Cobalt Element Effect of Ternary Mesoporous Cerium Lanthanum Solid Solution for the Catalytic Conversion of Methanol and CO2 into Dimethyl Carbonate
Abstract
:1. Introduction
2. Results and Discussion
2.1. XRD Analysis
2.2. TEM Images
2.3. N2 Adsorption-Desorption
2.4. CO2-TPD
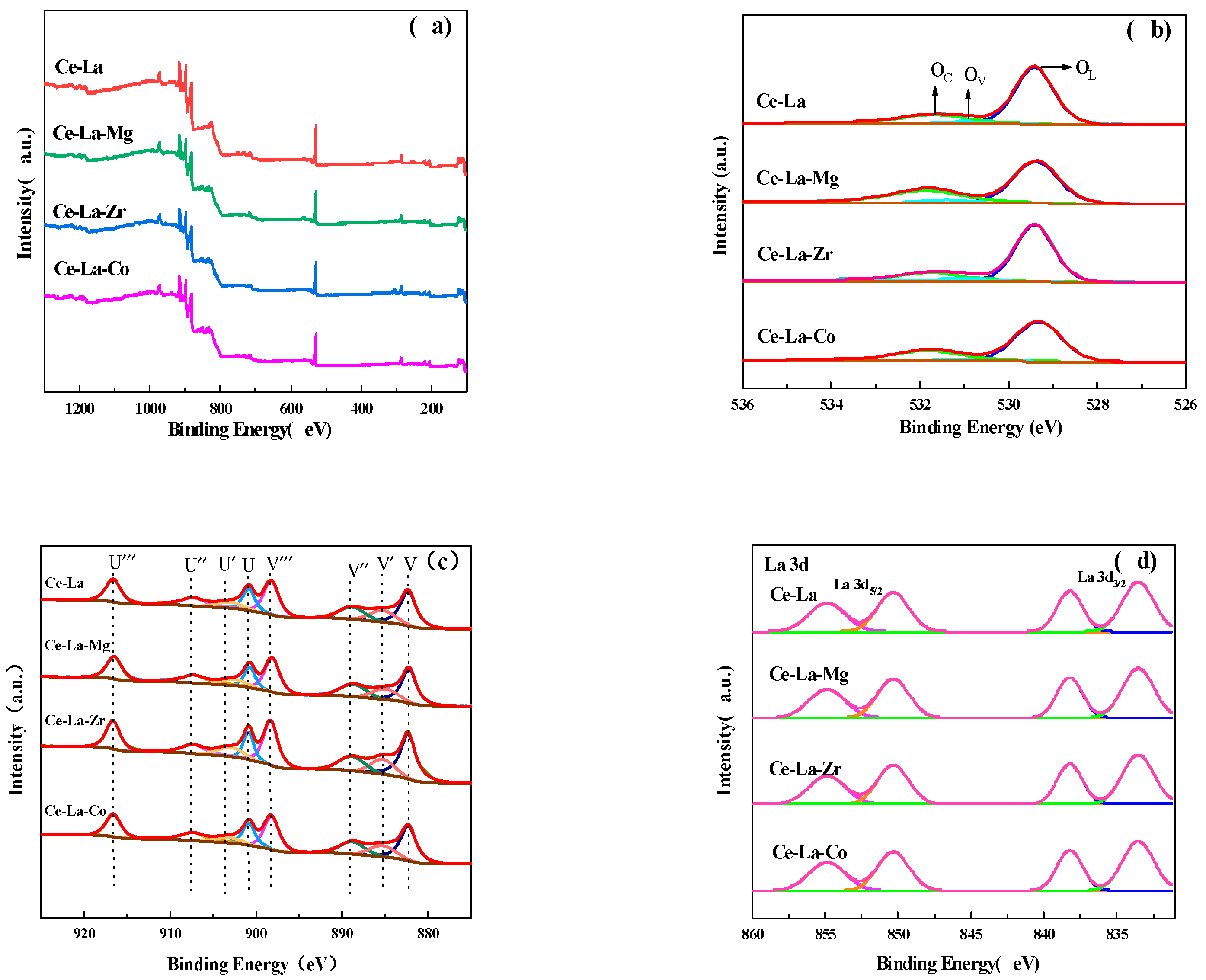
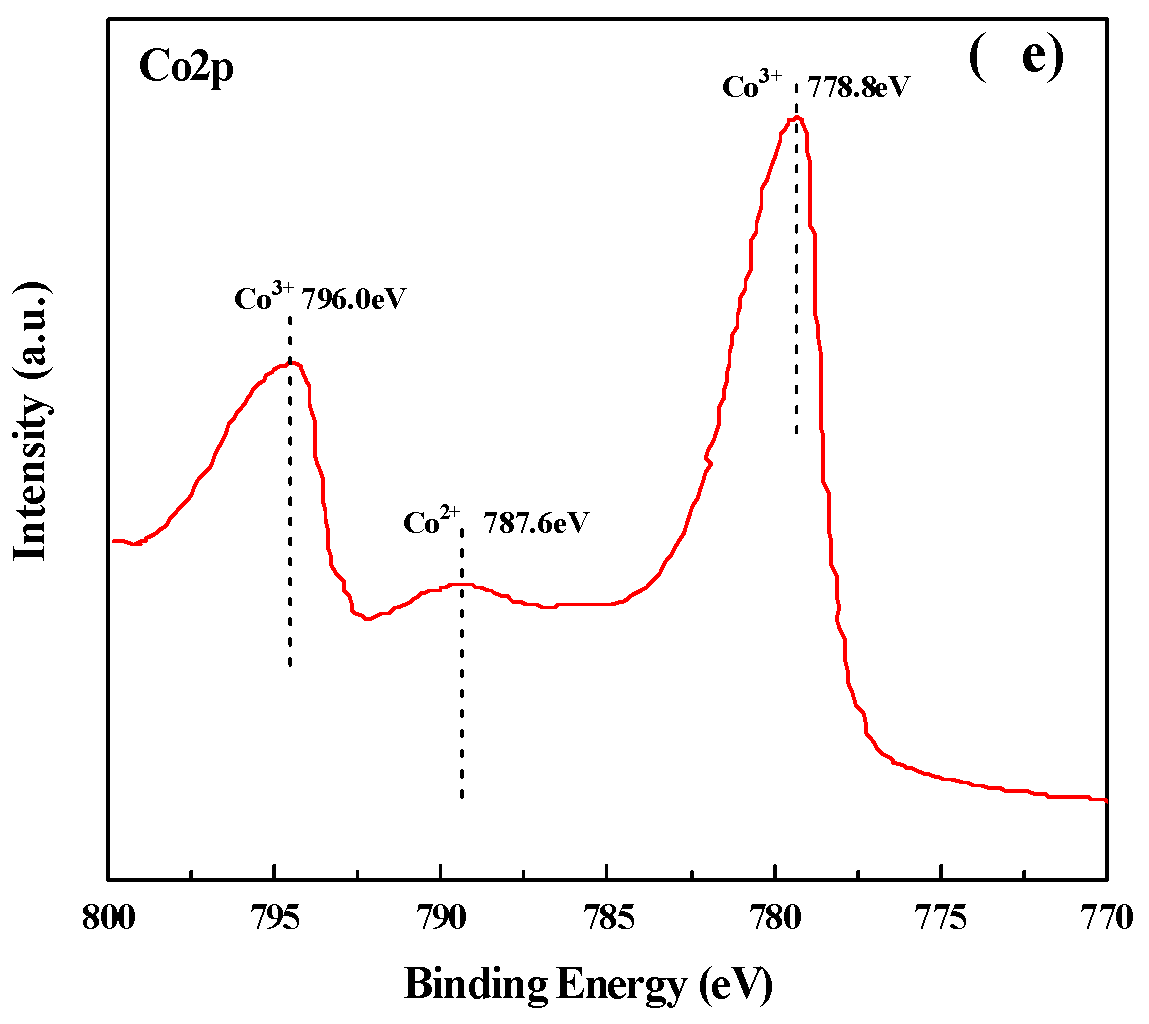
2.5. Concentration of the Surface Oxygen Vacancies and Chemical States of the Catalysts
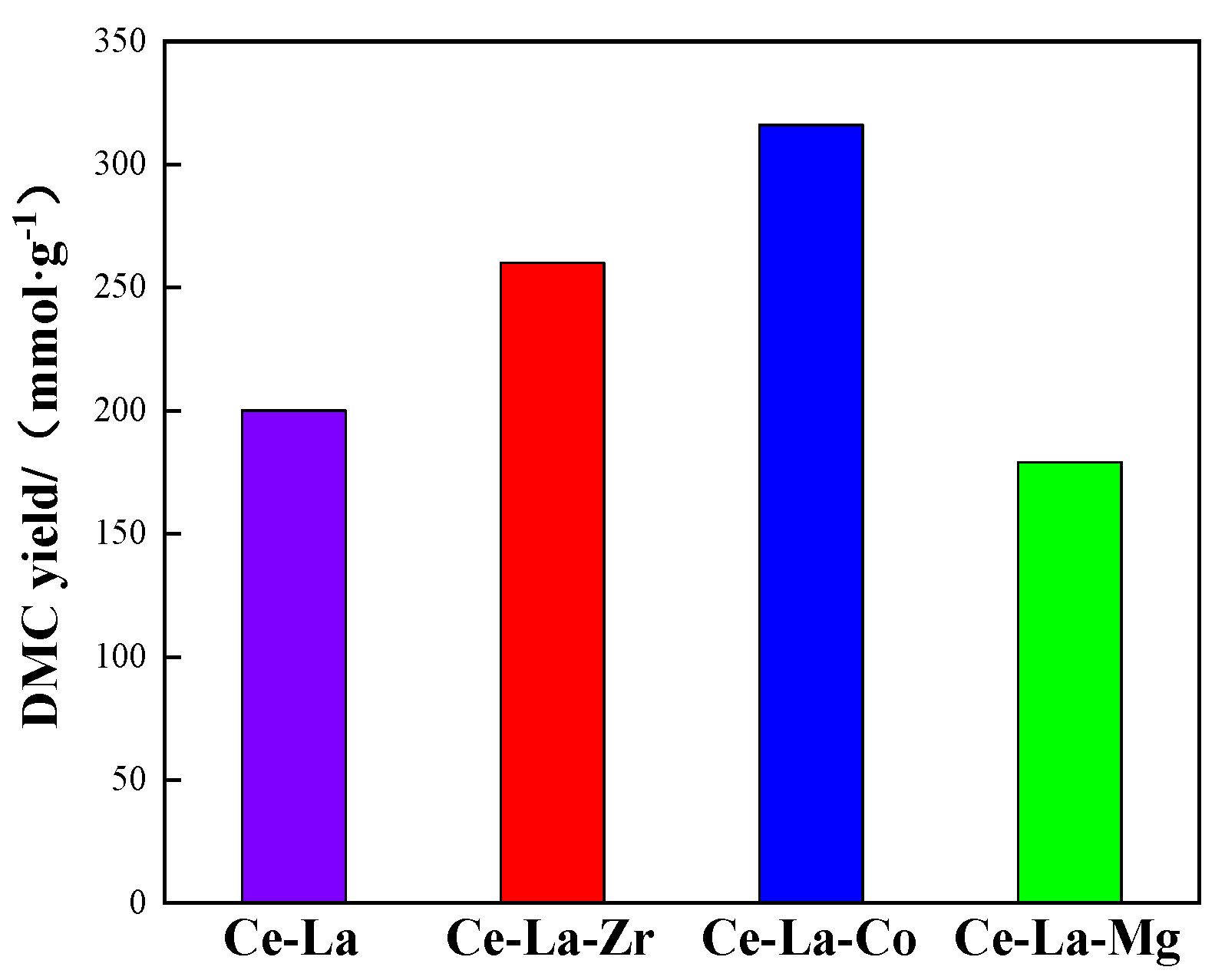
2.6. Catalytic Activity of the Catalysts for DMC Synthesis
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation
3.3. Physical Characterization
3.4. Catalytic Performance Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Tamboli, A.H.; Chaugule, A.A.; Kim, H. Catalytic developments in the direct dimethyl carbonate synthesis from carbon dioxide and methanol. Chem. Eng. J. 2017, 323, 530–544. [Google Scholar] [CrossRef]
- Aymes, D.; Ballivet-Tkatchenko, D.; Jeyalakshmi, K.; Saviot, L.; Vasireddy, S. A comparative study of methanol carbonation on unsupported SnO2 and ZrO2. Catal. Today 2009, 147, 62–67. [Google Scholar] [CrossRef]
- Cao, Y.; Cheng, H.; Ma, L.; Liu, F.; Liu, Z. Research Progress in the Direct Synthesis of Dimethyl Carbonate from CO2 and Methanol. Catal. Surv. Asia 2012, 16, 138–147. [Google Scholar] [CrossRef]
- Swallow, J.G.; Lee, J.K.; Defferriere, T.; Hughes, G.M.; Raja, S.N.; Tuller, H.L.; Warner, J.H.; Van Vliet, K.J. Atomic Resolution Imaging of Nanoscale Chemical Expansion in PrxCe1-xO2-delta during In Situ Heating. ACS Nano 2018, 12, 1359–1372. [Google Scholar] [CrossRef]
