical cultures. Methods Mol. Biol. 2007, 399, 15–29. [Google Scholar] [CrossRef]
- Farkhutdinov, R.R.; Likhovskikh, V.A. Chemiluminescent Methods for the Study of Free Radical Oxidation in Biology and Medicine; BGMI: Ufa, Russia, 1995. [Google Scholar]
- Gavrilov, V.B.; Gavrilova, A.R.; Mazhul’, L.M. Analysis of methods for measuring lipid peroxidation products in blood serum by the reaction with thiobarbituric acid. Vopr. Med. Khimii. 1987, 33, 118–122. [Google Scholar] [PubMed]
- Korolyuk, M.A.; Ivanova, L.I.; Maiorova, I.G.; Tokarev, V.E. Methods of measuring catalase activity. Lab. Delo 1988, 1, 16–19. [Google Scholar]
- Shurygina, L.V.; Zlishcheva, E.I.; Kravtsova, A.N.; Kravtsov, A.A. Antioxidant and Antiamnestic Effects of Potassium Comenate and Comenic Acid under Conditions of Normobaric Hypoxia with Hypercapnia. Bull. Exp. Biol. Med. 2017, 163, 344–348. [Google Scholar] [CrossRef]
- Dzhimak, S.S.; Barishev, M.G.; Basov, A.A.; Timakov, A.A. Influence of deuterium depleted water on freeze dried tissue isotopic composition and morphofunctional body performance in rats of different generations. Biophysics 2014, 59, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Maffezzini, C.; Calvo-Garrido, J.; Wredenberg, A.; Freyer, C. Metabolic regulation of neurodifferentiation in the adult brain. Cell. Mol. Life Sci. 2020, 77, 2483–2496. [Google Scholar] [CrossRef] [Green Version]
- Fathollahipour, S.; Patil, P.S.; Leipzig, N.D. Oxygen Regulation in Development: Lessons from Embryogenesis towards Tissue Engineering. Cells Tissues Organs. 2018, 205, 350–371. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.N.; Bondy, S.C. Increased oxidative stress, inflammation, and glutamate: Potential preventive and therapeutic targets for hearing disorders. Mech. Ageing Dev. 2020, 185, 111191. [Google Scholar] [CrossRef]
- Mukandala, G.; Tynan, R.; Lanigan, S.; O’Connor, J.J. The Effects of Hypoxia and Inflammation on Synaptic Signaling in the CNS. Brain Sci. 2016, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- Minhas, G.; Mathur, D.; Ragavendrasamy, B.; Sharma, N.K.; Paanu, V.; Anand, A. Hypoxia in CNS Pathologies: Emerging Role of miRNA-Based Neurotherapeutics and Yoga Based Alternative Therapies. Front. Neurosci. 2017, 11, 386. [Google Scholar] [CrossRef]
- Sun, M.S.; Jin, H.; Sun, X.; Huang, S.; Zhang, F.L.; Guo, Z.N.; Yang, Y. Free Radical Damage in Ischemia-Reperfusion Injury: An Obstacle in Acute Ischemic Stroke after Revascularization Therapy. Oxid. Med. Cell Longev. 2018, 3804979. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Candelario-Jalil, E. Emerging neuroprotective strategies for the treatment of ischemic stroke: An overview of clinical and preclinical studies. Exp. Neurol. 2020, 335, 113518. [Google Scholar] [CrossRef] [PubMed]
