Simultaneous Enzymatic Cellulose Hydrolysis and Product Separation in a Radial-Flow Membrane Bioreactor
Abstract
:1. Introduction
2. Results
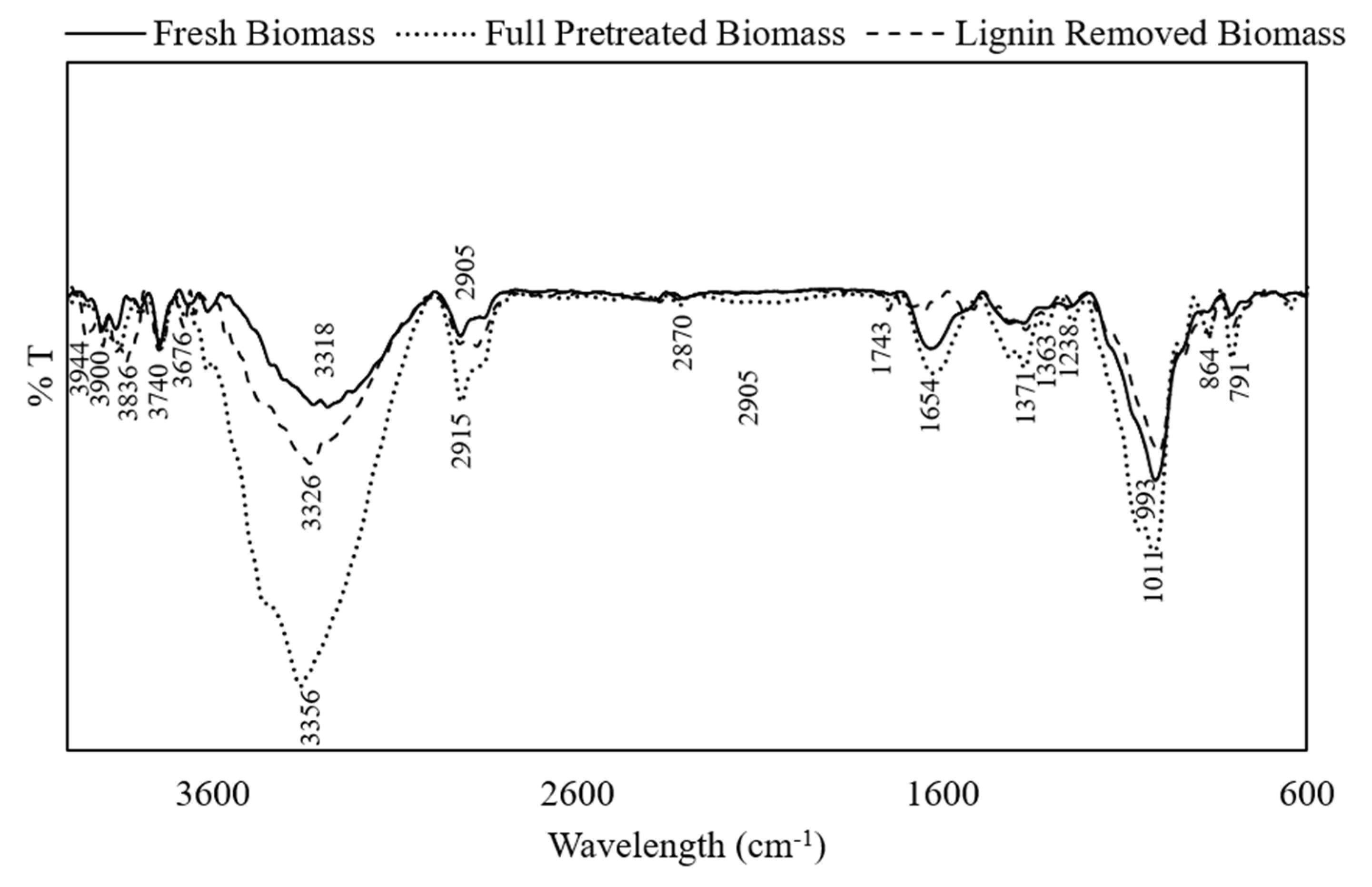
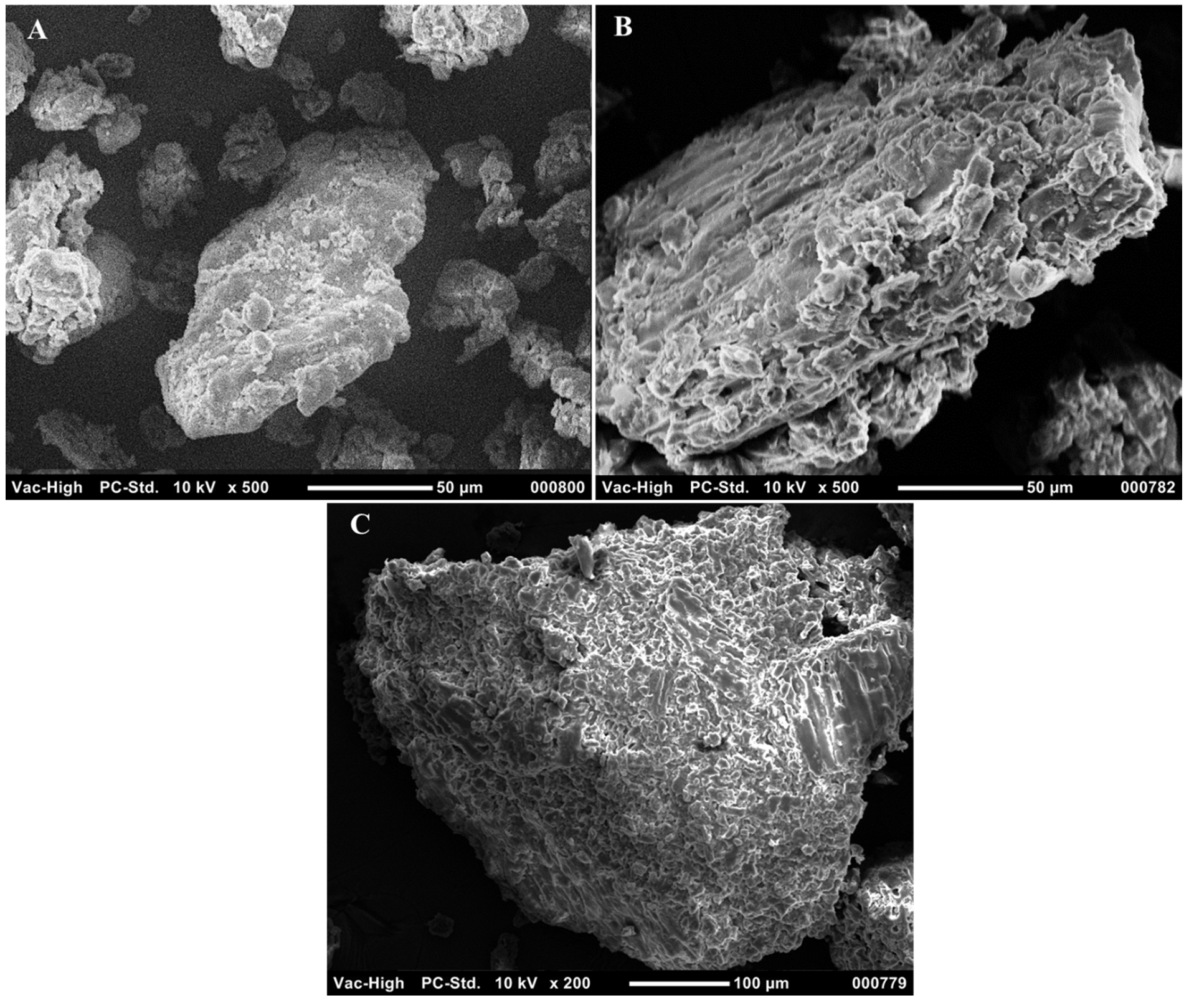
2.1. Biomass Characterization and the Effect of Pretreatment on the Substrate
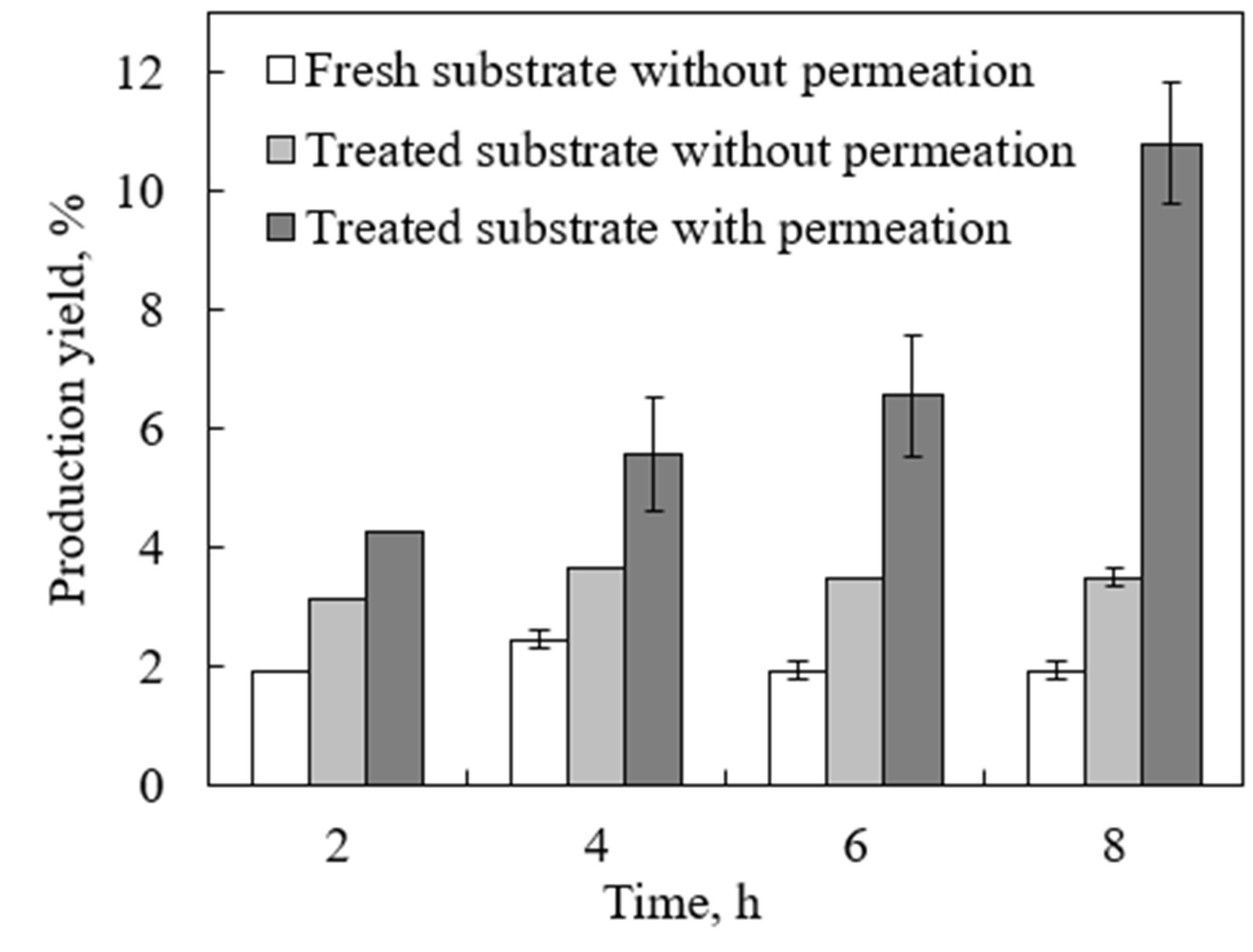
2.2. Enzymatic Hydrolysis with Product Separation
3. Materials and Methods
3.1. Chemicals and Enzymes
3.2. Biomass Preparation and Analysis
3.3. Enzymatic Hydrolysis with Product Separation
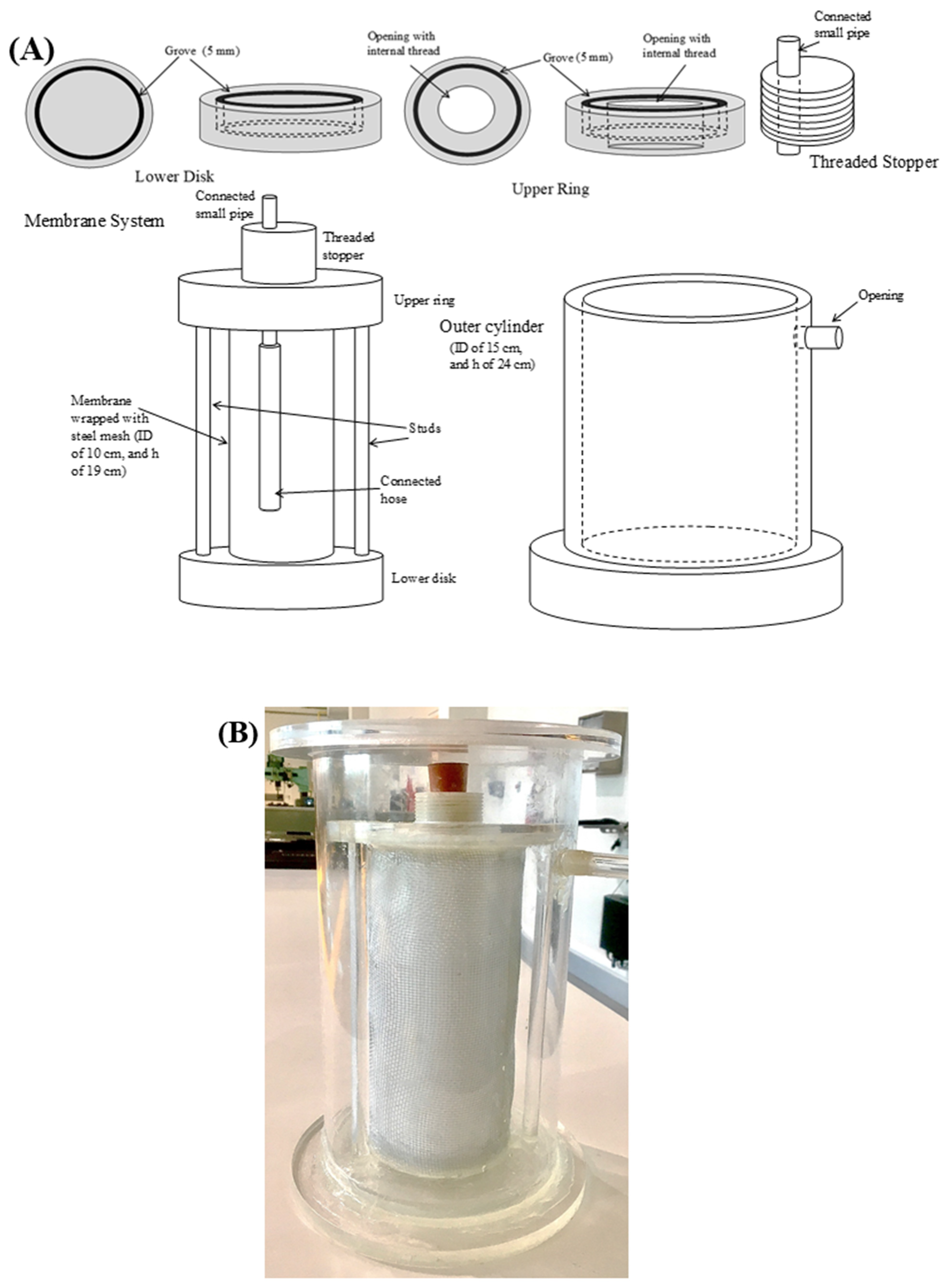
3.4. MBR Design
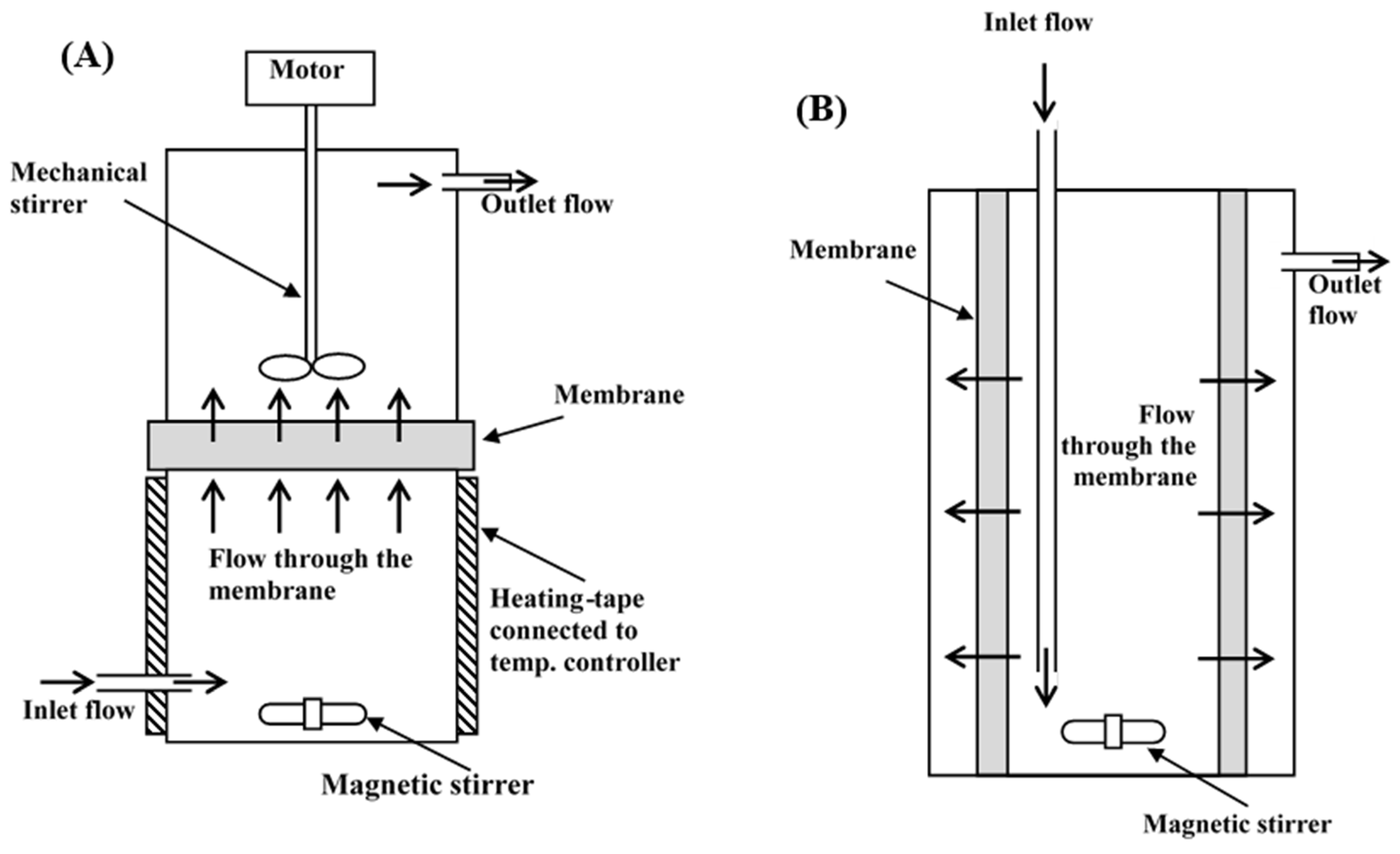
3.4.1. Inverted Dead-End Filtration MBR
3.4.2. Radial-Flow MBR
3.5. Analysis
3.5.1. Glucose Analysis
3.5.2. Protein Analysis
3.5.3. Lignin Analysis
3.5.4. Substrate Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Panahi, H.K.S.; Dehhaghi, M.; Aghbashlo, M.; Karimi, K.; Tabatabaei, M. Conversion of residues from agro-food industry into bioethanol in Iran: An under-valued biofuel additive to phase out MTBE in gasoline. Renew. Energy 2020, 145, 699–710. [Google Scholar] [CrossRef]
- Ala’a, H.; Jamil, F.; Al-Haj, L.; Myint, M.T.Z.; Mahmoud, E.; Ahmad, M.N.; Hasan, A.O.; Rafiq, S. Biodiesel production over a catalyst prepared from biomass-derived waste date pits. Biotechnol. Rep. 2018, 20, 00284. [Google Scholar]
