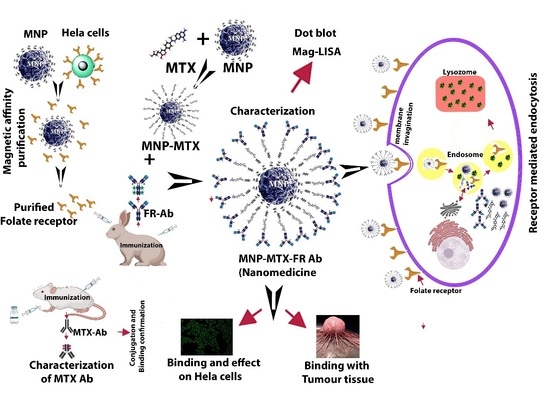
A Novel Method of Magnetic Nanoparticles Functionalized with Anti-Folate Receptor Antibody and Methotrexate for Antibody Mediated Targeted Drug Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization of MNPs
2.2. Magnetic Affinity Purification of Folate Receptors
2.3. Production and Characterization of Rabbit-Anti Folate Receptor Antibody
2.3.1. Characterization of Anti-Folate Receptor Antibodies
ELISA for Anti-Folate Receptor Antibody
Western Blot Analysis
2.4. Synthesis of Targeting Nanomedicines MNP-MTX-FR Ab
2.4.1. MTX Conjugation to MNP
2.4.2. Anti-Folate Receptor Conjugation with MNP-MTX
2.5. Immunocharacterization of Nanoformulations
2.6. pH-Dependent Stability of Nanomedicines
2.7. In Vitro Anticancer Effects of Nanocomposites
2.8. Receptor Binding Assay of MNP-MTX-FR Ab Nanomedicine
2.8.1. Intracellular Tracking of Nanomedicine (HeLa Cells)
2.8.2. Immunohistochemistry (Binding Efficiency with Tumor Tissue)
3. Results
3.1. Characterization of MNPs
3.2. FTIR Analysis
3.3. Stoichiometry
3.4. Characterization of Anti-Folate Receptor Antibodies
3.5. MagLISA
3.6. Nanocomposite Stability (In Vitro)
3.7. Cellular Cytotoxicity of Different Nanocomposites
3.8. Receptor Binding Assay of Nanomedicine with HeLa Cells
3.9. Receptor Binding Assay of Nanomedicine with Tumor Tissue (Immunohistochemistry)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADCs | Antibody-drug conjugates. |
| EDC | 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide. |
| DHFR | Dihydrofolate reductase. |
| ECD | Ethyl carbodiimide. |
| ELISA | Enzyme linked immunosorbent assay. |
| FITC | Fluorescein Isothiocyanate. |
| FA-MNP | folic acid-MNP |
| FRs | Folic acid receptors. |
| FBS | Fetal bovine serum. |
| FR | Rabbit-anti Ab. |
| HRP | Horse radish peroxidase. |
| MNP-MTX-FR Ab | Anti-folate receptor antibody and methotrexate. |
| MTX | Methotrexate. |
| MTX-MNPs | MTX-conjugated nanoparticles. |
| MNPs | Magnetic nanoparticles. |
| NPs | Nanoparticles. |
| TMB | Tetramethylbenzidine. |
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149. [Google Scholar] [CrossRef]
- Howell, A. The emerging breast cancer epidemic: Early diagnosis and treatment. Breast Cancer Res. 2010, 12, S10. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-E.; Kwak, J.-W.; Park, J.W. Nanotechnology for Early Cancer Detection. Sensors 2010, 10, 428–455. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-T.; Pang, J.-H.S.; Yang, R.-C. Anti-cancer effects of Phyllanthus urinaria and relevant mechanisms. Chang. Gung Med. J. 2010, 33, 477–487. [Google Scholar]
- Huang, X.; El-Sayed, M.A. Gold nanoparticles: Optical properties and implementations in cancer diagnosis and photothermal therapy. J. Adv. Res. 2010, 1, 13–28. [Google Scholar] [CrossRef] [Green Version]
- Madamsetty, V.S.; Mukherjee, A.; Mukherjee, S. Recent Trends of the Bio-Inspired Nanoparticles in Cancer Theranostics. Front. Pharmacol. 2019, 10, 1264. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.A.A.; Saleh, A.M. Applications of nanoparticle systems in drug delivery technology. Saudi Pharm. J. 2018, 26, 64–70. [Google Scholar] [CrossRef]
