IR-Spectroscopic Study of Complex Formation of Nitrogen Oxides (NO, N2O) with Cationic Forms of Zeolites and the Reactivity of Adsorbed Species in CO and CH4 Oxidation
Abstract
1. Introduction
2. Results and Discussion
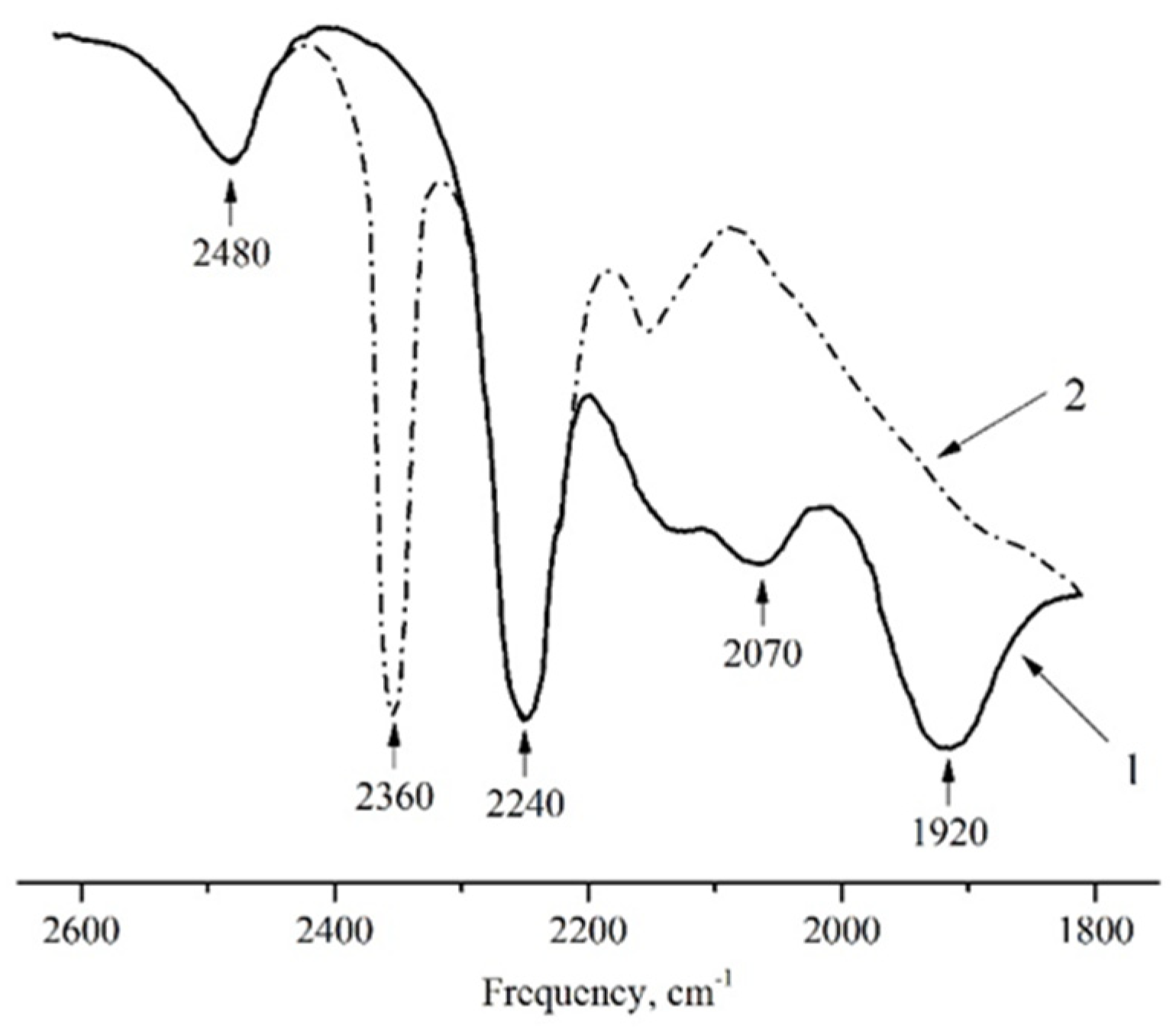
2.1. N2O Adsorption on Cationic Forms of Zeolites
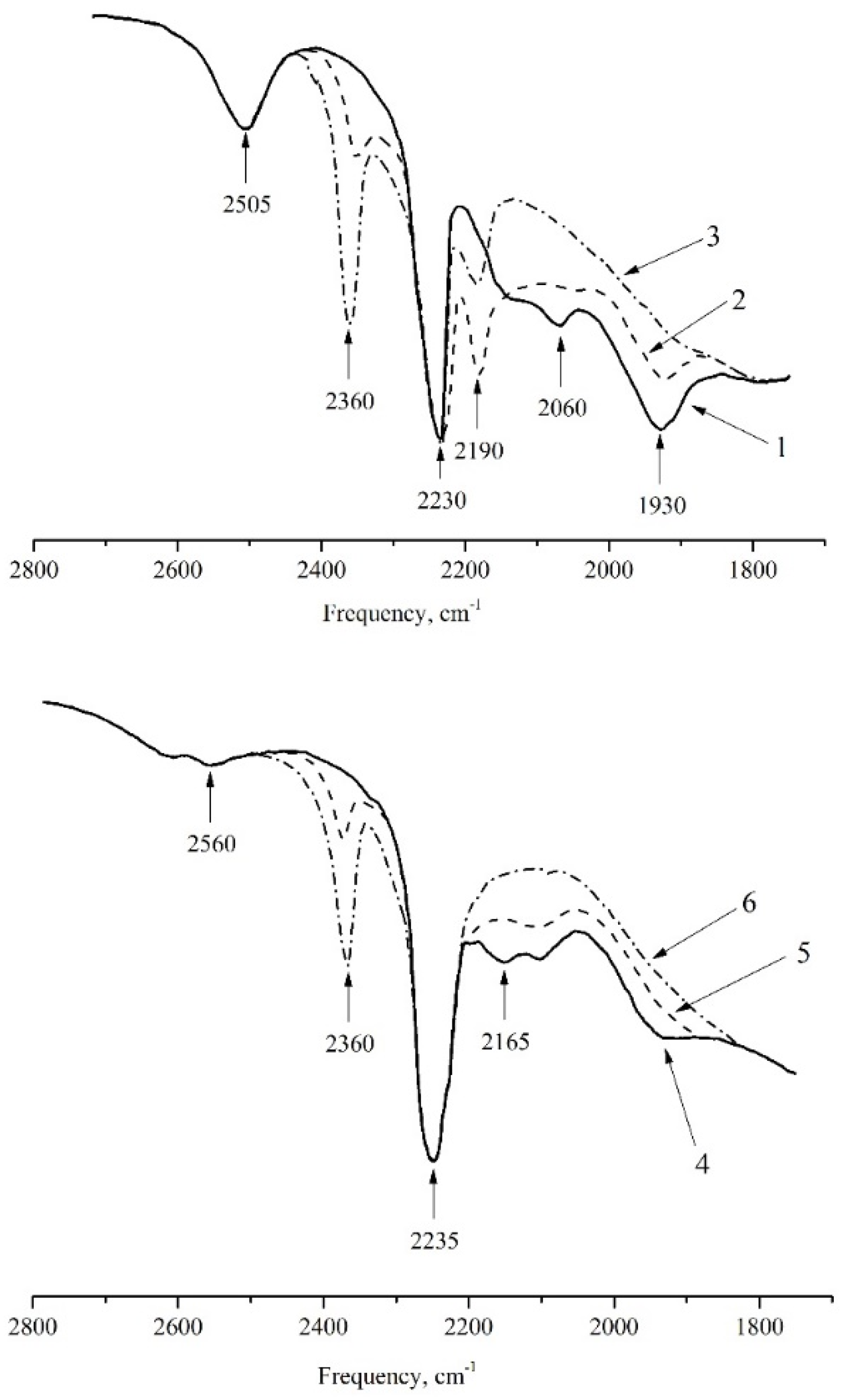
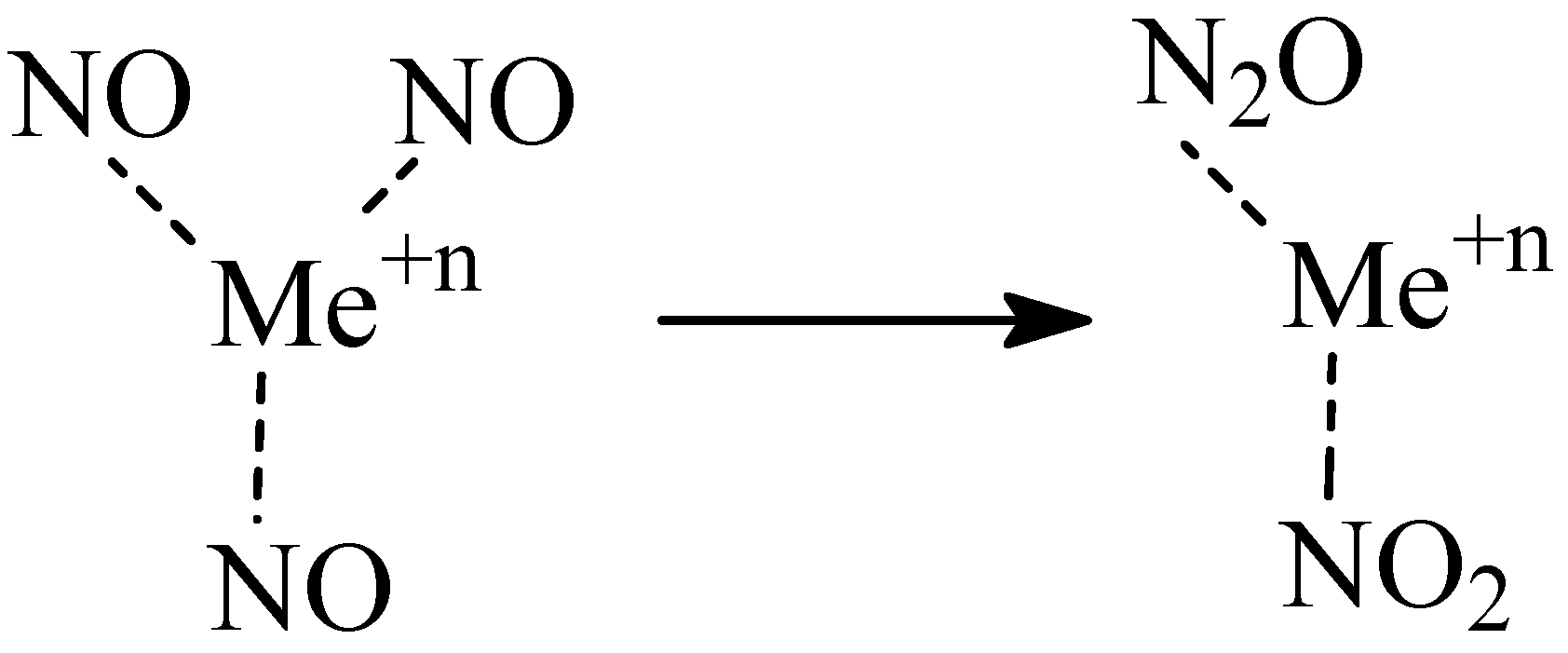
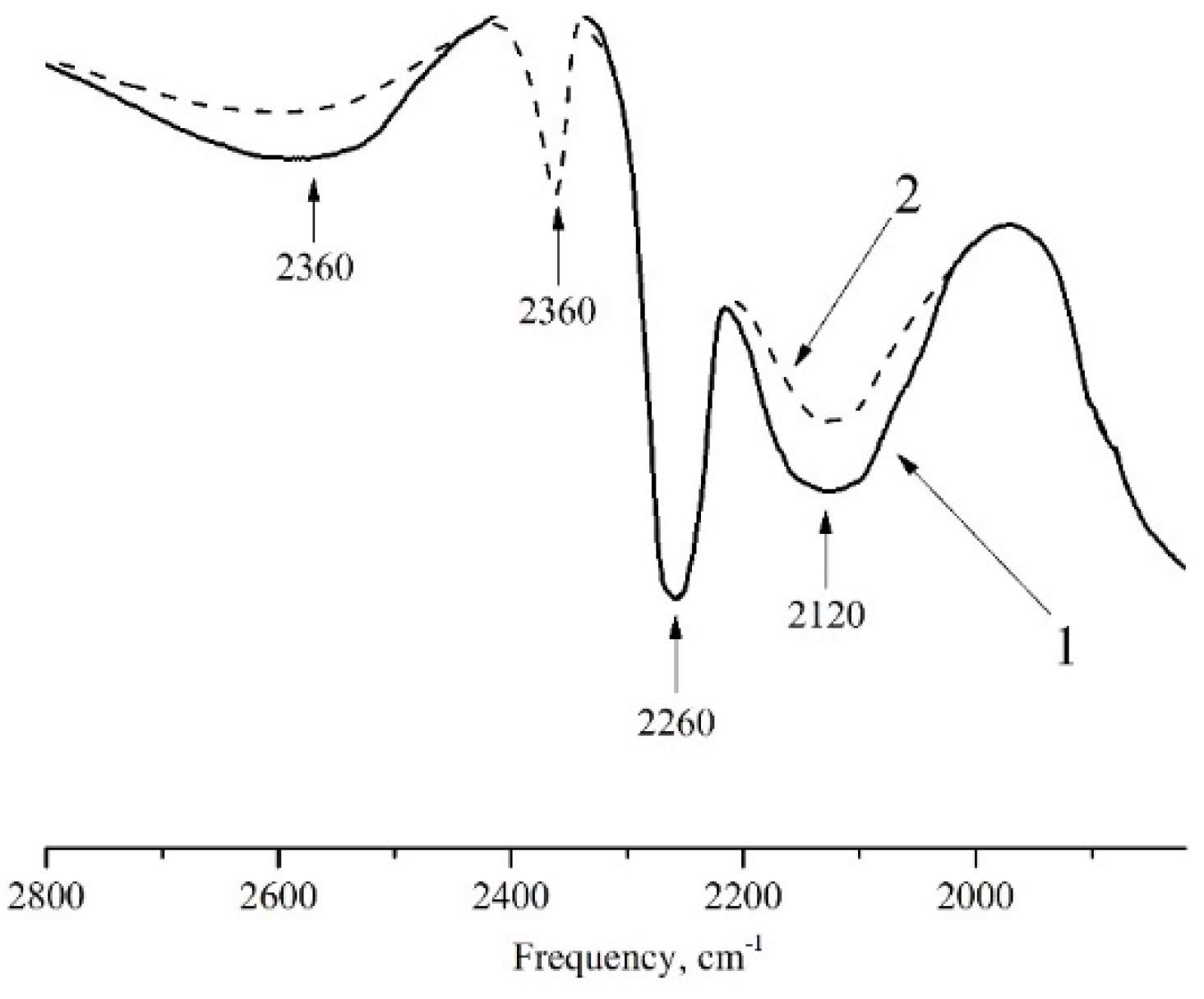
2.2. Adsorption of NO on Y Type Zeolites
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Iwamoto, M. Catalytic decomposition of nitrogen monoxide. Stud. Surf. Sci. Catal. 1990, 54, 121. [Google Scholar]
- Bosch, H.; Janssen, F. Catalytic reduction of nitrogen oxides: A review on the fundamentals and technology. Catal. Today 1988, 2. [Google Scholar]
- Hamada, H.; Kintaichi, Y.; Sasaki, M.; Ito, T.; Tabata, M. Highly selective reduction of nitrogen oxides with hydrocarbons over H-form zeolite catalysts in oxygen-rich atmosphere. Appl. Catal. 1990, 64, L1. [Google Scholar] [CrossRef]
- Davydov, A.A. Molecular Spectroscopy of Oxide Catalyst Surfaces; Wiley: New York, NY, USA, 2003. [Google Scholar]
- Kanno, Y.; Matsui, Y.; Imai, H. ESR spectroscopic study and kinetic discussion of the disproportionation reaction of nitrogen monoxide on CaHY-type zeolite. J. Incl. Phenom. 1987, 5, 385. [Google Scholar] [CrossRef]