- Akyuva, Y.; Nazıroğlu, M. Resveratrol attenuates hypoxia-induced neuronal cell death, inflammation and mitochondrial oxidative stress by modulation of TRPM2 channel. Sci. Rep. 2020, 10, 6449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, Z.; Yan, J.; Zhang, Y.; Pei, R. Applications of nanomaterials for scavenging reactive oxygen species in the treatment of central nervous system diseases. J. Mater. Chem. B. 2020, 8, 8748–8767. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Qin, C.; Huang, J.; Tang, X.; Liu, C.; Huang, K.; Xu, J.; Guo, G.; Tong, A.; Zhou, L. The role of astrocytes in oxidative stress of central nervous system: A mixed blessing. Cell Prolif. 2020, 53, e12781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuñez, A.; Benavente, I.; Blanco, D.; Boix, H.; Cabañas, F.; Chaffanel, M.; Fernández-Colomer, B.; Fernández-Lorenzo, J.R.; Loureiro, B.; Moral, M.T.; et al. Oxidative stress in perinatal asphyxia and hypoxic-ischaemic encephalopathy. An. Pediatr. 2018, 88, 228.e1–228.e9. [Google Scholar] [CrossRef]
- Zambuto, S.G.; Serrano, J.F.; Vilbert, A.C.; Lu, Y.; Harley, B.A.C.; Pedron, S. Response of neuroglia to hypoxia-induced oxidative stress using enzymatically crosslinked hydrogels. MRS Commun. 2020, 10, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Bailey, A.P.; Koster, G.; Guillermier, C.; Hirst, E.M.; MacRae, J.I.; Lechene, C.P.; Postle, A.D.; Gould, A.P. Antioxidant Role for Lipid Droplets in a Stem Cell Niche of Drosophila. Cell 2015, 163, 340–353. [Google Scholar] [CrossRef] [Green Version]
- Watts, M.E.; Pocock, R.; Claudianos, C. Brain Energy and Oxygen Metabolism: Emerging Role in Normal Function and Disease. Front. Mol. Neurosci. 2018, 11, 216. [Google Scholar] [CrossRef]
- Shukla, S.K.; King, R.J.; Singh, P.K. Transcriptional profiling using RNA-Seq to study hypoxia-mediated gene regulation. Methods Mol. Biol. 2018, 1742, 55–66. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Martin, C.A.; Dhayanithy, G.; Reddy, M.S.; Rela, M.; Kalkura, S.N.; Sellathamby, S. Hypoxic Preconditioning Induces Neuronal Differentiation of Infrapatellar Fat Pad Stem Cells through Epigenetic Alteration. ACS Chem. Neurosci. 2021, 12, 704–718. [Google Scholar] [CrossRef]
- Merelli, A.; Repetto, M.; Lazarowski, A.; Auzmendi, J. Hypoxia, Oxidative Stress, and Inflammation: Three Faces of Neurodegenerative Diseases. J. Alzheimers Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- Terraneo, L.; Paroni, R.; Bianciardi, P.; Giallongo, T.; Carelli, S.; Gorio, A.; Samaja, M. Brain adaptation to hypoxia and hyperoxia in mice. Redox. Biol. 2017, 11, 12–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonkowsky, J.L.; Son, J.H. Hypoxia and connectivity in the developing vertebrate nervous system. Dis. Model. Mech. 2018, 11, dmm037127. [Google Scholar] [CrossRef] [Green Version]
- Buxton, R.B. The thermodynamics of thinking: Connections between neural activity, energy metabolism and blood flow. Phil. Trans. R Soc. B 2021, 376, 20190624. [Google Scholar] [CrossRef] [PubMed]
- Raberin, A.; Nader, E.; Lopez Ayerbe, J.; Alfonsi, G.; Mucci, P.; Rytz, C.L.; Pialoux, V.; Durand, F. Pro-Oxidant/Antioxidant Balance during a Prolonged Exposure to Moderate Altitude in Athletes Exhibiting Exercise-Induced Hypoxemia at Sea-Level. Life 2021, 11, 228. [Google Scholar] [CrossRef]
- Drevets, W.C. Neuroplasticity in mood disorders. Dialogues Clin. Neurosci. 2004, 6, 199–216. [Google Scholar] [CrossRef]