- Zwicke, G.L.; Mansoori, G.A.; Jeffery, C.J. Utilizing the folate receptor for active targeting of cancer nanotherapeutics. Nano Rev. 2012, 3, 18496. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Gao, X.; Chen, Y.; Liu, T. Nanotechnology in cancer diagnosis: Progress, challenges and opportunities. J. Hematol. Oncol. 2019, 12, 137. [Google Scholar] [CrossRef] [Green Version]
- Jin, C.; Wang, K.; Oppong-Gyebi, A.; Hu, J. Application of Nanotechnology in Cancer Diagnosis and Therapy—A Mini-Review. Int. J. Med. Sci 2020, 17, 2964–2973. [Google Scholar] [CrossRef]
- Sinha, R.; Kim, G.J.; Nie, S.; Shin, D.M. Nanotechnology in cancer therapeutics: Bioconjugated nanoparticles for drug delivery. Mol. Cancer Ther. 2006, 5, 1909–1917. [Google Scholar] [CrossRef] [Green Version]
- Neuberger, T.; Schöpf, B.; Hofmann, H.; Hofmann, M.; von Rechenberg, B. Superparamagnetic nanoparticles for biomedical applications: Possibilities and limitations of a new drug delivery system. J. Magn. Magn. Mater. 2005, 293, 483–496. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Kalantari, K.; Saleh, B.; Webster, T.J. Biological Applications of Severely Plastically Deformed Nano-Grained Medical Devices: A Review. Nanomaterials 2021, 11, 748. [Google Scholar] [CrossRef]
- Agrawal, S.; Nooti, S.K.; Singh, H.; Rai, V. Nanomaterial-Mediated Theranostics for Vascular Diseases. J. Nanotheranostics 2021, 2, 1. [Google Scholar] [CrossRef]
- Tran, L.A.; Wilson, L.J. Nanomedicine: Making controllable magnetic drug delivery possible for the treatment of breast cancer. Breast Cancer Res. 2011, 13, 303. [Google Scholar] [CrossRef] [Green Version]
- Issa, B.; Obaidat, I.M.; Albiss, B.A.; Haik, Y. Magnetic nanoparticles: Surface effects and properties related to biomedicine applications. Int. J. Mol. Sci. 2013, 14, 21266–21305. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Low, P.S. Immunotherapy of folate receptor-expressing tumors: Review of recent advances and future prospects. J. Control. Release 2003, 91, 17–29. [Google Scholar] [CrossRef]
- Lu, Y.; Low, P.S. Folate-mediated delivery of macromolecular anticancer therapeutic agents. Adv. Drug Deliv. Rev. 2012, 64, 342–352. [Google Scholar] [CrossRef]
- Yadav, R.D.; Chaudhary, A. Nano–bio surface interactions, cellular internalisation in cancer cells and e-data portals of nanomaterials: A review. IET Nanobiotechnol. 2021, 15. [Google Scholar] [CrossRef] [PubMed]
- Marverti, G.; Marraccini, C.; Martello, A.; D’Arca, D.; Pacifico, S.; Guerrini, R.; Spyrakis, F.; Gozzi, G.; Lauriola, A.; Santucci, M.; et al. Folic Acid–Peptide Conjugates Combine Selective Cancer Cell Internalization with Thymidylate Synthase Dimer Interface Targeting. J. Med. Chem. 2021, 64, 3204–3221. [Google Scholar] [CrossRef]
- McCord, E.; Pawar, S.; Koneru, T.; Tatiparti, K.; Sau, S.; Iyer, A.K. Folate Receptors’ Expression in Gliomas May Possess Potential Nanoparticle-Based Drug Delivery Opportunities. ACS Omega 2021, 6, 4111–4118. [Google Scholar] [CrossRef] [PubMed]