- Kanno, Y.; Matsui, Y.; Imai, H. Mechanistic model of disproportionation of nitrogen monoxide on CaHY-type zeolite. J. Incl. Phenom. 1985, 3, 461. [Google Scholar] [CrossRef]
- Alekseev, A.A.; Filimonov, V.N.; Terenin, A.N. Infrared spectra of nitrogen oxide adsorbed on synthetic zeolites. Dokl. Akad. Nauk SSSR 1962, 147, 1392. [Google Scholar]
- Iwamoto, M.; Yahiro, H.; Torikai, Y.; Yoshioka, T.; Mizuno, N. Novel preparation method of highly copper ion-exchanged ZSM-5 zeolites and their catalytic activities for NO decomposition. Chem. Lett. 1990, 19, 1967. [Google Scholar] [CrossRef]
- Miessner, H.; Burkhardt, J.; Gutschick, D.J. Coadsorption and interaction of nitric oxide and carbon monoxide on Rh supported on highly dealuminated Y zeolite. Formation of mixed Rh carbonyl–nitrosyls on the surface. Chem. Soc. Faraday Trans. 1990, 86, 2329. [Google Scholar] [CrossRef]
- Chao, C.C.; Lunsford, J.H. Adsorption of nitric oxide on Y-type zeolites. A low-temperature infrared study. J. Am. Chem. Soc. 1971, 93, 6794. [Google Scholar] [CrossRef]
- Huang, Y.; Su, W.; Wang, R.; Zhao, T. Removal of Typical Industrial Gaseous Pollutants: From Carbon, Zeolite, and Metal-organic Frameworks to Molecularly Imprinted Adsorbents. Aerosol Air Qual. Res. 2019, 19, 2130. [Google Scholar] [CrossRef]