- Du, J.; Cao, Y.; Liu, G.; Zhao, J.; Li, X.; Qu, Y. Identifying and overcoming the effect of mass transfer limitation on decreased yield in enzymatic hydrolysis of lignocellulose at high solid concentrations. Bioresour. Technol. 2017, 229, 88–95. [Google Scholar] [CrossRef]
- Shokrkar, H.; Ebrahimi, S.; Zamani, M. A review of bioreactor technology used for enzymatic hydrolysis of cellulosic materials. Cellulose 2018, 25, 6279–6304. [Google Scholar] [CrossRef]
- Quiroga, A.G.; Costa, A.; Filho, R.M. Analysis of conversion and operation strategies for enzymatic hydrolysis of lignocellulosic biomass in a series of CSTRs with distributed feeding. Bioprocess Biosyst. Eng. 2010, 33, 901–910. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, J.; Bao, J. Cost evaluation of cellulase enzyme for industrial-scale cellulosic ethanol production based on rigorous Aspen Plus modeling. Bioprocess Biosyst. Eng. 2016, 39, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Bansal, P.; Hall, M.; Realff, M.J.; Lee, J.H.; Bommarius, A.S. Modeling cellulase kinetics on lignocellulosic substrates. Biotechnol. Adv. 2009, 27, 833–848. [Google Scholar] [CrossRef]
- Pino, M.S.; Rodríguez-Jasso, R.M.; Michelin, M.; Flores-Gallegos, A.C.; Morales-Rodriguez, R.; Teixeira, J.A.; Ruiz, H.A. Bioreactor design for enzymatic hydrolysis of biomass under the biorefinery concept. Chem. Eng. J. 2018, 347, 119–136. [Google Scholar] [CrossRef] [Green Version]
- Jørgensen, H.; Pinelo, M. Enzyme recycling in lignocellulosic biorefineries. Biofuels Bioprod. Biorefin. 2017, 11, 150–167. [Google Scholar] [CrossRef]
