Development and Characterization of Curcumin-Silver Nanoparticles as a Promising Formulation to Test on Human Pterygium-Derived Keratinocytes
Abstract
:1. Introduction
2. Results
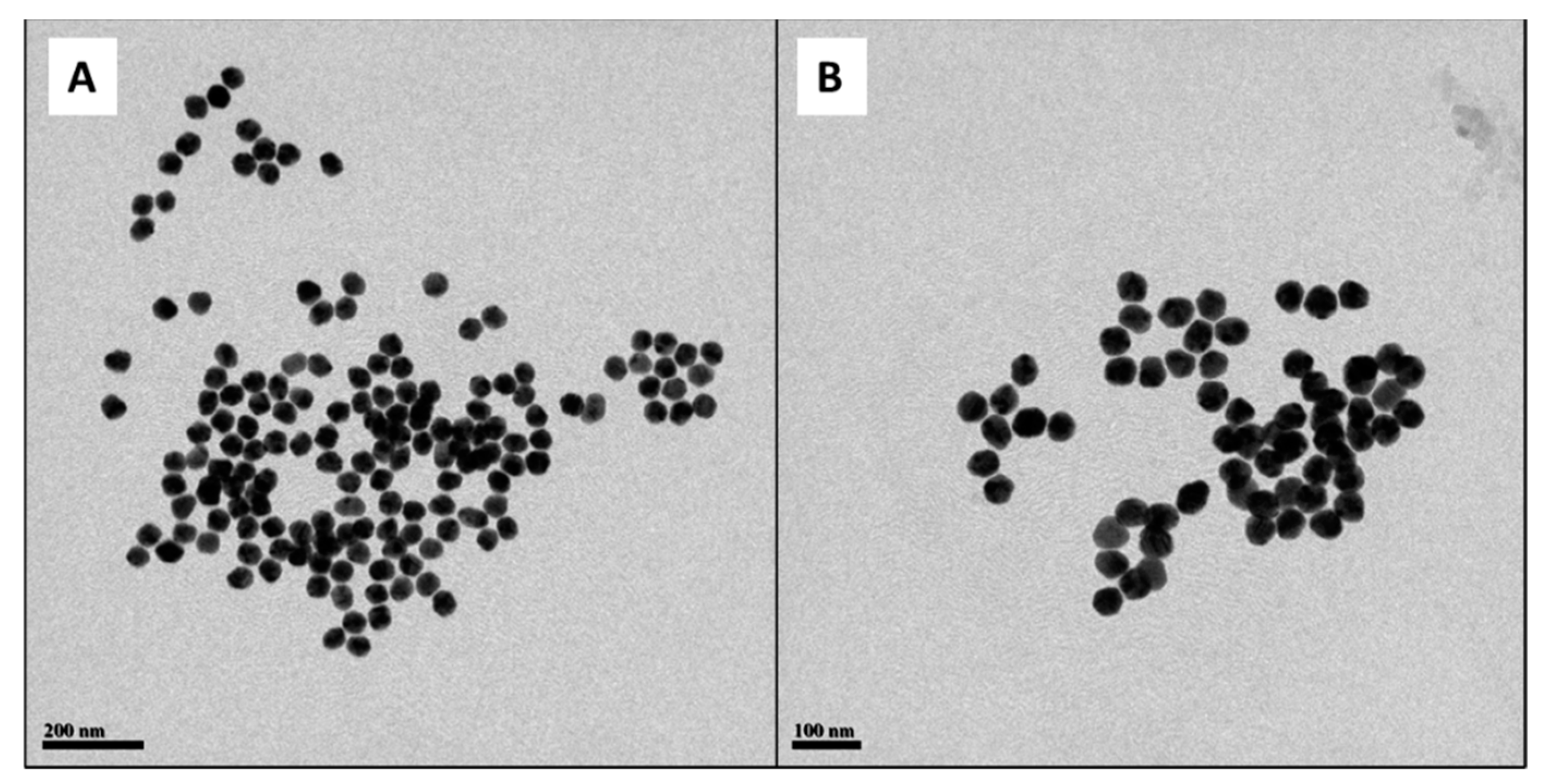
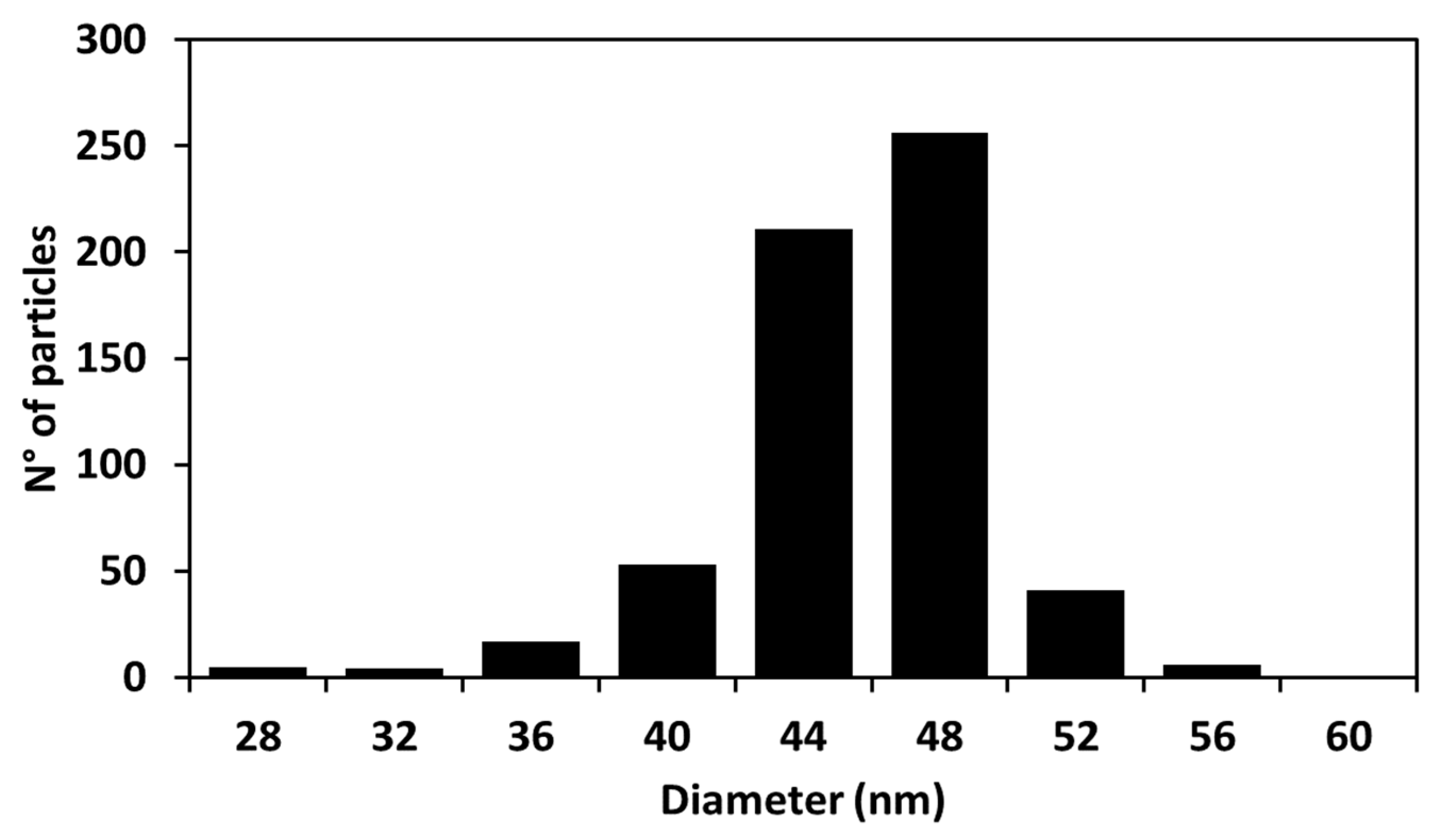
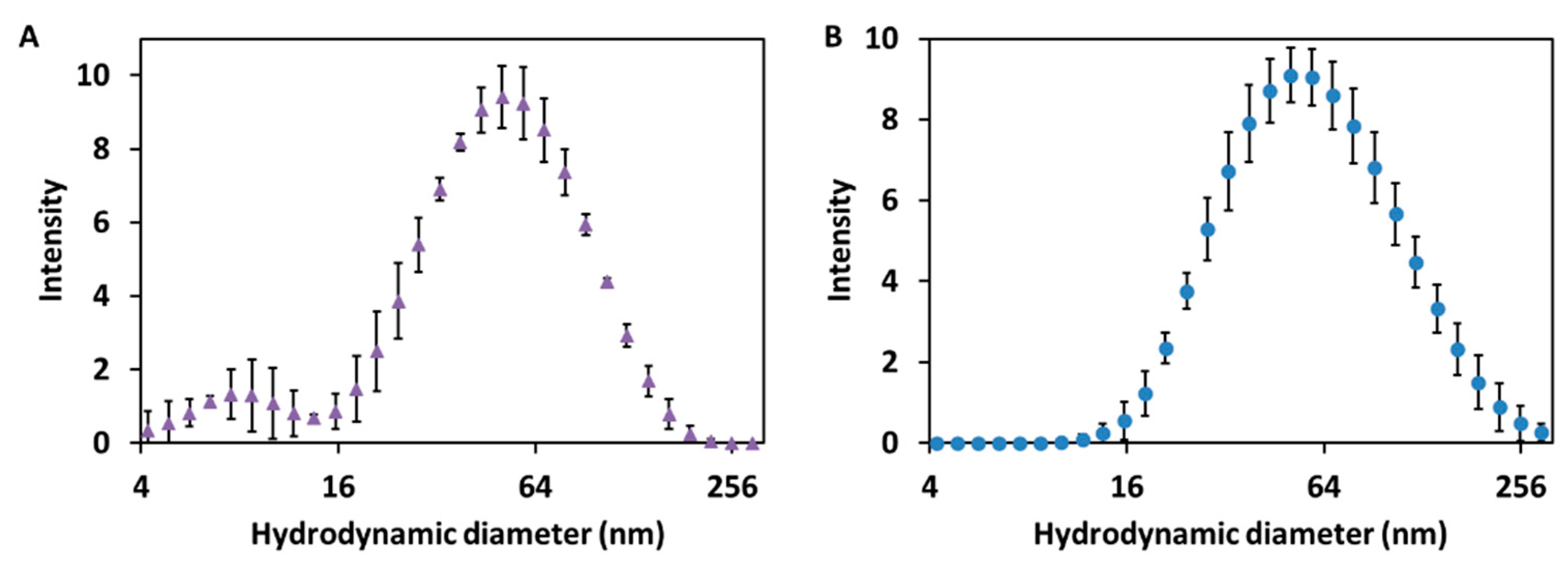
2.1. Study of the Size Distribution of Cur-AgNPs
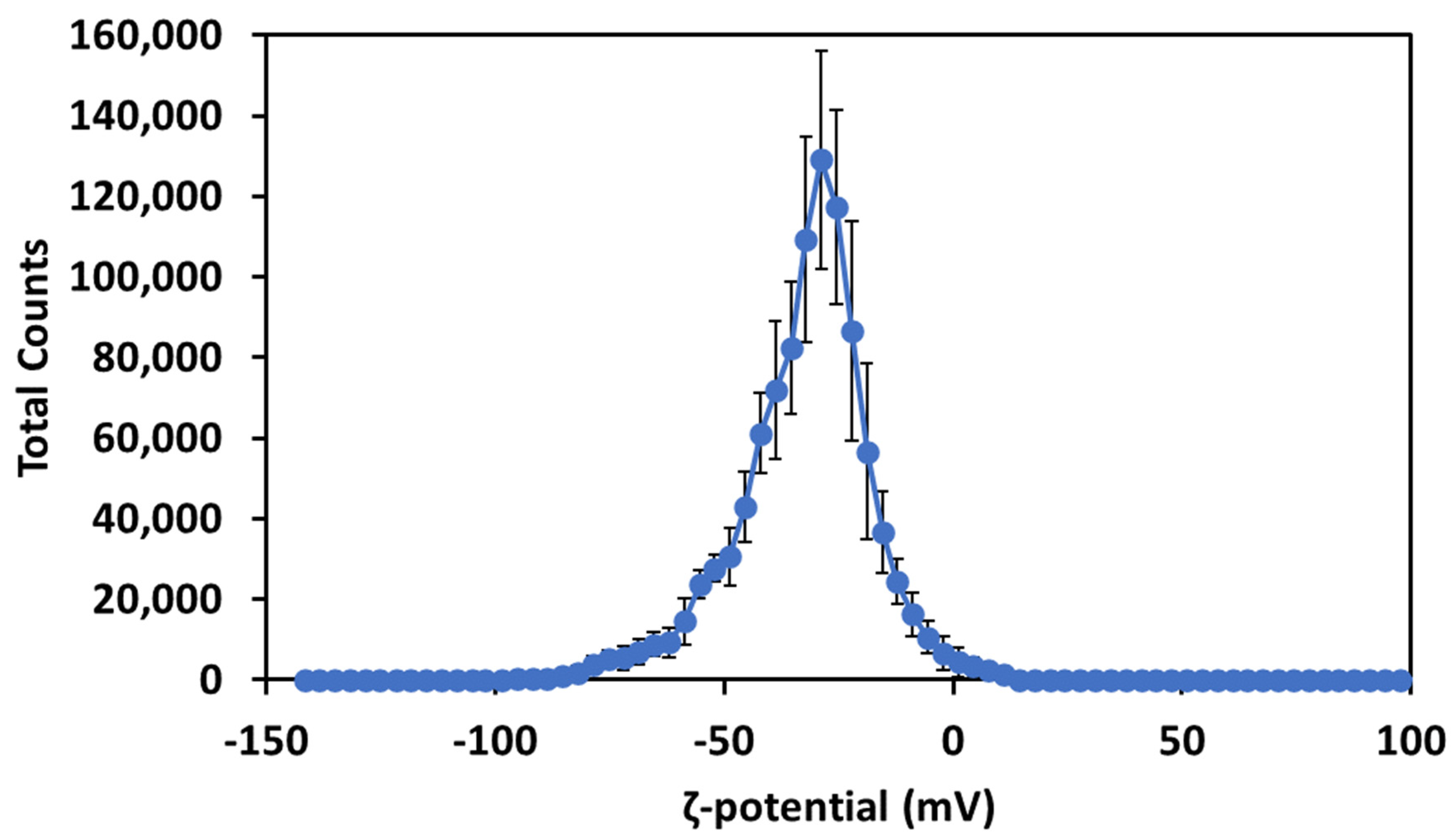
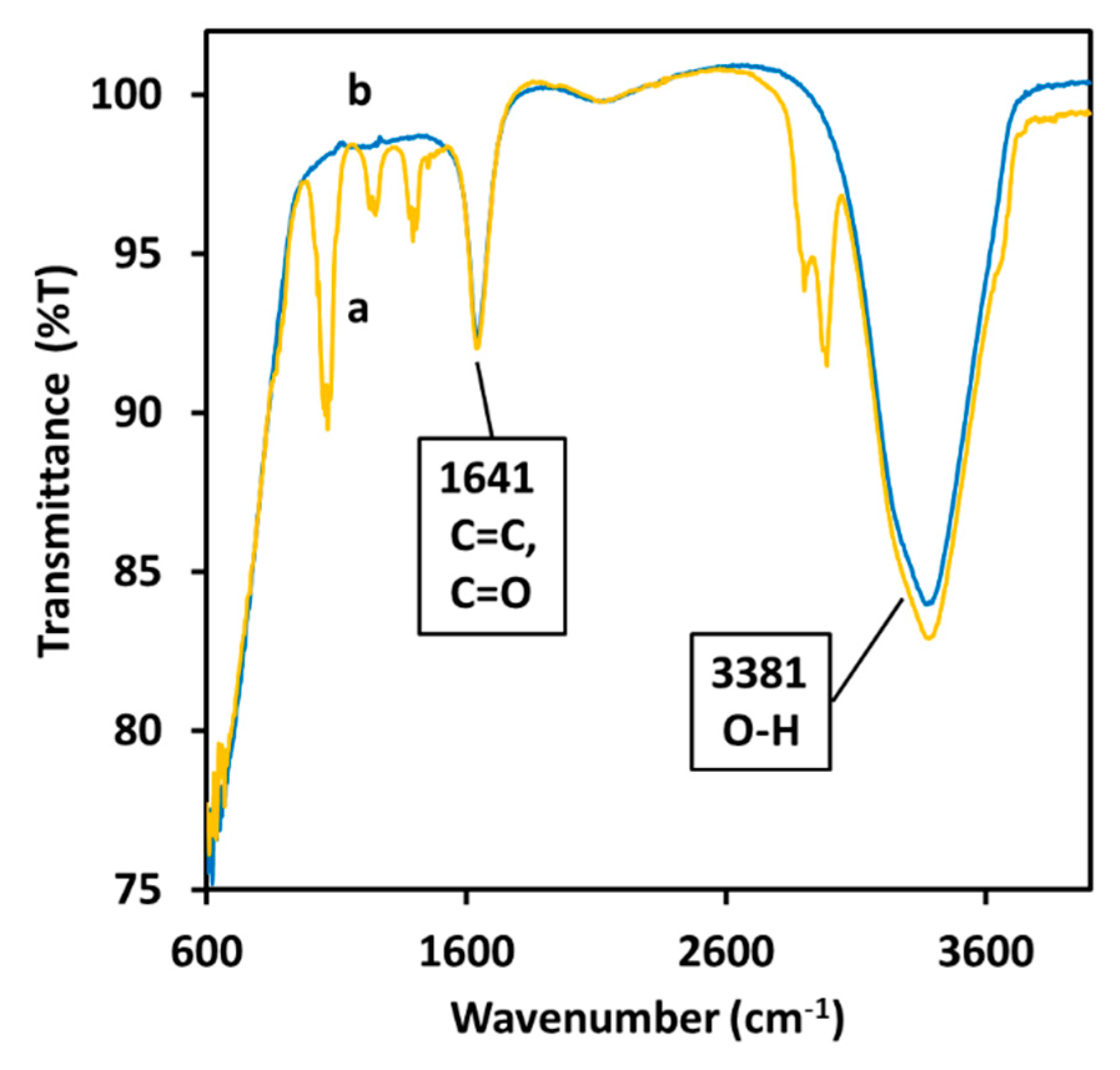
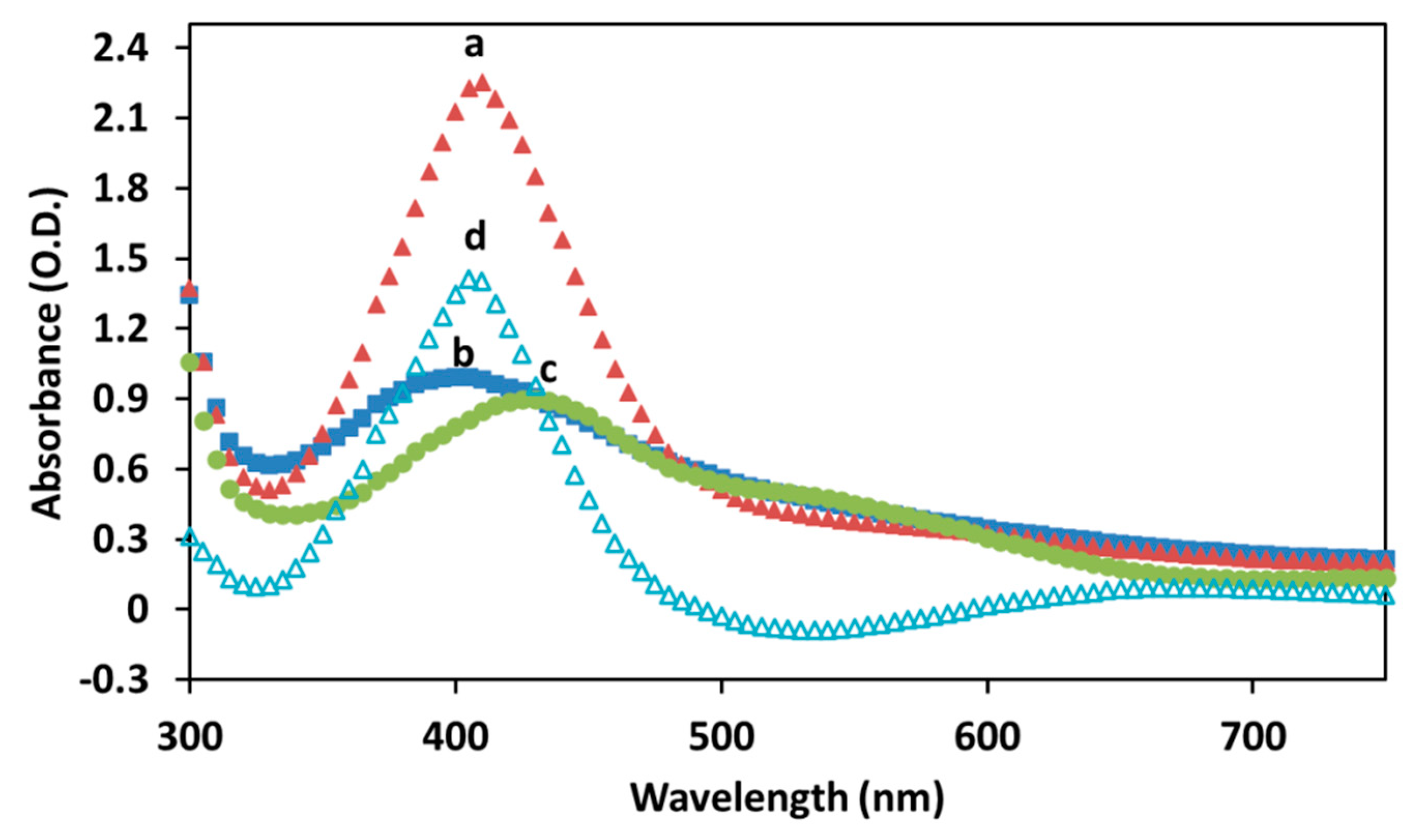
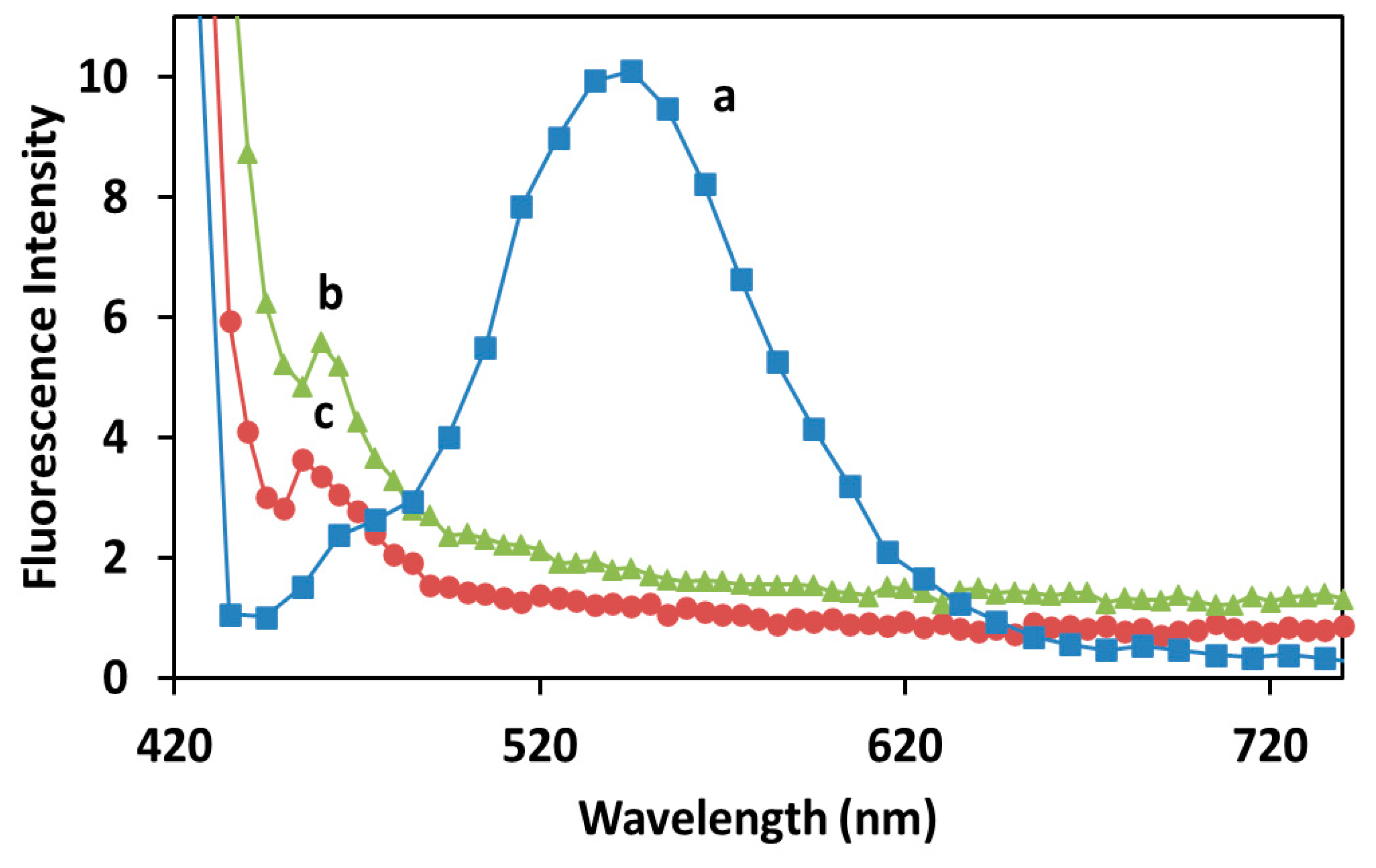
2.2. Study of the ζ-Potential and Optical Properties of Cur-AgNPs
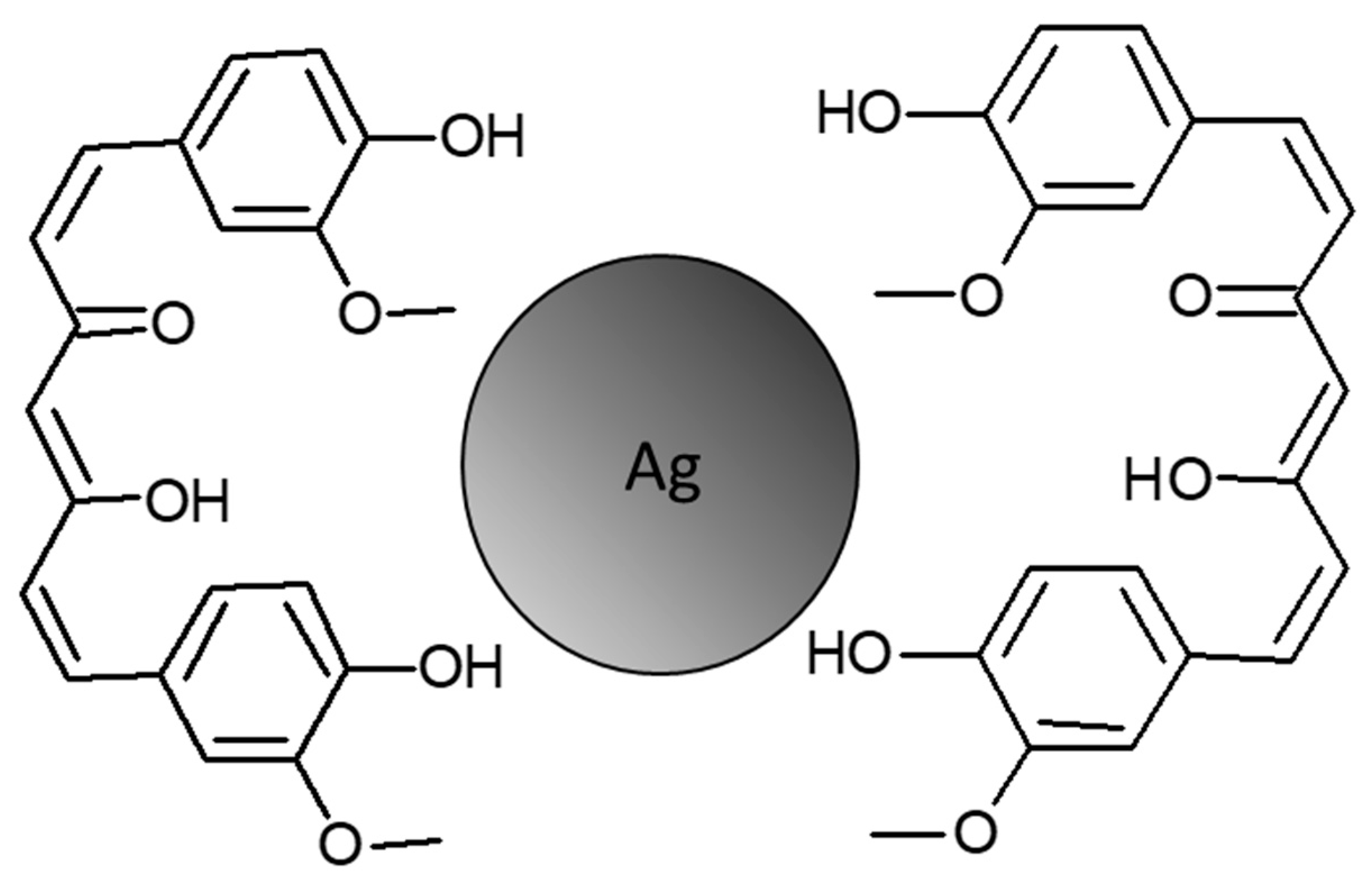
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Synthesis of Cur-AgNPs
4.2.2. Characterization of Cur-AgNPs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Sample Availability
References
- Singh, S.K. Pterygium: Epidemiology prevention and treatment. Community Eye Health 2017, 30, S5–S6. [Google Scholar]
- Hacıoğlu, D.; Erdöl, H. Developments and current approaches in the treatment of pterygium. Int. Ophthalmol. 2017, 37, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.P.; Sanghi, S.; Rohatgi, J.; Dhaliwal, U. Outcomes of preoperative intrapterygial injection of mitomycin C for pterygium excision with and without inferior conjunctival flap. Oman J. Ophthalmol. 2019, 12, 171. [Google Scholar] [CrossRef] [PubMed]