- Sun, W.; Lin, L.C.; Peng, X.; Smit, B. Computational screening of porous metal-organic frameworks and zeolites for the removal of SO2 and NOx from flue gases. AIChE J. 2014, 60, 2314. [Google Scholar] [CrossRef]
- Skarlis, S.A.; Berthout, D.; Nicolle, A.; Dujardin, C.; Granger, P. Combined IR spectroscopy and kinetic modeling of NOx storage and NO oxidation on Fe-BEA SCR catalysts. Appl. Catal. B Environ. 2014, 148, 446. [Google Scholar] [CrossRef]
- Liu, Z.; Wakihara, N.; Oshima, K.; Nishioka, D.; Hotta, Y.; Elangovan, S.P.; Yanaba, Y.; Yoshikawa, T.; Chaikittisilp, W.; Matsuo, T.; et al. Widening synthesis bottlenecks: Realization of ultrafast and continuous-flow synthesis of high-silica zeolite SSZ-13 for NOx removal. Angew. Chem. Int. Ed. 2015, 54, 5683. [Google Scholar] [CrossRef]
- Pan, H.; Guo, Y.; Bi, H.T. NOx adsorption and reduction with C3H6 over Fe/zeolite catalysts: Effect of catalyst support. Chem. Eng. J. 2015, 280, 66. [Google Scholar] [CrossRef]
- Ohnishi, T.; Kawakami, K.; Nishioka, M.; Ogura, M. Direct decomposition of NO on metal-loaded zeolites with coexistence of oxygen and water vapor under unsteady-state conditions by NO concentration and microwave rapid heating. Catal. Today 2017, 281, 566. [Google Scholar] [CrossRef]