- Wang, J.; Xiao, X.; Liu, Y.; Pan, K.; Pang, H.; Wei, S. The application of CeO2-based materials in electrocatalysis. J. Mater. Chem. A 2019, 7, 17675–17702. [Google Scholar] [CrossRef]
- Yan, X.-H.; Zhang, H.-F.; Wu, C.-L.; Zhang, C.; Li, S.-H. Pd/CeO2/γ-Al2O3 Catalyst with Low Loading for Catalytic Oxidation of VOCs. J. Inorg. Mater. 2019, 34, 827–883. [Google Scholar]
- Kondratenko, E.V.; Sakamoto, Y.; Okumura, K.; Shinjoh, H. Time-resolved in situ UV/vis spectroscopy for analyzing the dynamics of surface and bulk redox processes in materials used as oxygen storage capacitors. Catal. Today 2011, 164, 46–51. [Google Scholar] [CrossRef]
- Zhang, B.; Li, D.; Wang, X. Catalytic performance of La–Ce–O mixed oxide for combustion of methane. Catal. Today 2010, 158, 348–353. [Google Scholar] [CrossRef]
- Reddy, B.M.; Katta, L.; Thrimurthulu, G. Novel Nanocrystalline Ce1−xLaxO2−δ (x = 0.2) Solid Solutions: Structural Characteristics and Catalytic Performance. Chem. Mater. 2009, 22, 467–475. [Google Scholar] [CrossRef]
- Wilkes, M. Catalytic studies on ceria lanthana solid solutions I. Oxidation of methane. J. Catal. 2003, 219, 286–294. [Google Scholar] [CrossRef]
- Mishra, B.G.; Rao, G.R. Promoting effect of ceria on the physicochemical and catalytic properties of CeO2–ZnO composite oxide catalysts. J. Mol. Catal. A Chem. 2006, 243, 204–213. [Google Scholar] [CrossRef]
- Joe, W.; Lee, H.J.; Hong, U.G.; Ahn, Y.S.; Song, C.J.; Kwon, B.J.; Song, I.K. Synthesis of dimethyl carbonate from urea and methanol over ZnO(X)–CeO2(1−X) catalysts prepared by a sol–gel method. J. Ind. Eng. Chem. 2012, 18, 1018–1022. [Google Scholar] [CrossRef]