- Stati, G.; Sancilio, S.; Basile, M.; Angelini, A.; Di Pietro, R. Curcuma longa aqueous extract: A potential solution for the prevention of corneal scarring as a result of pterygium surgical excision (Review). Int. J. Mol. Med. 2020, 46, 1951–1957. [Google Scholar] [CrossRef] [PubMed]
- Sancilio, S.; Di Staso, S.; Sebastiani, S.; Centurione, L.; Di Girolamo, N.; Ciancaglini, M.; Di Pietro, R. Curcuma longa Is Able to Induce Apoptotic Cell Death of Pterygium-Derived Human Keratinocytes. BioMed Res. Int. 2017, 2017, 2956597. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Bian, F.; Wen, C.; Hao, N. Inhibitory effect of curcumin on proliferation of human pterygium fibroblasts. J. Huazhong Univ. Sci. Technol. 2007, 27, 339–342. [Google Scholar] [CrossRef]
- Abdollahi, E.; Momtazi, A.A.; Johnston, T.P.; Sahebkar, A. Therapeutic effects of curcumin in inflammatory and immune-mediated diseases: A nature-made jack-of-all-trades? J. Cell. Physiol. 2018, 233, 830–848. [Google Scholar] [CrossRef]
- Shah, D.; Savaliya, R.; Patel, P.; Kansara, K.; Pandya, A.; Dhawan, A.; Singh, S. Curcumin Ag nanoconjugates for improved therapeutic effects in cancer. Int. J. Nanomed. 2018, 13, 75–77. [Google Scholar] [CrossRef] [Green Version]