- Fedeyko, J.M.; Chen, B.; Chen, H.-Y. Mechanistic study of the low temperature activity of transition metal exchanged zeolite SCR catalysts. Catal. Today 2010, 151, 231. [Google Scholar] [CrossRef]
- Landi, G.; Lisi, L.; Pirone, R.; Tortorelli, M.; Russo, G. NO decomposition over La-doped Cu-ZSM5 monolith under adsorption-reaction conditions. Appl. Catal. A Gen. 2013, 464–465, 61. [Google Scholar] [CrossRef]
- Guesmi, H.; Berthomieu, D.; Kiwi-Minsker, L. Nitrous oxide decomposition on the binuclear [FeII(μ-O)(μ-OH)FeII] Center in Fe-ZSM-5 zeolite. J. Phys. Chem. C 2008, 112, 20319. [Google Scholar] [CrossRef][Green Version]
- Elzey, S.; Mubayi, A.; Larsen, S.C.; Grassian, V.H. FTIR study of the selective catalytic reduction of NO2 with ammonia on nanocrystalline NaY and CuY. J. Molec. Catal. A Chem. 2008, 285, 48. [Google Scholar] [CrossRef]
- Salama, T.M.; Mohamed, M.M.; Othman, A.I.; El-Shobaky, G.A. Structural and textural characteristics of Ce-containing mordenite and ZSM-5 solids and FT-IR spectroscopic investigation of the reactivity of NO gas adsorbed on them. Appl. Catal. A Gen. 2005, 286, 85. [Google Scholar] [CrossRef]
