The Caenorhabditis elegans DEG-3/DES-2 Channel Is a Betaine-Gated Receptor Insensitive to Monepantel
Abstract
:1. Introduction
2. Results
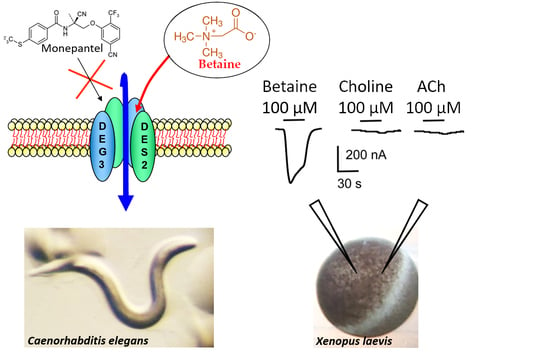
2.1. The C. elegans DEG-3/DES-2 Channel Is a Betaine-Sensitive Receptor
2.2. The C. elegans DEG-3/DES-2 Channel Is Not Modulated by Monepantel and Monepantel Sulfone
3. Discussion
4. Materials and Methods
4.1. Drugs
4.2. Cloning of the des-2 and deg-3 Subunits from C. elegans
4.3. Electrophysiological Experiments in Xenopus laevis Oocytes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Chitwood, D.J. Research on plant-parasitic nematode biology conducted by the United States Department of Agriculture-Agricultural Research Service. Pest Manag. Sci. 2003, 59, 748–753. [Google Scholar] [CrossRef]
- Charlier, J.; Rinaldi, L.; Musella, V.; Ploeger, H.W.; Chartier, C.; Vineer, H.R.; Hinney, B.; von Samson-Himmelstjerna, G.; Băcescu, B.; Mickiewicz, M.; et al. Initial assessment of the economic burden of major parasitic helminth infections to the ruminant livestock industry in Europe. Prev. Vet. Med. 2020, 182, 105103. [Google Scholar] [CrossRef]
- Ghareeb, R.Y.; Adss, I.A.; Bayoumi, S.R.; El-Habashy, D.E. The nematicidal potentiality of some algal extracts and their role in enhancement the tomato defense genes against root knot-nematodes. Egypt J. Biol. Pest Control 2019, 29, 53. [Google Scholar] [CrossRef]
- Rose, H.; Rinaldi, L.; Bosco, A.; Mavrot, F.; de Waal, T.; Skuce, P.; Charlier, J.; Torgerson, P.R.; Hertzberg, H.; Hendrickx, G.; et al. Widespread anthelmintic resistance in European farmed ruminants: A systematic review. Vet. Rec. 2015, 176, 546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prichard, R. Anthelmintic resistance. Vet. Parasitol. 1994, 54, 259–268. [Google Scholar] [CrossRef]
- Wu, Y.; Jenkins, T.; Blunden, G.; Whapham, C.; Hankins, S.D. The role of betaines in alkaline extracts of Ascophyllum nodosum in the reduction of Meloidogyne javanica and M. incognita infestations of tomato plants. Fundam. Appl. Nematol. 1997, 20, 99–102. [Google Scholar]
- Wu, Y.; Jenkins, T.; Blunden, G.; Von Mende, N.; Hankins, S.D. Suppression of fecundity of the root-knot nematode, Meloidogyne javanica, in monoxenic cultures of Arabidopsis thaliana treated with an alkaline extract of Ascophyllum nodosum. J. Appl. Phycol. 1998, 10, 91–94. [Google Scholar] [CrossRef]
- Whapham, C.A.; Jenkins, T.; Blunden, G.; Hankins, S.D. The role of seaweed extracts, Ascophyllum nodosum, in the reduction in fecundity of Meloidogyne javanica. Fundam. Appl. Nematol. 1994, 17, 181–183. [Google Scholar]
- Zhao, G.; He, F.; Wu, C.; Li, P.; Li, N.; Deng, J.; Zhu, G.; Ren, W. Betaine in inflammation: Mechanistic Aspects and Applications. Front. Immunol. 2018, 9, 1070. [Google Scholar] [CrossRef] [Green Version]
- Peden, A.S.; Mac, P.; Fei, Y.J.; Castro, C.; Jiang, G.; Murfitt, K.J.; Miska, E.A.; Griffin, J.L.; Ganapathy, V.; Jorgensen, E.M. Betaine acts on a ligand-gated ion channel in the nervous system of the nematode C. elegans. Nat. Neurosci. 2013, 16, 1794–1801. [Google Scholar] [CrossRef] [Green Version]
- Albuquerque, E.X.; Pereira, E.F.R.; Alkondon, M.; Rogers, S.W. Mammalian Nicotinic Acetylcholine Receptors: From Structure to Function. Physiol. Rev. 2009, 89, 73–120. [Google Scholar] [CrossRef] [Green Version]
- Holden-Dye, L.; Joyner, M.; O’Connor, V.; Walker, R.J. Nicotinic acetylcholine receptors: A comparison of the nAChRs of Caenorhabditis elegans and parasitic nematodes. Parasitol. Int. 2013, 62, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.K.; Sattelle, D.B. Functional genomics of the nicotinic acetylcholine receptor gene family of the nematode, Caenorhabditis elegans. Bioessays 2004, 26, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.A.; Jones, A.K.; Buckingham, S.D.; Mee, C.J.; Sattelle, D.B. Contributions from Caenorhabditis elegans functional genetics to antiparasitic drug target identification and validation: Nicotinic acetylcholine receptors, a case study. Int. J. Parasitol. 