- Guo, R.; Zheng, X.; Wang, Y.; Yang, Y.; Ma, Y.; Zou, D.; Liu, Y. Optimization of Cellulase Immobilization with Sodium Alginate-Polyethylene for Enhancement of Enzymatic Hydrolysis of Microcrystalline Cellulose Using Response Surface Methodology. Appl. Biochem. Biotechnol. 2021, 193, 2043–2060. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, S.; Ingle, A.P.; da Silva, S.S.; Rai, M. Immobilized Nanoparticles-Mediated Enzymatic Hydrolysis of Cellulose for Clean Sugar Production: A Novel Approach. Curr. Nanosci. 2019, 15, 296–303. [Google Scholar] [CrossRef]
- Ingle, A.P.; Rathod, J.; Pandit, R.; da Silva, S.S.; Rai, M. Comparative evaluation of free and immobilized cellulase for enzymatic hydrolysis of lignocellulosic biomass for sustainable bioethanol production. Cellulose 2017, 24, 5529–5540. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Neo, K.R.S.; Yang, K.-L. Continuous hydrolysis of carboxymethyl cellulose with cellulase aggregates trapped inside membranes. Enzym. Microb. Technol. 2015, 78, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, G.; Wijffels, R.H.; Marzocchella, A.; Russo, M.E. Bioreactor and bioprocess design issues in enzymatic hydrolysis of lignocellulosic biomass. Catalysts 2021, 11, 680. [Google Scholar] [CrossRef]