- Joe, W.; Lee, H.J.; Hong, U.G.; Ahn, Y.S.; Song, C.J.; Kwon, B.J.; Song, I.K. Urea methanolysis to dimethyl carbonate over ZnO–CeO2–MO (MO: La2O3, Y2O3, Co2O3, Ga2O3, and ZrO2) catalysts. J. Ind. Eng. Chem. 2012, 18, 1730–1735. [Google Scholar] [CrossRef]
- Lu, X.; Wang, W.; Wei, S.; Guo, C.; Shao, Y.; Zhang, M.; Deng, Z.; Zhu, H.; Guo, W. Initial reduction of CO2 on perfect and O-defective CeO2 (111) surfaces: Towards CO or COOH? RSC Adv. 2015, 5, 97528–97535. [Google Scholar] [CrossRef]
- Stoian, D.; Medina, F.; Urakawa, A. Improving the Stability of CeO2 Catalyst by Rare Earth Metal Promotion and Molecular Insights in the Dimethyl Carbonate Synthesis from CO2 and Methanol with 2-Cyanopyridine. ACS Catal. 2018, 8, 3181–3193. [Google Scholar] [CrossRef] [Green Version]
- Atribak, I.; Buenolopez, A.; Garciagarcia, A. Combined removal of diesel soot particulates and NOx over CeO2–ZrO2 mixed oxides. J. Catal. 2008, 259, 123–132. [Google Scholar] [CrossRef] [Green Version]
- Harada, K.; Oishi, T.; Hamamoto, S.; Ishihara, T. Lattice Oxygen Activity in Pr- and La-Doped CeO2 for Low-Temperature Soot Oxidation. J. Phys. Chem. C 2013, 118, 559–568. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, L.; Wang, W.; Zhao, Y.; Zhang, G.; Ma, X.; Gong, J. Morphology control of ceria nanocrystals for catalytic conversion of CO2 with methanol. Nanoscale 2013, 5, 5582–5588. [Google Scholar] [CrossRef]
- Ansari, A.A.; Adil, S.F.; Alam, M.; Ahmad, N.; Assal, M.E.; Labis, J.P.; Alwarthan, A. Catalytic performance of the Ce-doped LaCoO3 perovskite nanoparticles. Sci. Rep. 2020, 10, 15012. [Google Scholar] [CrossRef]