2006, 36, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Yassin, L.; Gillo, B.; Kahan, T.; Halevi, S.; Eshel, M.; Treinin, M. Characterization of the DEG-3/DES-2 receptor: A nicotinic acetylcholine receptor that mutates to cause neuronal degeneration. Mol. Cell Neurosci. 2001, 17, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Treinin, M.; Gillo, B.; Liebman, L.; Chalfie, M. Two functionally dependent acetylcholine subunits are encoded in a single Caenorhabditis elegans operon. Proc. Natl. Acad. Sci. USA 1998, 95, 15492–15495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, E.; Chatzigeorgiou, M.; Husson, S.J.; Steuer-Costa, W.; Gottschalk, A.; Schafer, W.R.; Treinin, M. Caenorhabditis elegans nicotinic acetylcholine receptors are required for nociception. Mol. Cell Neurosci. 2014, 59, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Baur, R.; Beech, R.; Sigel, E.; Rufener, L. Monepantel irreversibly binds to and opens Haemonchus contortus MPTL-1 and Caenorhabditis elegans ACR-20 receptors. Mol. Pharmacol. 2014, 87, 96–102. [Google Scholar] [CrossRef] [Green Version]
- Kaminsky, R.; Ducray, P.; Jung, M.; Clover, R.; Rufener, L.; Bouvier, J.; Weber, S.S.; Wenger, A.; Wieland-Berghausen, S.; Goebel, T.; et al. A new class of anthelmintics effective against drug-resistant nematodes. Nature 2008, 452, 176–180. [Google Scholar] [CrossRef]
- Karadzovska, D.; Seewald, W.; Browning, A.; Smal, M.; Bouvier, J.; Giraudel, J.M. Pharmacokinetics of monepantel and its sulfone metabolite, monepantel sulfone, after intravenous and oral administration in sheep. J. Vet. Pharmacol. Ther. 2009, 32, 359–367. [Google Scholar] [CrossRef]
- Rufener, L.; Baur, R.; Kaminsky, R.; Mäser, P.; Sigel, E. Monepantel allosterically activates DEG-3/DES-2 channels of the gastrointestinal nematode Haemonchus contortus. Mol. Pharmacol. 2010, 78, 895–902. [Google Scholar] [CrossRef] [Green Version]
- Rufener, L.; Bedoni, N.; Baur, R.; Rey, S.; Glauser, D.A.; Bouvier, J.; Beech, R.; Sigel, E.; Puoti, A. acr-23 Encodes a monepantel-sensitive channel in Caenorhabditis elegans. PLoS Pathog. 2013, 9, e1003524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bekdash, R.A. Neuroprotective Effects of Choline and Other Methyl Donors. Nutrients 2019, 11, 2995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rufener, L.; Keiser, J.; Kaminsky, R.; Mäser, P.; Nilsson, D. Phylogenomics of Ligand-Gated Ion Channels Predicts Monepantel Effect. PLoS Pathog. 2010, 6, e1001091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, T.V.A.; Grencis, R.K.; Issouf, M.; Neveu, C.; Charvet, C.L. Functional characterization of the oxantel-sensitive acetylcholine receptor from Trichuris muris. Pharmaceuticals 2021, 14, 698. [Google Scholar] [CrossRef]
- Boulin, T.; Gielen, M.; Richmond, J.E.; Williams, D.C.; Paoletti, P.; Bessereau, J.-L. Eight genes are required for functional reconstitution of the Caenorhabditis elegans levamisole-sensitive acetylcholine receptor. Proc. Natl. Acad. Sci. USA 2008, 105, 18590–18595. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hansen, T.V.A.; Sager, H.; Toutain, C.E.; Courtot, E.; Neveu, C.; Charvet, C.L. The Caenorhabditis elegans DEG-3/DES-2 Channel Is a Betaine-Gated Receptor Insensitive to Monepantel. Molecules 2022, 27, 312. https://doi.org/10.3390/molecules27010312
Hansen TVA, Sager H, Toutain CE, Courtot E, Neveu C, Charvet CL. The Caenorhabditis elegans DEG-3/DES-2 Channel Is a Betaine-Gated Receptor Insensitive to Monepantel. Molecules. 2022; 27(1):312. https://doi.org/10.3390/molecules27010312
Chicago/Turabian StyleHansen, Tina V. A., Heinz Sager, Céline E. Toutain, Elise Courtot, Cédric Neveu, and Claude L. Charvet. 2022. "The Caenorhabditis elegans DEG-3/DES-2 Channel Is a Betaine-Gated Receptor Insensitive to Monepantel" Molecules 27, no. 1: 312. https://doi.org/10.3390/molecules27010312