- Lozano, P.; Bernal, B.; Jara, A.G.; Belleville, M.-P. Enzymatic membrane reactor for full saccharification of ionic liquid-pretreated microcrystalline cellulose. Bioresour. Technol. 2014, 151, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.Y.; Ghazali, N.F. Product Removal Strategy and Fouling Mechanism for Cellulose Hydrolysis in Enzymatic Membrane Reactor. Waste Biomass Valorization 2020, 11, 5575–5590. [Google Scholar] [CrossRef]
- Sueb, M.S.M.; Luo, J.; Meyer, A.S.; Jørgensen, H.; Pinelo, M. Impact of the fouling mechanism on enzymatic depolymerization of xylan in different configurations of membrane reactors. Sep. Purif. Technol. 2017, 178, 154–162. [Google Scholar] [CrossRef]
- Zain, M.M.; Mohammad, A.W.; Hairom, N.H.H. Flux and permeation behaviour of ultrafiltration in sugaring out cellulose hydrolysate solution: A membrane screening. J. Phys. Sci. 2017, 28, 25. [Google Scholar] [CrossRef] [Green Version]
- Al-Mardeai, S.; Elnajjar, E.; Hashaikeh, R.; Kruczek, B.; Al-Zuhair, S. Dynamic model of simultaneous enzymatic cellulose hydrolysis and product separation in a membrane bioreactor. Biochem. Eng. J. 2021, 174, 108107. [Google Scholar] [CrossRef]
- Bélafi-Bakó, K.; Koutinas, A.; Nemestóthy, N.; Gubicza, L.; Webb, C. Continuous enzymatic cellulose hydrolysis in a tubular membrane bioreactor. Enzym. Microb. Technol. 2006, 38, 155–161. [Google Scholar] [CrossRef]
- Saldarriaga, J.; Pablos, A.; Aguado, R.; Amutio, M.; Olazar, M. Characterization of lignocellulosic biofuels by TGA. Int. Rev. Chem. Eng. 2021, 4, 585–588. [Google Scholar]
- Park, Y.C.; Kim, J.S. Comparison of various alkaline pretreatment methods of lignocellulosic biomass. Energy 2012, 47, 31–35. [Google Scholar] [CrossRef]