- Rijnboutt, S.; Jansen, G.; Posthuma, G.; Hynes, J.B.; Schornagel, J.H.; Strous, G.J. Endocytosis of GPI-linked membrane folate receptor-alpha. J. Cell Biol. 1996, 132, 35–47. [Google Scholar] [CrossRef]
- Leone, J.P.; Bhargava, R.; Theisen, B.K.; Hamilton, R.L.; Lee, A.V.; Brufsky, A.M. Expression of high affinity folate receptor in breast cancer brain metastasis. Oncotarget 2015, 6, 30327–30333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jolivet, J.; Cowan, K.H.; Curt, G.A.; Clendeninn, N.J.; Chabner, B.A. The Pharmacology and Clinical Use of Methotrexate. N. Engl. J. Med. 1983, 309, 1094–1104. [Google Scholar] [CrossRef]
- Giri, B.R.; Kim, J.S.; Park, J.H.; Jin, S.G.; Kim, K.S.; ud Din, F.; Choi, H.G.; Kim, D.W. Improved Bioavailability and High Photostability of Methotrexate by Spray-Dried Surface-Attached Solid Dispersion with an Aqueous Medium. Pharmaceutics 2021, 13, 111. [Google Scholar] [CrossRef]
- Khalili, L.; Dehghan, G.; Hosseinpour Feizi, M.A.; Sheibani, N.; Hamishekar, H. Development of an albumin decorated lipid-polymer hybrid nanoparticle for simultaneous delivery of methotrexate and conferone to cancer cells. Int. J. Pharm. 2021, 599, 120421. [Google Scholar] [CrossRef]
- Shi, G.-N.; Hu, M.; Chen, C.; Fu, J.; Shao, S.; Zhou, Y.; Wu, L.; Zhang, T. Methotrexate enhances antigen presentation and maturation of tumour antigen-loaded dendritic cells through NLRP3 inflammasome activation: A strategy for dendritic cell-based cancer vaccine. Adv. Med. Oncol. 2021, 13, 1758835920987056. [Google Scholar] [CrossRef]
- Rajalingam, K.; Krishnaswami, V.; Alagarsamy, S.; Kandasamy, R. Solubility Enhancement of Methotrexate by Solid Nanodispersion Approach for the Improved Treatment of Small Cell Lung Carcinoma. Curr. Top. Med. Chem. 2021, 21, 140–150. [Google Scholar] [CrossRef]
- Kohler, N.; Sun, C.; Wang, J.; Zhang, M. Methotrexate-Modified Superparamagnetic Nanoparticles and Their Intracellular Uptake into Human Cancer Cells. Langmuir 2005, 21, 8858–8864. [Google Scholar] [CrossRef]
- Wróbel, A.; Baradyn, M.; Ratkiewicz, A.; Drozdowska, D. Synthesis, Biological Activity, and Molecular Dynamics Study of Novel Series of a Trimethoprim Analogs as Multi-Targeted Compounds: Dihydrofolate Reductase (DHFR) Inhibitors and DNA-Binding Agents. Int. J. Mol. Sci. 2021, 22, 3685. [Google Scholar] [CrossRef]
- Davies, J.F.; Delcamp, T.J.; Prendergast, N.J.; Ashford, V.A.; Freisheim, J.H.; Kraut, J. Crystal structures of recombinant human dihydrofolate reductase complexed with folate and 5-deazafolate. Biochemistry 1990, 29, 9467–9479. [Google Scholar] [CrossRef] [PubMed]
- White, J.C.; Goldman, I.D. Mechanism of action of methotrexate. IV. Free intracellular methotrexate required to suppress dihydrofolate reduction to tetrahydrofolate by Ehrlich ascites tumor cells in vitro. Mol. Pharm. 1976, 12, 711–719. [Google Scholar]