- Li, L.; Chen, F.; Lu, J.Q.; Luo, M.F. Study of defect sites in Ce1-xMxO2-delta (x = 0.2) solid solutions using Raman spectroscopy. J. Phys. Chem. A 2011, 115, 7972–7977. [Google Scholar] [CrossRef]
- La, K.W.; Youn, M.H.; Chung, J.S.; Baeck, S.H.; Song, I.K. Synthesis of Dimethyl Carbonate from Methanol and Carbon Dioxide by Heteropolyacid/Metal Oxide Catalysts. Solid State Phenom. 2007, 119, 287–290. [Google Scholar] [CrossRef]
- Liu, X.; Ding, J.; Lin, X.; Gao, R.; Li, Z.; Dai, W.-L. Zr-doped CeO2 nanorods as versatile catalyst in the epoxidation of styrene with tert-butyl hydroperoxide as the oxidant. Appl. Catal. A Gen. 2015, 503, 117–123. [Google Scholar] [CrossRef]
- Matarrese, R.; Morandi, S.; Castoldi, L.; Villa, P.; Lietti, L. Removal of NOx and soot over Ce/Zr/K/Me (Me = Fe, Pt, Ru, Au) oxide catalysts. Appl. Catal. B Environ. 2017, 201, 318–330. [Google Scholar] [CrossRef]
- Zhu, S.; Lian, X.; Fan, T.; Chen, Z.; Dong, Y.; Weng, W.; Yi, X.; Fang, W. Thermally stable core-shell Ni/nanorod-CeO2@SiO2 catalyst for partial oxidation of methane at high temperatures. Nanoscale 2018, 10, 14031–14038. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Wang, W.; Zhang, L.; Zheng, Y.; Wang, Z. Insights into the Surface-Defect Dependence of Photoreactivity over CeO2 Nanocrystals with Well-Defined Crystal Facets. ACS Catal. 2017, 7, 4716–4735. [Google Scholar] [CrossRef]
- Zhang, X.; Li, H.; Hou, F.; Yang, Y.; Dong, H.; Liu, N.; Wang, Y.; Cui, L. Synthesis of highly efficient Mn2O3 catalysts for CO oxidation derived from Mn-MIL-100. Appl. Surf. Sci. 2017, 411, 27–33. [Google Scholar] [CrossRef]