- Aboragah, A.; Embaby, M.; Günal, M.; AbuGhazaleh, A. Effect of alkaline and sonication pretreatments on the rumen degradability of date palm seeds. Trop. Anim. Health Prod. 2020, 52, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, X.; Zhang, Y.; Yu, Q.; Tan, X.; Zhuang, X.; Yuan, Z. Effect of sodium hydroxide pretreatment on physicochemical changes and enzymatic hydrolysis of herbaceous and woody lignocelluloses. Ind. Crops Prod. 2020, 145, 112145. [Google Scholar] [CrossRef]
- Al-Zuhair, S.; Abualreesh, M.; Ahmed, K.; Razak, A.A. Enzymatic delignification of biomass for enhanced fermentable sugars production. Energy Technol. 2015, 3, 121–127. [Google Scholar] [CrossRef]
- Abu-Thabit, N.Y.; Judeh, A.A.; Hakeem, A.S.; Ul-Hamid, A.; Umar, Y.; Ahmad, A. Isolation and characterization of microcrystalline cellulose from date seeds (Phoenix dactylifera L.). Int. J. Biol. Macromol. 2020, 155, 730–739. [Google Scholar] [CrossRef]
- Huang, C.; Lin, W.; Lai, C.; Li, X.; Jin, Y.; Yong, Q. Coupling the post-extraction process to remove residual lignin and alter the recalcitrant structures for improving the enzymatic digestibility of acid-pretreated bamboo residues. Bioresour. Technol. 2019, 285, 121355. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, J.; Zhang, X.; Tan, T. The correlation between cellulose allomorphs (I and II) and conversion after removal of hemicellulose and lignin of lignocellulose. Bioresour. Technol. 2015, 193, 164–170. [Google Scholar] [CrossRef]