- Chabner, B.A.; Roberts, T.G. Chemotherapy and the war on cancer. Nat. Rev. Cancer 2005, 5, 65–72. [Google Scholar] [CrossRef]
- Hada, A.-M.; Craciun, A.-M.; Focsan, M.; Borlan, R.; Soritau, O.; Todea, M.; Astilean, S. Folic acid functionalized gold nanoclusters for enabling targeted fluorescence imaging of human ovarian cancer cells. Talanta 2021, 225, 121960. [Google Scholar] [CrossRef]
- de Oliveira, A.L.C.; Zerillo, L.; Cruz, L.J.; Schomann, T.; Chan, A.B.; de Carvalho, T.G.; Souza, S.V.D.P.; Araújo, A.A.; de Geus-Oei, L.F.; de Araújo Júnior, R.F. Maximizing the potency of oxaliplatin coated nanoparticles with folic acid for modulating tumor progression in colorectal cancer. Mater. Sci. Eng. C 2021, 120, 111678. [Google Scholar] [CrossRef] [PubMed]
- Marko, A.J.; Borah, B.M.; Siters, K.E.; Missert, J.R.; Gupta, A.; Pera, P.; Isaac-Lam, M.F.; Pandey, R.K. Targeted Nanoparticles for Fluorescence Imaging of Folate Receptor Positive Tumors. Biomolecules 2020, 10, 1651. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Han, S.; Zhang, Z.; Wang, J.; Zhang, G. Preparation and Performance of Chemotherapy Drug-Loaded Graphene Oxide-Based Nanosheets That Target Ovarian Cancer Cells via Folate Receptor Mediation. J. Biomed. Nanotechnol. 2021, 17, 960–970. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wu, C.; Duan, J.; Miao, J.; Ren, H.; Liu, J. Anti-Glioma Effect with Targeting Therapy Using Folate Modified Nano-Micelles Delivery Curcumin. J. Biomed. Nanotechnol. 2020, 16, 1–13. [Google Scholar] [CrossRef]
- Yang, R.; An, Y.; Miao, F.; Li, M.; Liu, P.; Tang, Q. Preparation of folic acid-conjugated, doxorubicin-loaded, magnetic bovine serum albumin nanospheres and their antitumor effects in vitro and in vivo. Int. J. Nanomed. 2014, 9, 4231–4243. [Google Scholar] [CrossRef] [Green Version]
- Matsunaga, Y.; Yamaoka, T.; Ohba, M.; Miura, S.; Masuda, H.; Sangai, T.; Takimoto, M.; Nakamura, S.; Tsurutani, J. Novel Anti-FOLR1 Antibody-Drug Conjugate MORAb-202 in Breast Cancer and Non-Small Cell Lung Cancer Cells. Antibodies 2021, 10, 6. [Google Scholar] [CrossRef] [PubMed]
- Shahzad Lodhi, M.; Qadir Samra, Z. Purification of transferrin by magnetic nanoparticles and conjugation with cysteine capped gold nanoparticles for targeting diagnostic probes. Prep. Biochem. Biotechnol. 2019, 49, 961–973. [Google Scholar] [CrossRef] [PubMed]
- Lodhi, M.S.; Shaheen, A.; Khan, M.T.; Shafiq, M.I.; Samra, Z.Q.; Wei, D.-Q. A novel method of affinity purification and characterization of polygalacturonase of Aspergillus flavus by galacturonic acid engineered magnetic nanoparticle. Food Chem. 2022, 372, 131317. [Google Scholar] [CrossRef]
- Lodhi, M.S.; Samra, Z.Q. Engineering Quantum Dot (Cadmium Sulfide) on Antibodies for Fluoroimmunoassays. J. Nanomater. 2020, 2020, 4707123. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Kochneva-Pervukhova, N.V.; Alexandrov, A.I.; Ter-Avanesyan, M.D. Amyloid-Mediated Sequestration of Essential Proteins Contributes to Mutant Huntingtin Toxicity in Yeast. PLoS ONE 2012, 7, e29832. [Google Scholar] [CrossRef] [Green Version]