- Postole, G.; Chowdhury, B.; Karmakar, B.; Pinki, K.; Banerji, J.; Auroux, A. Knoevenagel condensation reaction over acid–base bifunctional nanocrystalline CexZr1−xO2 solid solutions. J. Catal. 2010, 269, 110–121. [Google Scholar] [CrossRef]
- Tomishige, K.; Furusawa, Y.; Ikeda, Y.; Asadullah, M.; Fujimoto, K. CeO2–ZrO2 solid solution catalyst for selective synthesis ofdimethyl carbonate from methanol and carbon dioxide. Catal. Lett. 2001, 76, 71–74. [Google Scholar] [CrossRef]
- Kumar, P.; With, P.; Srivastava, V.C.; Shukla, K.; Gläser, R.; Mishra, I.M. Dimethyl carbonate synthesis from carbon dioxide using ceria–zirconia catalysts prepared using a templating method: Characterization, parametric optimization and chemical equilibrium modeling. RSC Adv. 2016, 6, 110235–110246. [Google Scholar] [CrossRef] [Green Version]
- Bêche, E.; Charvin, P.; Perarnau, D.; Abanades, S.; Flamant, G. Ce 3d XPS investigation of cerium oxides and mixed cerium oxide (CexTiyOz). Surf. Interface Anal. 2008, 40, 264–267. [Google Scholar] [CrossRef]
- Nagai, Y.; Yamamoto, T.; Tanaka, T.; Yoshida, S.; Nonaka, T.; Okamoto, T.; Suda, A.; Sugiura, M. X-ray absorption fine structure analysis of local structure of CeO2–ZrO2 mixed oxides with the same composition ratio (Ce/Zr = 1). Catal. Today 2002, 74, 225–234. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, Z.; Zheng, B.; Xie, Z.; Zheng, L. Synthesis and shape-dependent catalytic properties of CeO2 nanocubes and truncated octahedra. CrystEngComm 2012, 14, 7579–7582. [Google Scholar] [CrossRef]