- Jaiswal, S.; Mishra, P. Antimicrobial and antibiofilm activity of curcumin-silver nanoparticles with improved stability and selective toxicity to bacteria over mammalian cells. Med. Microbiol. Immunol. 2018, 207, 39–53. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Wang, J.; Nie, X.; Wen, T.; Ji, Y.L.; Wu, X.C.; Zhao, Y.L.; Chen, C.Y. Near Infrared Laser-Induced Targeted Cancer Therapy Using Thermoresponsive Polymer Encapsulated Gold Nanorods. J. Am. Chem. Soc. 2014, 136, 7317–7326. [Google Scholar] [CrossRef]
- Sharma, S.K.; Shrivastava, N.; Rossi, F.; Tung, L.D.; Thanh, N.T.K. Nanoparticles-based magnetic and photo induced hyperthermia for cancer treatment. Nano Today 2019, 29, 100795. [Google Scholar] [CrossRef]
- Rossi, F.; Khoo, E.H.; Su, X.; Thanh, N.T.K. Study of the Effect of Anisotropic Gold Nanoparticles on Plasmonic Coupling with a Photosensitizer for Antimicrobial Film. ACS Appl. Bio Mater. 2020, 3, 315–326. [Google Scholar] [CrossRef] [Green Version]
- Waszczykowska, A.; Żyro, D.; Ochocki, J.; Jurowski, P. Clinical application and efficacy of silver drug in ophthalmology: A literature review and new formulation of eye drops with drug silver (i) complex of metronidazole with improved dosage form. Biomedicines 2021, 9, 210. [Google Scholar] [CrossRef] [PubMed]
