Transgelin-2 in Multiple Myeloma: A New Marker of Renal Impairment?
Abstract
:1. Introduction
2. Results
2.1. Characteristics of the Studied Group
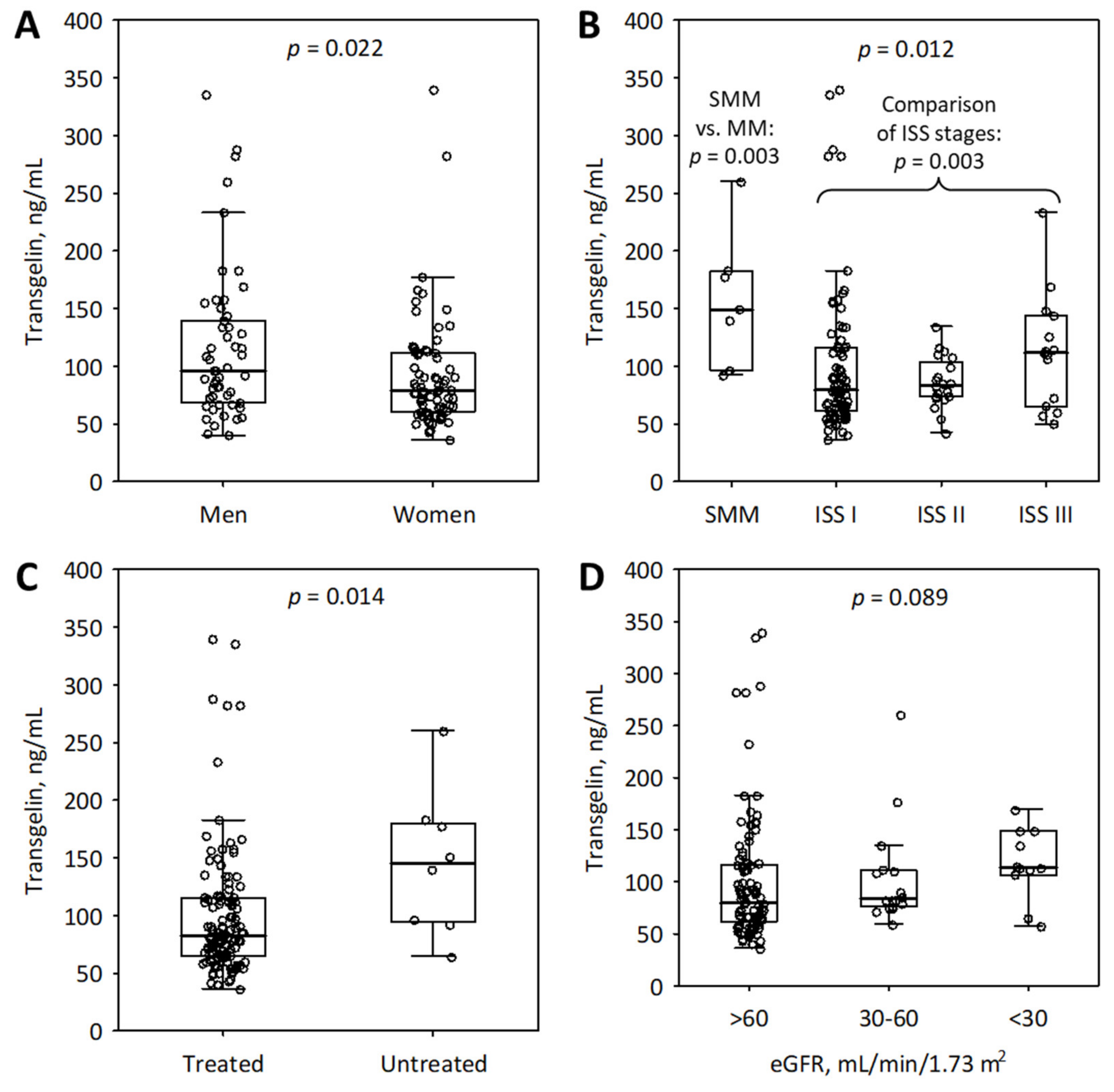
2.2. Variables Associated with Serum Transgelin at Baseline
2.3. The Association between Serum Transgelin and Renal Function after the Follow-Up Period
3. Discussion
4. Materials and Methods
4.1. Study Design and Patients
4.2. Ethics Statement
4.3. Blood Samples and Laboratory Tests
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Cowan, A.J.; Allen, C.; Barac, A.; Basaleem, H.; Bensenor, I.; Curado, M.P.; Foreman, K.; Gupta, R.; Harvey, J.; Hosgood, H.D.; et al. Global Burden of Multiple Myeloma: A Systematic Analysis for the Global Burden of Disease Study 2016. JAMA Oncol. 2018, 4, 1221–1227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajkumar, S.V.; Dimopoulos, M.A.; Palumbo, A.; Blade, J.; Merlini, G.; Mateos, M.-V.; Kumar, S.; Hillengass, J.; Kastritis, E.; Richardson, P.; et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma: Presenting features and predictors of outcome in 94 patients from a single institution. Lancet Oncol. 2014, 15, e538–e548. [Google Scholar] [CrossRef]
- Bladé, J.; Fernández-Llama, P.; Bosch, F.; Montolíu, J.; Lens, X.M.; Montoto, S.; Cases, A.; Darnell, A.; Rozman, C.; Montserrat, E. Renal Failure in Multiple Myeloma. Arch. Intern. Med. 1998, 158, 1889–1893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gertz, M.A.; Lacy, M.Q.; Dispenzieri, A.; Hayman, S.R.; Kumar, S.; Leung, N.; Gastineau, D.A. Impact of age and serum creatinine value on outcome after autologous blood stem cell transplantation for patients with multiple myeloma. Bone Marrow Transplant. 2007, 39, 605–611. [Google Scholar] [CrossRef] [Green Version]
- Kyle, R.A.; Gertz, M.A.; Witzig, T.E.; Lust, J.A.; Lacy, M.Q.; Dispenzieri, A.; Fonseca, R.; Rajkumar, S.V.; Offord, J.R.; Larson, D.R.; et al. Review of 1027 Patients with Newly Diagnosed Multiple Myeloma. Mayo Clin. Proc. 2003, 78, 21–33. [Google Scholar] [CrossRef]
- Augustson, B.M.; Begum, G.; Dunn, J.A.; Barth, N.J.; Davies, F.; Morgan, G.; Behrens, J.; Smith, A.; Child, J.A.; Drayson, M.T. Early mortality after diagnosis of multiple myeloma: Analysis of patients entered onto the united kingdom medical research council trials between 1980 and 2002—Medical research council adult leukaemia working party. J. Clin. Oncol. 2005, 23, 9219–9226. [Google Scholar] [CrossRef]