- Zhao, F.; Yang, J.; Cui, R. Effect of Hypoxic Injury in Mood Disorder. Neural. Plast. 2017, 2017, 6986983. [Google Scholar] [CrossRef] [Green Version]
- Kann, O.; Hollnagel, J.O.; Elzoheiry, S.; Schneider, J. Energy and Potassium Ion Homeostasis during Gamma Oscillations. Front. Mol. Neurosci. 2016, 9, 47. [Google Scholar] [CrossRef]
- Kann, O.; Papageorgiou, I.E.; Draguhn, A. Highly energized inhibitory interneurons are a central element for information processing in cortical networks. J. Cereb. Blood Flow Metab. 2014, 34, 1270–1282. [Google Scholar] [CrossRef] [Green Version]
- Khuu, M.A.; Pagan, C.M.; Nallamothu, T.; Hevner, R.F.; Hodge, R.D.; Ramirez, J.M.; Garcia, A.J., 3rd. Intermittent Hypoxia Disrupts Adult Neurogenesis and Synaptic Plasticity in the Dentate Gyrus. J. Neurosci. 2019, 39, 1320–1331. [Google Scholar] [CrossRef] [Green Version]
- Somjen, G.G. Ion regulation in the brain: Implications for pathophysiology. Neuroscientist 2002, 8, 254–267. [Google Scholar] [CrossRef]
- Buzsáki, G.; Kaila, K.; Raichle, M. Inhibition and brain work. Neuron 2007, 56, 771–783. [Google Scholar] [CrossRef] [Green Version]
- Verma, V.; Bali, A.; Singh, N.; Jaggi, A.S. Implications of sodium hydrogen exchangers in various brain diseases. J. Basic Clin. Physiol. Pharmacol. 2015, 26, 417–426. [Google Scholar] [CrossRef]
- Song, S.; Luo, L.; Sun, B.; Sun, D. Roles of glial ion transporters in brain diseases. Glia 2020, 68, 472–494. [Google Scholar] [CrossRef]
- Jung, H.; Kim, S.Y.; Canbakis Cecen, F.S.; Cho, Y.; Kwon, S.K. Dysfunction of Mitochondrial Ca2+ Regulatory Machineries in Brain Aging and Neurodegenerative Diseases. Front. Cell Dev. Biol. 2020, 8, 599792. [Google Scholar] [CrossRef]
- Bak, L.K.; Schousboe, A.; Waagepetersen, H.S. The glutamate/GABA-glutamine cycle: Aspects of transport, neurotransmitter homeostasis and ammonia transfer. J. Neurochem. 2006, 98, 641–653. [Google Scholar] [CrossRef]
- Roth, F.C.; Draguhn, A. GABA metabolism and transport: Effects on synaptic efficacy. Neural. Plast. 2012, 2012, 805830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vien, T.N.; Moss, S.J.; Davies, P.A. Regulating the Efficacy of Inhibition Through Trafficking of γ-Aminobutyric Acid Type A Receptors. Anesth Analg. 2016, 123, 1220–1227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groc, L.; Choquet, D. Linking glutamate receptor movements and synapse function. Science 2020, 368, eaay4631. [Google Scholar] [CrossRef] [PubMed]
- Ryan, R.M.; Ingram, S.L.; Scimemi, A. Regulation of Glutamate, GABA and Dopamine Transporter Uptake, Surface Mobility and Expression. Front. Cell Neurosci. 2021, 15, 670346. [Google Scholar] [CrossRef]
- Ghosh Dastidar, S.; Das Sharma, S.; Chakraborty, S.; Chattarji, S.; Bhattacharya, A.; Muddashetty, R.S. Distinct regulation of bioenergetics and translation by group I mGluR and NMDAR. EMBO Rep. 2020, 21, e48037. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, M.; Valez, V.; Cimarra, C.; Blasina, F.; Radi, R. Hypoxic-Ischemic Encephalopathy and Mitochondrial Dysfunction: Facts, Unknowns, and Challenges. Antioxid. Redox Signal. 2020, 33, 247–262. [Google Scholar] [CrossRef]