- Abeleda, H.E.P.; Javier, A.P.; Murillo, A.Q.M.; Baculi, R.Q. Alpha-amylase conjugated biogenic silver nanoparticles as innovative strategy against biofilm-forming multidrug resistant bacteria. Biocatal. Agric. Biotechnol. 2020, 29, 101784. [Google Scholar] [CrossRef]
- Khristunova, E.; Barek, J.; Kratochvil, B.; Korotkova, E.; Dorozhko, E.; Vyskocil, V. Electrochemical immunoassay for the detection of antibodies to tick-borne encephalitis virus by using various types of bioconjugates based on silver nanoparticles. Bioelectrochemistry 2020, 135, 107576. [Google Scholar] [CrossRef]
- Prasher, P.; Sharma, M.; Mudila, H.; Gupta, G.; Sharma, A.K.; Kumar, D.; Bakshi, H.A.; Negi, P.; Kapoor, D.N.; Chellappan, D.K.; et al. Emerging trends in clinical implications of bio-conjugated silver nanoparticles in drug delivery. Colloids Interface Sci. Commun. 2020, 35, 100244. [Google Scholar] [CrossRef]
- Silva, L.R.; Gurgel, R.Q.; Lima, D.R.R.; Cuevas, L.E. Current usefulness of Credé’s method of preventing neonatal ophthalmia. Ann. Trop. Paediatr. 2013, 28, 45–48. [Google Scholar] [CrossRef] [Green Version]
- Ferdous, Z.; Nemmar, A. Health Impact of Silver Nanoparticles: A Review of the Biodistribution and Toxicity Following Various Routes of Exposure. Int. J. Mol. Sci. 2020, 21, 2375. [Google Scholar] [CrossRef] [Green Version]