- Akolekar, D.B.; Bhargava, S.K.; Foger, K. FTIR investigations of the adsorption and disproportionation of NO on Cu-exchanged silicoaluminophosphate of type 34. J. Chem. Soc. Faraday Trans. 1998, 94, 155. [Google Scholar] [CrossRef]
- Katoh, M.; Yamazaki, T.; Kamijo, H.; Ozawa, S. Infrared studies on nitrogen oxides adsorbed on alkali metal ion-exchanged ZSM-5 zeolites. Zeolites 1995, 15, 591. [Google Scholar] [CrossRef]
- Zecchina, A.; Otero Areán, C.; Turnes Palomino, G.; Geobaldo, F.; Lamberti, C.; Spoto, G.; Bordiga, S. The vibrational spectroscopy of H2, N2, CO and NO adsorbed on the titanosilicate molecular sieve ETS-10. Phys. Chem. Chem. Phys. 1999, 1, 1649. [Google Scholar] [CrossRef]
- Monticelli, O.; Loenders, R.; Jacobs, P.A.; Martens, J.A. NOx removal from exhaust gas from lean burn internal combustion engines through adsorption on FAU type zeolites cation exchanged with alkali metals and alkaline earth metals. Appl. Catal. B Environ. 1999, 21, 215. [Google Scholar] [CrossRef]
- Ivanova, E.; Hadjiivanov, K.; Klissurski, D.; Bevilacqua, M.; Armaroli, T.; Busca, G. FTIR study of species arising after NO adsorption and NO+O2 co-adsorption on CoY: Comparison with Co-ZSM-5. Micropor. Mesopor. Mater. 2001, 46, 299. [Google Scholar] [CrossRef]