- Şahin, Ö.; Saka, C. Preparation and characterization of activated carbon from acorn shell by physical activation with H2O–CO2 in two-step pretreatment. Bioresour. Technol. 2013, 136, 163–168. [Google Scholar] [CrossRef]
- Salim, R.M.; Asik, J.; Sarjadi, M.S. Chemical functional groups of extractives, cellulose and lignin extracted from native Leucaena leucocephala bark. Wood Sci. Technol. 2021, 55, 295–313. [Google Scholar] [CrossRef]
- Nabili, A.; Fattoum, A.; Passas, R.; Elaloui, E. Extraction and characterization of cellulose from date palm seeds (Phoenix dactylifera L.). Cellul. Chem. Technol. 2016, 50, 1015–1023. [Google Scholar]
- Cuaya, H.T.; Segura, L.P.; Bravo, S.M.; García, I.G.; Medrano, R.C.V.; Torres, E.F. Characterization of lignocellulosic biomass using five simple steps. J. Chem. Biol. Phys. Sci. 2014, 4, 28–47. [Google Scholar]
- Belouadah, Z.; Ati, A.; Rokbi, M. Characterization of new natural cellulosic fiber from Lygeum spartum L. Carbohydr. Polym. 2015, 134, 429–437. [Google Scholar] [CrossRef]
- Agarwal, U.P.; Zhu, J.; Ralph, S.A. Enzymatic hydrolysis of biomass: Effects of crystallinity, particle size, and lignin removal. In Proceedings of the 6th International Symposium on Wood, Fiber and Pulping Chemistry, Tianjin, China, 8–10 June 2011. [Google Scholar]