- Qin, G.; Tang, S.; Li, S.; Lu, H.; Wang, Y.; Zhao, P.; Li, B.; Zhang, J.; Peng, L. Toxicological evaluation of silver nanoparticles and silver nitrate in rats following 28 days of repeated oral exposure. Environ. Toxicol. 2017, 32, 609–618. [Google Scholar] [CrossRef]
- Maneewattanapinyo, P.; Banlunara, W.; Thammacharoen, C.; Ekgasit, S.; Kaewamatawong, T. An evaluation of acute toxicity of colloidal silver nanoparticles. J. Vet. Med. Sci. 2011, 73, 1417–1423. [Google Scholar] [CrossRef] [Green Version]
- Weng, Y.; Liu, J.; Jin, S.; Guo, W.; Liang, X.; Hu, Z. Nanotechnology-based strategies for treatment of ocular disease. Acta Pharm. Sin. B 2017, 7, 281–291. [Google Scholar] [CrossRef] [Green Version]
- Bettini, S.; Pagano, R.; Valli, L.; Giancane, G. Drastic nickel ion removal from aqueous solution by curcumin-capped Ag nanoparticles. Nanoscale 2014, 6, 10113–10117. [Google Scholar] [CrossRef]
- Lee, W.-H.; Loo, C.-Y.; Bebawy, M.; Luk, F.; Mason, R.; Rohanizadeh, R. Curcumin and its Derivatives: Their Application in Neuropharmacology and Neuroscience in the 21st Century. Curr. Neuropharmacol. 