- Adisson, W.E.; Barrer, R.M. Sorption and reactivity of nitrous oxide and nitric oxide in crystalline and amorphous siliceous sorbents. J. Chem. Soc. 1955, 70, 757. [Google Scholar] [CrossRef]
- Hinokuma, S.; Iwasa, T.; Kon, Y.; Taketsugu, T.; Sato, K. Effects of support materials and Ir loading on catalytic N2O decomposition properties. Catal. Commun. 2021, 149, 106208. [Google Scholar] [CrossRef]
- Rac, V.; Rakic, V.; Damjanovic-Vasilic, L.; Dondur, V.; Auroux, A. Complementary approach to the adsorption of CO and N2O on bimetallic ion exchanged ZMS-5 zeolite: Microcalorimetric and FTIR spectroscopy study. Appl. Surf. Sci. 2017, 423, 1134. [Google Scholar] [CrossRef]
- Jabłonska, M.; Aran, M.A.; Beale, A.M.; Gora-Marek, K.; Delahay, G.; Petitto, C.; Pacultov, K.; Palkovits, R. Catalytic decomposition of N2O over Cu-Al-Ox mixed metal oxides. RSC Adv. 2019, 9, 3979. [Google Scholar] [CrossRef]
- Mihaylov, M.Y.; Ivanova, E.Z.; Vayssilov, G.N.; Hadjiivanov, K.I. Revisiting ceria-NOx interaction: FTIR studies. Catal. Today 2020, 357, 613. [Google Scholar] [CrossRef]
- Herzberg, G. Molecular Spectra and Molecular Structure; Spectra of Diatomic Molecules; D. Van Nostrand: New York, NY, USA, 1939; Volume 1. [Google Scholar]