- Wong, Y.C.; Tan, Y.P.; Taufiq-Yap, Y.H.; Ramli, I.; Tee, H.S. Biodiesel production via transesterification of palm oil by using CaO–CeO2 mixed oxide catalysts. Fuel 2015, 162, 288–293. [Google Scholar] [CrossRef]
- Taufiq-Yap, Y.H.; Teo, S.H.; Rashid, U.; Islam, A.; Hussien, M.Z.; Lee, K.T. Transesterification of Jatropha curcas crude oil to biodiesel on calcium lanthanum mixed oxide catalyst: Effect of stoichiometric composition. Energy Convers. Manag. 2014, 88, 1290–1296. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Y.; Liu, M.; Ma, Z. Complete catalytic oxidation of o-xylene over Mn–Ce oxides prepared using a redox-precipitation method. Catal. Today 2010, 153, 170–175. [Google Scholar] [CrossRef]
- Idriss, H.; Diagne, C.; Hindermann, J.P.; Kiennemann, A.; Barteau, M.A. Reactions of acetaldehyde on CeO2 and CeO2-supported catalysts. J. Catal. 1995, 14, 219–237. [Google Scholar] [CrossRef]
- O’Connell, M.; Norman, A.K.; Hüttermann, C.F.; Morris, M.A. Catalytic oxidation over lanthanum transition metal perovskite materials. Catal. Today 1999, 47, 123–132. [Google Scholar] [CrossRef]
- Taguchi, H.; Kido, H.; Tabata, K. Relationship between crystal structure and electrical property of K2NiF4-type (Ca1−xNd1+x)CoO4−δ. Phys. B Condens. Matter 2004, 344, 271–277. [Google Scholar] [CrossRef]
- Fu, Z.; Zhong, Y.; Yu, Y.; Long, L.; Xiao, M.; Han, D.; Wang, S.; Meng, Y. TiO2-Doped CeO2 Nanorod Catalyst for Direct Conversion of CO2 and CH3OH to Dimethyl Carbonate: Catalytic Performance and Kinetic Study. ACS Omega 2018, 3, 198–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, J.; Liu, C.; Mei, D.; Ge, Q. Active Oxygen Vacancy Site for Methanol Synthesis from CO2 Hydrogenation on In2O3(110): A DFT Study. ACS Catal. 2013, 3, 1296–1306. [Google Scholar] [CrossRef]