- Ladak, Z.; Garcia, E.; Yoon, J.; Landry, T.; Armstrong, E.A.; Yager, J.Y.; Persad, S. Sulforaphane (SFA) protects neuronal cells from oxygen & glucose deprivation (OGD). PLoS ONE 2021, 16, e0248777. [Google Scholar] [CrossRef]
- Gava-Junior, G.; Roque, C.; Mendes-Oliveira, J.; Bernardino, A.C.; Serrenho, I.; Pires, J.P.; Baltazar, G. A Cell Culture Model for Studying the Role of Neuron-Glia Interactions in Ischemia. J. Vis. Exp. 2020, 165. [Google Scholar] [CrossRef]
- He, Z.; Hu, M.; Zha, Y.H.; Li, Z.C.; Zhao, B.; Yu, L.L.; Yu, M.; Qian, Y. Piracetam ameliorated oxygen and glucose deprivation-induced injury in rat cortical neurons via inhibition of oxidative stress, excitatory amino acids release and P53/Bax. Cell. Mol. Neurobiol. 2014, 34, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Zhang, J.; Wang, Y.; Hao, Q.; Chen, H.; Cheng, X. Sirt1 Regulates Oxidative Stress in Oxygen-Glucose Deprived Hippocampal Neurons. Front. Pediatr. 2020, 8, 455. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, C.; Wang, J.; Zhu, M.; Yao, Y.; Liu, J. Hypoxia preconditioning protects neuronal cells against traumatic brain injury through stimulation of glucose transport mediated by HIF-1α/GLUTs signaling pathway in rat. Neurosurg. Rev. 2021, 44, 411–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muzzi, L.; Hassink, G.; Levers, M.; Jansman, M.; Frega, M.; Hofmeijer, J.; van Putten, M.; le Feber, J. Mild stimulation improves neuronal survival in an in vitro model of the ischemic penumbra. J. Neural Eng. 2019, 17, 016001. [Google Scholar] [CrossRef]
- Ozugur, S.; Kunz, L.; Straka, H. Relationship between oxygen consumption and neuronal activity in a defined neural circuit. BMC Biol. 2020, 18, 76. [Google Scholar] [CrossRef]
- Zhang, X.; Gaetani, M.; Chernobrovkin, A.; Zubarev, R.A. Anticancer Effect of Deuterium Depleted Water—Redox Disbalance Leads to Oxidative Stress. Mol. Cell Proteom. 2019, 18, 2373–2387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kravtsov, A.; Kozin, S.; Basov, A.; Butina, E.; Baryshev, M.; Malyshko, V.; Moiseev, A.; Elkina, A.; Dzhimak, S. Reduction of Deuterium Level Supports Resistance of Neurons to Glucose Deprivation and Hypoxia: Study in Cultures of Neurons and on Animals. Molecules 2022, 27, 243. https://doi.org/10.3390/molecules27010243
Kravtsov A, Kozin S, Basov A, Butina E, Baryshev M, Malyshko V, Moiseev A, Elkina A, Dzhimak S. Reduction of Deuterium Level Supports Resistance of Neurons to Glucose Deprivation and Hypoxia: Study in Cultures of Neurons and on Animals. Molecules. 2022; 27(1):243. https://doi.org/10.3390/molecules27010243
Chicago/Turabian StyleKravtsov, Alexandr, Stanislav Kozin, Alexandr Basov, Elena Butina, Mikhail Baryshev, Vadim Malyshko, Arkady Moiseev, Anna Elkina, and Stepan Dzhimak. 2022. "Reduction of Deuterium Level Supports Resistance of Neurons to Glucose Deprivation and Hypoxia: Study in Cultures of Neurons and on Animals" Molecules 27, no. 1: 243. https://doi.org/10.3390/molecules27010243