- Basha, G.; Yap, P.; Penninckx, F. Comparative Study of Classical, Colorimetric and Immunologic Staining Methods for the Assessment of Tumor Cell Viability. Tumor Biol. 1996, 17, 354–361. [Google Scholar] [CrossRef]
- Lodhi, M.S.; Khan, M.T.; Aftab, S.; Samra, Z.Q.; Wang, H.; Wei, D.Q. A novel formulation of theranostic nanomedicine for targeting drug delivery to gastrointestinal tract cancer. Cancer Nano 2021, 12, 26. [Google Scholar] [CrossRef]
- Koźmiński, P.; Halik, P.K.; Chesori, R.; Gniazdowska, E. Common Shortcomings in Study on Radiopharmaceutical Design Research: A Case Study of 99mTc-Labelled Methotrexate. Molecules 2021, 26, 5862. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.; Javaid, F.; Chudasama, V. Advances in targeting the folate receptor in the treatment/imaging of cancers. Chem. Sci. 2018, 9, 790–810. [Google Scholar] [CrossRef] [Green Version]
- Fenech, M.; Rinaldi, J. The relationship between micronuclei in human lymphocytes and plasma levels of vitamin C, vitamin E, vitamin B12 and folic acid. Carcinogenesis 1994, 15, 1405–1411. [Google Scholar] [CrossRef]
- O’Shannessy, D.J.; Somers, E.B.; Albone, E.; Cheng, X.; Park, Y.C.; Tomkowicz, B.E.; Hamuro, Y.; Kohl, T.O.; Forsyth, T.M.; Smale, R.; et al. Characterization of the Human Folate Receptor Alpha Via Novel Antibody-Based Probes. Oncotarget 2011, 2, 1227–1243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez, J.A.; González, F.; Bonilla, F.A.; Zambrano, G.; Gómez, M.E. Synthesis and characterization of Fe3O4 magnetic nanofluid. Rev. Latinoam. Metal. Mater. 2010, 30, 60–66. [Google Scholar]
- Hussein-Al-Ali, S.H.; El Zowalaty, M.E.; Hussein, M.Z.; Ismail, M.; Webster, T.J. Synthesis, characterization, controlled release, and antibacterial studies of a novel streptomycin chitosan magnetic nanoantibiotic. Int. J. Nanomed. 2014, 9, 549. [Google Scholar]
- Firer, M.A.; Gellerman, G. Targeted drug delivery for cancer therapy: The other side of antibodies. J. Hematol. Oncol. 2012, 5, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, B.; Tomizawa, K.; Michiue, H.; Miyatake, S.; Han, X.-J.; Fujimura, A.; Seno, M.; Kirihata, M.; Matsui, H. Delivery of sodium borocaptate to glioma cells using immunoliposome conjugated with anti-EGFR antibodies by ZZ-His. Biomaterials 2009, 30, 1746–1755. [Google Scholar] [CrossRef]
- Bramhachari, P.V.; Mohana Sheela, G.; Prathyusha, A.M.V.N.; Madhavi, M.; Satish Kumar, K.; Reddy, N.N.R.; Berde, C.P. Advanced Immunotechnological Methods for Detection and Diagnosis of Viral Infections: Current Applications and Future Challenges. Dyn. Immune Act. Viral Dis. 2019, 261–275. [Google Scholar] [CrossRef] [Green Version]
- Romijn, J.C.; Verkoelen, C.F.; Schroeder, F.H. Application of the MTT assay to human prostate cancer cell lines in vitro: Establishment of test conditions and assessment of hormone-stimulated growth and drug-induced cytostatic and cytotoxic effects. Prostate 1988, 12, 99–110. [Google Scholar] [CrossRef]