- Kumari, N.; Haider, M.A.; Agarwal, M.; Sinha, N.; Basu, S. Role of Reduced CeO2 (110) Surface for CO2 Reduction to CO and Methanol. J. Phys. Chem. C 2016, 120, 16626–16635. [Google Scholar] [CrossRef]
- Zhao, S.-Y.; Wang, S.-P.; Zhao, Y.-J.; Ma, X.-B. An in situ infrared study of dimethyl carbonate synthesis from carbon dioxide and methanol over well-shaped CeO2. Chin. Chem. Lett. 2017, 28, 65–69. [Google Scholar] [CrossRef]
- Zhang, M.; Xiao, M.; Wang, S.; Han, D.; Lu, Y.; Meng, Y. Cerium oxide-based catalysts made by template-precipitation for the dimethyl carbonate synthesis from Carbon dioxide and methanol. J. Clean. Prod. 2015, 103, 847–853. [Google Scholar] [CrossRef]
- Liu, B.; Li, C.; Zhang, G.; Yan, L.; Li, Z. Direct synthesis of dimethyl carbonate from CO2 and methanol over CaO–CeO2 catalysts: The role of acid–base properties and surface oxygen vacancies. New J. Chem. 2017, 41, 12231–12240. [Google Scholar] [CrossRef]
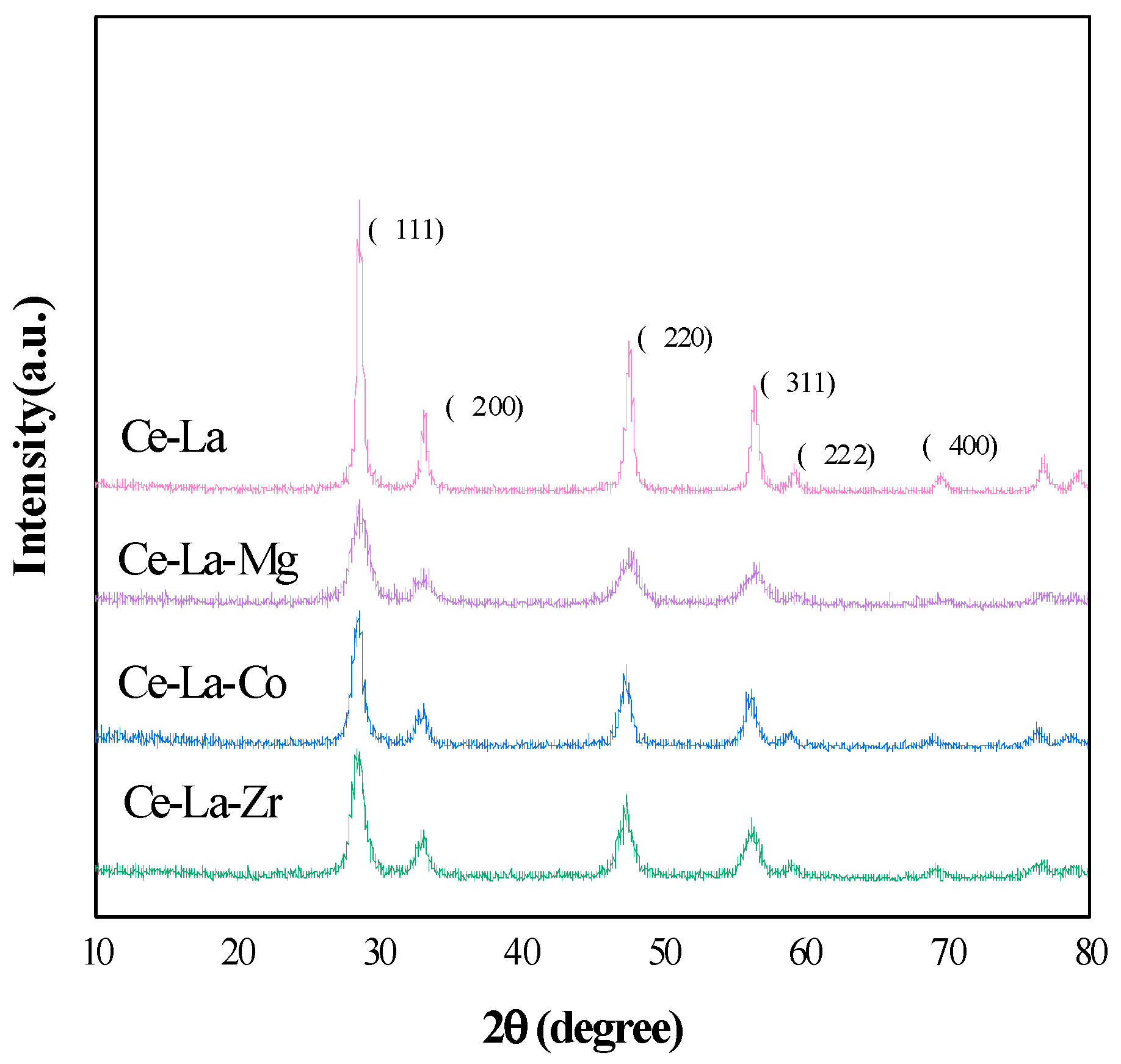
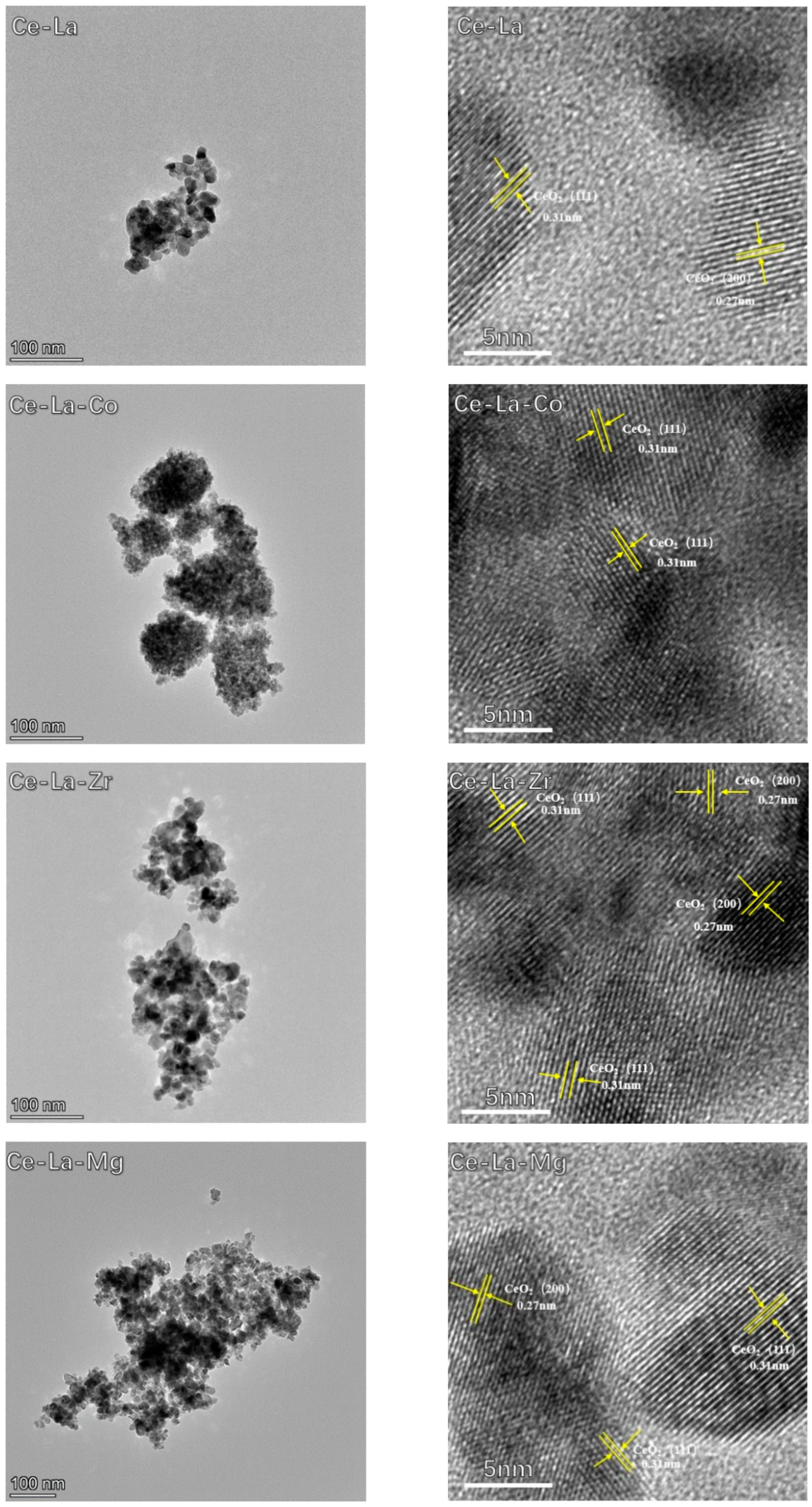
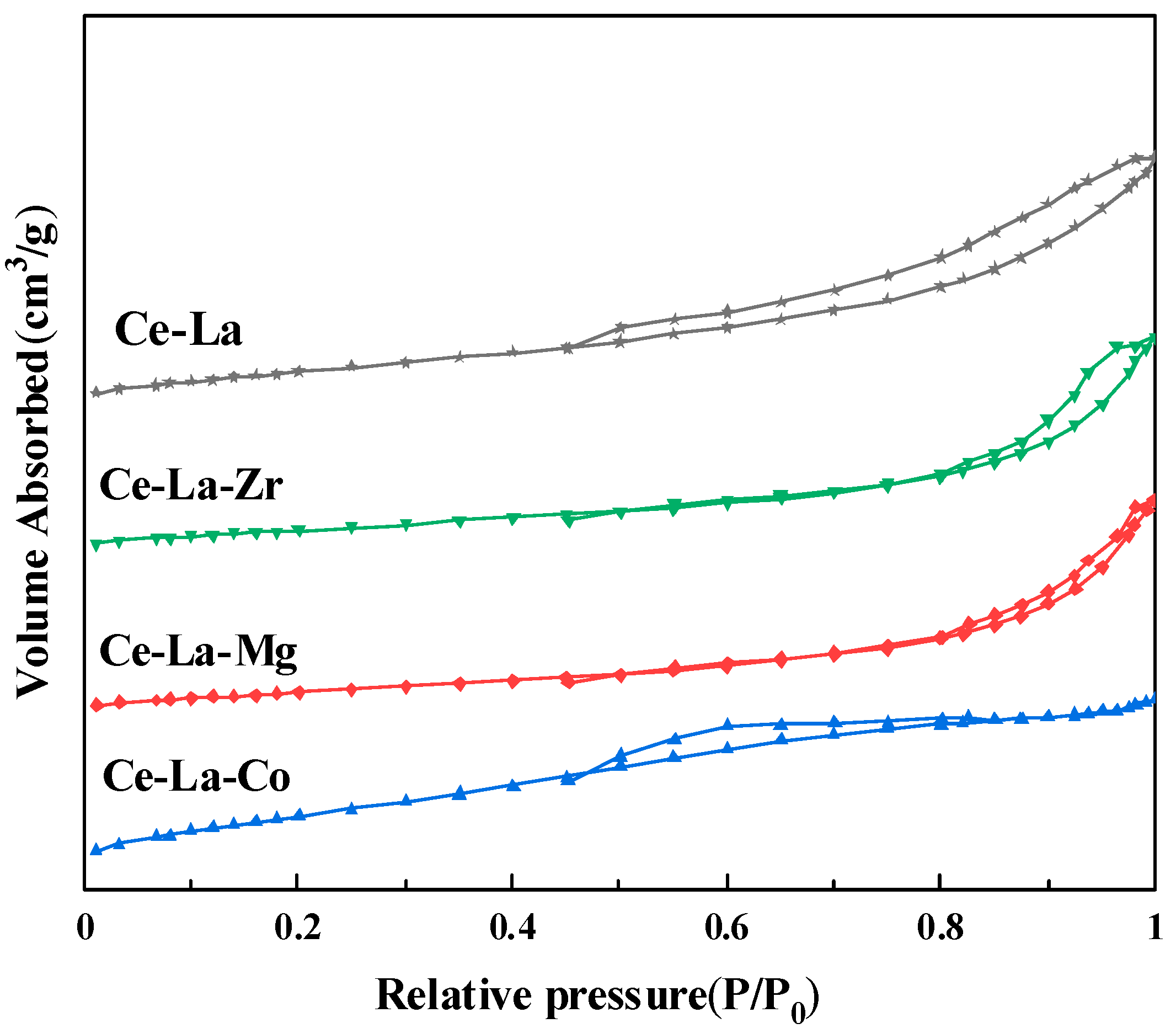
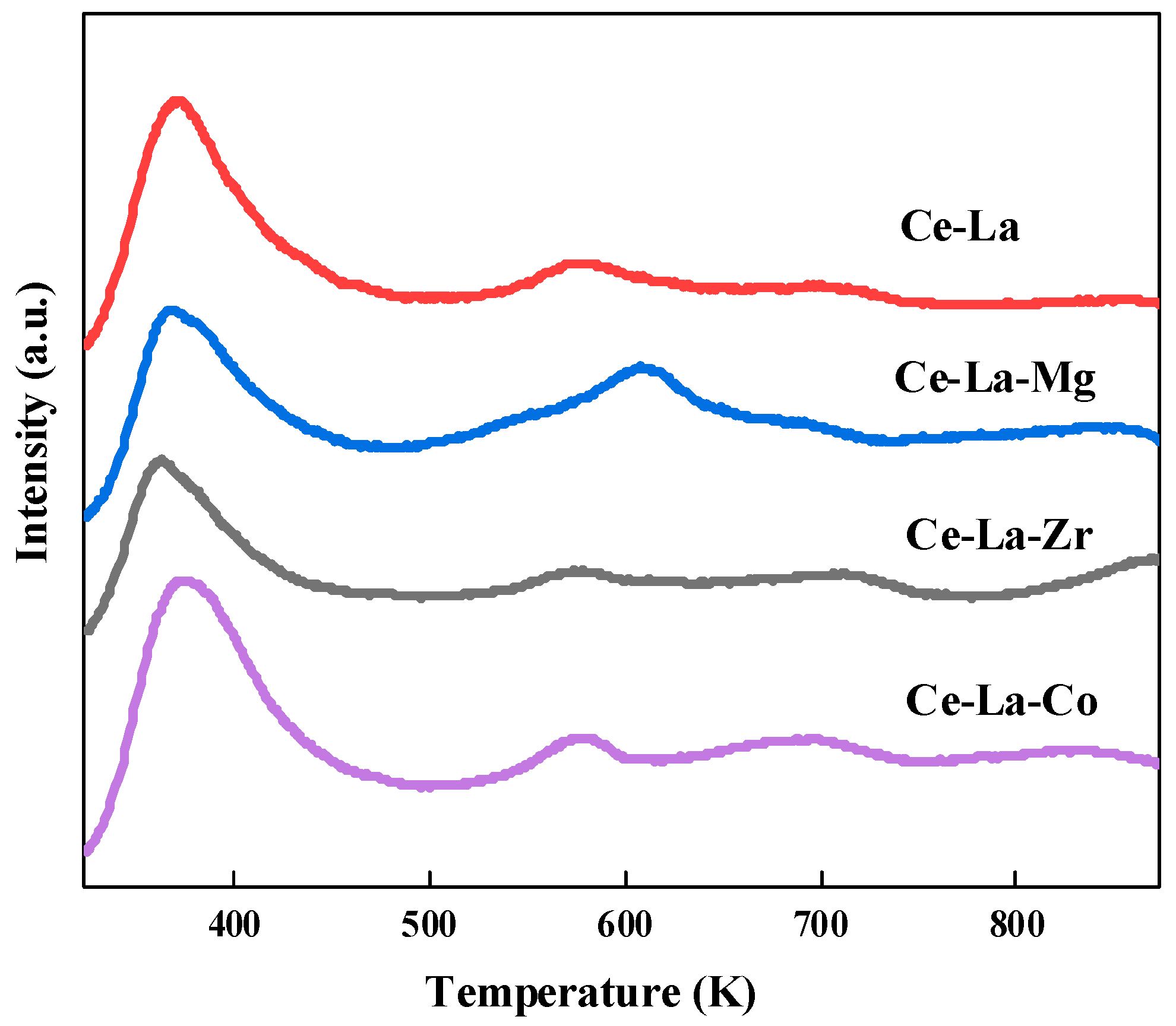

| Sample | (111) Plane | Lattice Parameter | Particle Size | |
|---|---|---|---|---|
| 2θ (°) | D (nm) | (nm) | (nm) | |
| Ce-La | 28.46 | 0.3132 | 0.54255 | 9.036 |
| Ce-La-Mg | 28.49 | 0.3129 | 0.54199 | 7.437 |
| Ce-La-Co | 28.56 | 0.3121 | 0.54069 | 8.718 |
| Ce-La-Zr | 28.61 | 0.3116 | 0.53977 | 9.111 |