2013, 11, 338–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hackley, V.A.; Clogston, J.D. Measuring the Hydrodynamic Size of Nanoparticles in Aqueous Media Using Batch-Mode Dynamic Light Scattering. Methods Mol. Biol. 2011, 697, 35–52. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Bott, S.; Huo, Q. Techniques for Accurate Sizing of Gold Nanoparticles Using Dynamic Light Scattering with Particular Application to Chemical and Biological Sensing Based on Aggregate Formation. ACS Appl. Mater. Interfaces 2016, 8, 21585–21594. [Google Scholar] [CrossRef]
- Lim, J.; Yeap, S.P.; Che, H.X.; Low, S.C. Characterization of magnetic nanoparticle by dynamic light scattering. Nanoscale Res. Lett. 2013, 8, 381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeta Potential—An Overview|ScienceDirect Topics. Available online: https://www.sciencedirect.com/topics/nursing-and-health-professions/zeta-potential (accessed on 18 May 2021).
- Xing, L.; Xiahou, Y.; Zhang, P.; Du, W.; Xia, H. Size Control Synthesis of Monodisperse, Quasi-Spherical Silver Nanoparticles to Realize Surface-Enhanced Raman Scattering Uniformity and Reproducibility. ACS Appl. Mater. Interfaces 2019, 11, 17637–17646. [Google Scholar] [CrossRef]
- Pescosolido, N.; Giannotti, R.; Plateroti, A.M.; Pascarella, A.; Nebbioso, M. Curcumin: Therapeutical potential in ophthalmology. Planta Med. 2014, 80, 249–254. [Google Scholar] [CrossRef] [Green Version]