- Zholobenko, V.L.; Senchenya, I.N.; Kustov, L.M.; Kazansky, V.B. Complexation and decomposition of N2O on Broensted acid sites in high-silicon zeolites. Spectral and quantum chemical study. Kinet. Katal. 1991, 32, 151. [Google Scholar]
- Mortier, W.J. Compilation of Extra-Framework Sites in Zeolites. Acta Cryst. 1982, B38, 2986. [Google Scholar] [CrossRef]
- Kustov, L.M.; Smekalina, E.V.; Uvarova, E.B.; Kazansky, V.B. NOx adsorption complexes on zeolites containing metal cations and strong Lewis acid sites and their reactivity in CO and CH4 oxidation: A spectroscopic study. Stud. Surf. Sci. Catal. 1995, 97, 409. [Google Scholar]
- Brauer, G. (Ed.) Handbook of Preparative Inorganic Synthesis; Elsevier: Amsterdam, The Netherlands, 1963. [Google Scholar] [CrossRef]
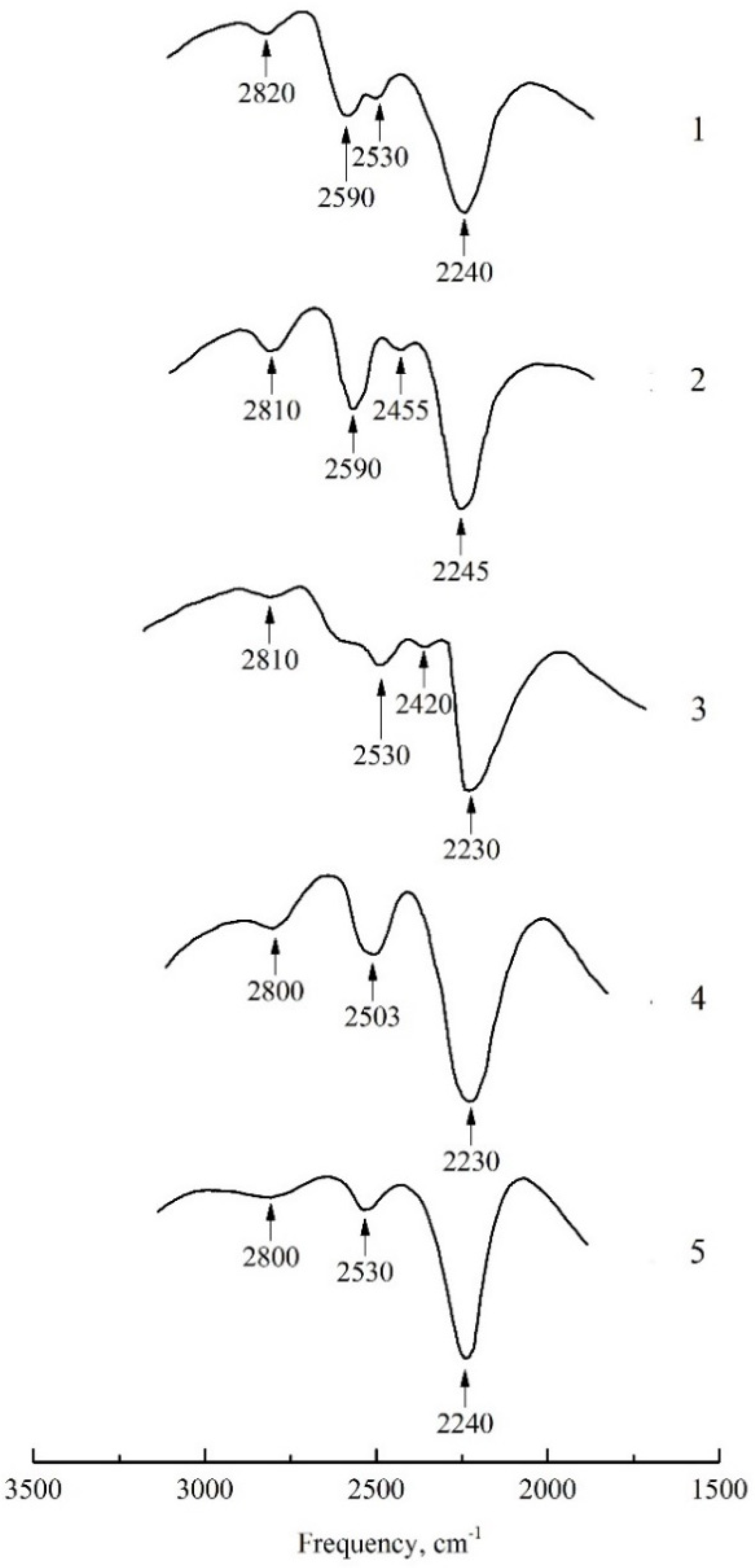
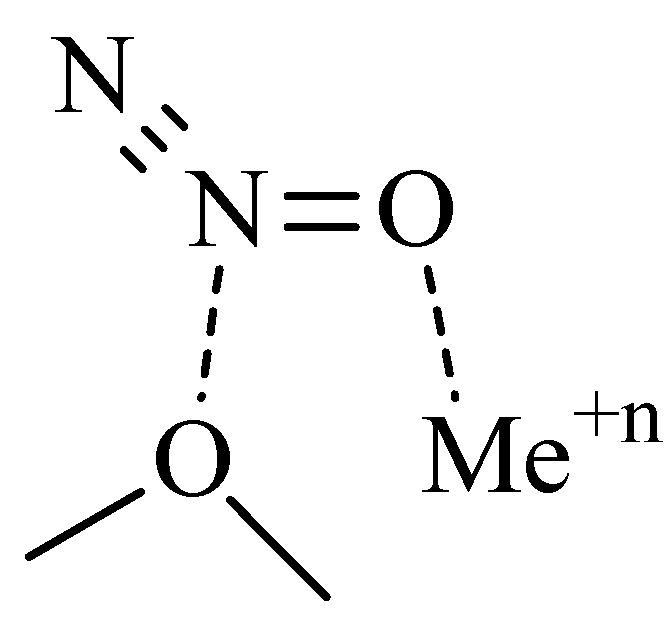
| Spectral Transition | N2O in the Gas Phase | Ca-FAU | Na-LTA | Na-FAU | Na-MOR | Mg-MOR |
|---|---|---|---|---|---|---|
| ν30–1 | 2224 | 2230 | 2245 | 2230 | 2240 | 2240 |
| ν10–1 | 1285 | - | - | - | - | - |
| δ0–1 | 589 | - | - | - | - | - |
| δ0–2 | 1169 | - | - | - | - | - |
| ν10–1 + δ0–2 | 2460 | - | 2455 | 2420 | - | - |
| ν10–2 | 2562 | 2503 | 2590 | 2600 | 2530, 2590 | 2530, 2600 |
| ν30–1 + δ0–1 | 2796 | 2800 | 2810 | 2810 | 2820 | 2800 |