- Andrić, P.; Meyer, A.S.; Jensen, P.A.; Dam-Johansen, K. Reactor design for minimizing product inhibition during enzymatic lignocellulose hydrolysis: II. Quantification of inhibition and suitability of membrane reactors. Biotechnol. Adv. 2010, 28, 407–425. [Google Scholar] [CrossRef] [PubMed]
- Gan, Q.; Allen, S.; Taylor, G. Design and operation of an integrated membrane reactor for enzymatic cellulose hydrolysis. Biochem. Eng. J. 2002, 12, 223–229. [Google Scholar] [CrossRef]
- Gavlighi, H.A.; Meyer, A.S.; Mikkelsen, J.D. Enhanced enzymatic cellulose degradation by cellobiohydrolases via product removal. Biotechnol. Lett. 2013, 35, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Al-Zuhair, S.; Al-Hosany, M.; Zooba, Y.; Al-Hammadi, A.; Al-Kaabi, S. Development of a membrane bioreactor for enzymatic hydrolysis of cellulose. Renew. Energy 2013, 56, 85–89. [Google Scholar] [CrossRef]
- Yang, C.; Lü, X. Chapter 5—Composition of plant biomass and its impact on pretreatment. In Advances in 2nd Generation of Bioethanol Production; Lü, X., Ed.; Woodhead Publishing: Sawston, UK, 2021; pp. 71–85. [Google Scholar]
- Galiwango, E.; Al-Marzouqi, A.H. Investigation of Nonisothermal Combustion Kinetics of Isolated Lignocellulosic Biomass: A Case Study of Cellulose from Date Palm Biomass Waste; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar]
- Vogler, D.; Gikonyo, B. Bioethanol Production: Glucose Testing and Quantification Using DNS Analysis and Addition Analytical Methods. 2021. Available online: https://knightscholar.geneseo.edu/mcnair-scholars/7/ (accessed on 17 November 2021).