- Schepers, E.; Glorieux, G.; Eloot, S.; Hulko, M.; Boschetti-de-Fierro, A.; Beck, W.; Krause, B.; Van Biesen, W. Assessment of the association between increasing membrane pore size and endotoxin permeability using a novel experimental dialysis simulation set-up. BMC Nephrol. 2018, 19, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanaor, D.; Michelazzi, M.; Leonelli, C.; Sorrell, C.C. The effects of carboxylic acids on the aqueous dispersion and electrophoretic deposition of ZrO2. J. Eur. Ceram. Soc. 2012, 32, 235–244. [Google Scholar] [CrossRef] [Green Version]
- IR Spectrum Table. Available online: https://www.sigmaaldrich.com/IT/it/technical-documents/technical-article/analytical-chemistry/photometry-and-reflectometry/ir-spectrum-table (accessed on 18 June 2021).
- Guzatov, D.V.; Vaschenko, S.V.; Stankevich, V.V.; Lunevich, A.Y.; Glukhov, Y.F.; Gaponenko, S.V. Plasmonic enhancement of molecular fluorescence near silver nanoparticles: Theory, modeling, and experiment. J. Phys. Chem. C 2012, 116, 10723–10733. [Google Scholar] [CrossRef]
- Ghosh, D.; Chattopadhyay, N. Gold and silver nanoparticles based superquenching of fluorescence: A review. J. Lumin. 2015, 160, 223–232. [Google Scholar] [CrossRef]