| ν30–1 + δ0–2 | 3360 | - | - | - | - | - |
| ν30–1 + ν10–1 | 3489 | 3475 | - | 3475, 3530 | 3440, 3540 | 3480 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kustov, A.L.; Kustov, L.M. IR-Spectroscopic Study of Complex Formation of Nitrogen Oxides (NO, N2O) with Cationic Forms of Zeolites and the Reactivity of Adsorbed Species in CO and CH4 Oxidation. Molecules 2022, 27, 55. https://doi.org/10.3390/molecules27010055
Kustov AL, Kustov LM. IR-Spectroscopic Study of Complex Formation of Nitrogen Oxides (NO, N2O) with Cationic Forms of Zeolites and the Reactivity of Adsorbed Species in CO and CH4 Oxidation. Molecules. 2022; 27(1):55. https://doi.org/10.3390/molecules27010055
Chicago/Turabian StyleKustov, Alexander L., and Leonid M. Kustov. 2022. "IR-Spectroscopic Study of Complex Formation of Nitrogen Oxides (NO, N2O) with Cationic Forms of Zeolites and the Reactivity of Adsorbed Species in CO and CH4 Oxidation" Molecules 27, no. 1: 55. https://doi.org/10.3390/molecules27010055
APA StyleKustov, A. L., & Kustov, L. M. (2022). IR-Spectroscopic Study of Complex Formation of Nitrogen Oxides (NO, N2O) with Cationic Forms of Zeolites and the Reactivity of Adsorbed Species in CO and CH4 Oxidation. Molecules, 27(1), 55. https://doi.org/10.3390/molecules27010055