| Sample | BET Surface Area/(m2/g) | Pore Size/(nm) | Pore Volume/(cm3/g) |
|---|---|---|---|
| Ce-La | 71 | 9.1 | 0.08 |
| Ce-La-Zr | 72 | 9.6 | 0.11 |
| Ce-La-Mg | 74 | 9.8 | 0.09 |
| Ce-La-Co | 77 | 9.9 | 0.13 |
| Sample | CO2 Adsorption (mmol/g) | |||
|---|---|---|---|---|
| Weak < 473 K | Medium-strength (473~673 K) | Strong > 673 K | Total | |
| Ce-La | 0.56 | 0.06 | 0.04 | 0.66 |
| Ce-La-Mg | 0.44 | 0.16 | 0.15 | 0.75 |
| Ce-La-Zr | 0.45 | 0.13 | 0.12 | 0.70 |
| Ce-La-Co | 0.55 | 0.17 | 0.07 | 0.79 |
| Sample | Molar Fraction (%) | ||
|---|---|---|---|
| Ce3+ (%) | Ce4+ (%) | OV (%) | |
| Ce-La | 15.32 | 84.68 | 9.47 |
| Ce-La-Mg | 15.43 | 84.57 | 9.94 |
| Ce-La-Zr | 15.74 | 84.26 | 10.28 |
| Ce-La-Co | 16.73 | 83.27 | 12.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Shi, H.; Gu, Y.; Cheng, Q.; Wang, Y. Cobalt Element Effect of Ternary Mesoporous Cerium Lanthanum Solid Solution for the Catalytic Conversion of Methanol and CO2 into Dimethyl Carbonate. Molecules 2022, 27, 270. https://doi.org/10.3390/molecules27010270
Li X, Shi H, Gu Y, Cheng Q, Wang Y. Cobalt Element Effect of Ternary Mesoporous Cerium Lanthanum Solid Solution for the Catalytic Conversion of Methanol and CO2 into Dimethyl Carbonate. Molecules. 2022; 27(1):270. https://doi.org/10.3390/molecules27010270
Chicago/Turabian StyleLi, Xu, Hua Shi, Yunhan Gu, Qingyan Cheng, and Yanji Wang. 2022. "Cobalt Element Effect of Ternary Mesoporous Cerium Lanthanum Solid Solution for the Catalytic Conversion of Methanol and CO2 into Dimethyl Carbonate" Molecules 27, no. 1: 270. https://doi.org/10.3390/molecules27010270