- Hegazy, M.G.; Imam, A.M.; Abdelghany, B.E. Evaluation of cytotoxic and anticancer effect of Orobanche crenata methanolic extract on cancer cell lines. Tumour Biol. 2020, 42, 1010428320918685. [Google Scholar] [CrossRef]
- Duan, D.; Zhang, J.; Yao, J.; Liu, Y.; Fang, J. Targeting Thioredoxin Reductase by Parthenolide Contributes to Inducing Apoptosis of HeLa Cells*. J. Biol. Chem. 2016, 291, 10021–10031. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Wang, J.; Kaul, S.C.; Wadhwa, R.; Miyako, E. Folic Acid Receptor-Mediated Targeting Enhances the Cytotoxicity, Efficacy, and Selectivity of Withania somnifera Leaf Extract: In vitro and in vivo Evidence. Front. Oncol. 2019, 9, 602. [Google Scholar] [CrossRef] [Green Version]
- Chenthamara, D.; Subramaniam, S.; Ramakrishnan, S.G.; Krishnaswamy, S.; Essa, M.M.; Lin, F.-H.; Qoronfleh, M.W. Therapeutic efficacy of nanoparticles and routes of administration. Biomater. Res. 2019, 23, 20. [Google Scholar] [CrossRef]
- Doucette, M.M.; Stevens, V.L. Folate Receptor Function Is Regulated in Response to Different Cellular Growth Rates in Cultured Mammalian Cells. J. Nutr. 2001, 131, 2819–2825. [Google Scholar] [CrossRef] [PubMed]
- El-Boubbou, K. Magnetic iron oxide nanoparticles as drug carriers: Preparation, conjugation and delivery. Nanomedicine 2018, 13, 929–952. [Google Scholar] [CrossRef]
- Huang, J.; Li, Y.; Orza, A.; Lu, Q.; Guo, P.; Wang, L.; Yang, L.; Mao, H. Magnetic Nanoparticle Facilitated Drug Delivery for Cancer Therapy with Targeted and Image-Guided Approaches. Adv. Funct. Mater. 2016, 26, 3818–3836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chegini, S.P.; Varshosaz, J.; Taymouri, S. Recent approaches for targeted drug delivery in rheumatoid arthritis diagnosis and treatment. Artif. Cells Nanomed. Biotechnol. 2018, 46, 502–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, J.; Bhargava, P.; Bahadur, D. Methotrexate conjugated magnetic nanoparticle for targeted drug delivery and thermal therapy. J. Appl. Phys. 2014, 115, 17B516. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lodhi, M.S.; Khalid, F.; Khan, M.T.; Samra, Z.Q.; Muhammad, S.; Zhang, Y.-J.; Mou, K. A Novel Method of Magnetic Nanoparticles Functionalized with Anti-Folate Receptor Antibody and Methotrexate for Antibody Mediated Targeted Drug Delivery. Molecules 2022, 27, 261. https://doi.org/10.3390/molecules27010261
Lodhi MS, Khalid F, Khan MT, Samra ZQ, Muhammad S, Zhang Y-J, Mou K. A Novel Method of Magnetic Nanoparticles Functionalized with Anti-Folate Receptor Antibody and Methotrexate for Antibody Mediated Targeted Drug Delivery. Molecules. 2022; 27(1):261. https://doi.org/10.3390/molecules27010261
Chicago/Turabian StyleLodhi, Madeeha Shahzad, Fatima Khalid, Muhammad Tahir Khan, Zahoor Qadir Samra, Shabbir Muhammad, Yu-Juan Zhang, and Kejie Mou. 2022. "A Novel Method of Magnetic Nanoparticles Functionalized with Anti-Folate Receptor Antibody and Methotrexate for Antibody Mediated Targeted Drug Delivery" Molecules 27, no. 1: 261. https://doi.org/10.3390/molecules27010261