- Skirtach, A.G.; Déjugnat, C.; Braun, D.; Susha, A.S.; Rogach, A.L.; Sukhorukov, G.B. Nanoparticles distribution control by polymers: Aggregates versus nonaggregates. J. Phys. Chem. C 2007, 111, 555–564. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stati, G.; Rossi, F.; Trakoolwilaiwan, T.; Tung, L.D.; Mourdikoudis, S.; Thanh, N.T.K.; Di Pietro, R. Development and Characterization of Curcumin-Silver Nanoparticles as a Promising Formulation to Test on Human Pterygium-Derived Keratinocytes. Molecules 2022, 27, 282. https://doi.org/10.3390/molecules27010282
Stati G, Rossi F, Trakoolwilaiwan T, Tung LD, Mourdikoudis S, Thanh NTK, Di Pietro R. Development and Characterization of Curcumin-Silver Nanoparticles as a Promising Formulation to Test on Human Pterygium-Derived Keratinocytes. Molecules. 2022; 27(1):282. https://doi.org/10.3390/molecules27010282
Chicago/Turabian StyleStati, Gianmarco, Francesco Rossi, Thithawat Trakoolwilaiwan, Le Duc Tung, Stefanos Mourdikoudis, Nguyễn Thi Kim Thanh, and Roberta Di Pietro. 2022. "Development and Characterization of Curcumin-Silver Nanoparticles as a Promising Formulation to Test on Human Pterygium-Derived Keratinocytes" Molecules 27, no. 1: 282. https://doi.org/10.3390/molecules27010282