- Templeton, D.; Ehrman, T. Determination of Acid-Insoluble Lignin in Biomass: Chemical Analysis and Testing Task Laboratory Analytical Procedure (LAP-003). National Renewable Energy Laboratory. 1995. Available online: https://www.nrel.gov/biomass/analytical_procedures.html (accessed on 17 November 2021).


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Mardeai, S.; Elnajjar, E.; Hashaikeh, R.; Kruczek, B.; Van der Bruggen, B.; Al-Zuhair, S. Simultaneous Enzymatic Cellulose Hydrolysis and Product Separation in a Radial-Flow Membrane Bioreactor. Molecules 2022, 27, 288. https://doi.org/10.3390/molecules27010288
Al-Mardeai S, Elnajjar E, Hashaikeh R, Kruczek B, Van der Bruggen B, Al-Zuhair S. Simultaneous Enzymatic Cellulose Hydrolysis and Product Separation in a Radial-Flow Membrane Bioreactor. Molecules. 2022; 27(1):288. https://doi.org/10.3390/molecules27010288
Chicago/Turabian StyleAl-Mardeai, Saleha, Emad Elnajjar, Raed Hashaikeh, Boguslaw Kruczek, Bart Van der Bruggen, and Sulaiman Al-Zuhair. 2022. "Simultaneous Enzymatic Cellulose Hydrolysis and Product Separation in a Radial-Flow Membrane Bioreactor" Molecules 27, no. 1: 288. https://doi.org/10.3390/molecules27010288
APA StyleAl-Mardeai, S., Elnajjar, E., Hashaikeh, R., Kruczek, B., Van der Bruggen, B., & Al-Zuhair, S. (2022). Simultaneous Enzymatic Cellulose Hydrolysis and Product Separation in a Radial-Flow Membrane Bioreactor. Molecules, 27(1), 288. https://doi.org/10.3390/molecules27010288