- Dimopoulos, M.; Delimpasi, S.; Katodritou, E.; Vassou, A.; Kyrtsonis, M.C.; Repousis, P.; Kartasis, Z.; Parcharidou, A.; Michael, M.; Michalis, E.; et al. Significant improvement in the survival of patients with multiple myeloma presenting with severe renal impairment after the introduction of novel agents. Ann. Oncol. 2014, 25, 195–200. [Google Scholar] [CrossRef]
- Bernstein, S.P.; Humes, H.D. Reversible renal insufficiency in multiple myeloma. Arch. Intern. Med. 1982, 142, 2083–2086. [Google Scholar] [CrossRef]
- Knudsen, L.M.; Hjorth, M.; Hippe, E. Nordic Myeloma Study Group Renal failure in multiple myeloma: Reversibility and impact on the prognosis. Eur. J. Haematol. 2000, 65, 175–181. [Google Scholar] [CrossRef]
- Sakhuja, V.; Jha, V.; Varma, S.; Joshi, K.; Gupta, K.L.; Sud, K.; Kohli, H. Renal involvement in multiple myeloma: A 10-year study. Ren. Fail. 2000, 22, 465–477. [Google Scholar] [CrossRef]
- Gonsalves, W.I.; Leung, N.; Rajkumar, S.V.; Dispenzieri, A.; Lacy, M.Q.; Hayman, S.R.; Buadi, F.K.; Dingli, D.; Kapoor, P.; Go, R.S.; et al. Improvement in renal function and its impact on survival in patients with newly diagnosed multiple myeloma. Blood Cancer J. 2015, 5, e296. [Google Scholar] [CrossRef] [Green Version]
- Bernard, R.S.; Chodirker, L.; Masih-Khan, E.; Jiang, H.; Franke, N.; Kukreti, V.; Tiedemann, R.; Trudel, S.; Reece, D.; Chen, C.I. Efficacy, toxicity and mortality of autologous SCT in multiple myeloma patients with dialysis-dependent renal failure. Bone Marrow Transplant. 2015, 50, 95–99. [Google Scholar] [CrossRef] [Green Version]
- Tosi, P.; Zamagni, E.; Tacchetti, P.; Ceccolini, M.; Perrone, G.; Brioli, A.; Pallotti, M.C.; Pantani, L.; Petrucci, A.; Baccarani, M.; et al. Thalidomide-Dexamethasone as Induction Therapy before Autologous Stem Cell Transplantation in Patients with Newly Diagnosed Multiple Myeloma and Renal Insufficiency. Biol. Blood Marrow Transplant. 2010, 16, 1115–1121. [Google Scholar] [CrossRef] [Green Version]
- Uttervall, K.; Duru, A.; Lund, J.; Liwing, J.; Gahrton, G.; Holmberg, E.; Aschan, J.; Alici, E.; Nahi, H. The Use of Novel Drugs Can Effectively Improve Response, Delay Relapse and Enhance Overall Survival in Multiple Myeloma Patients with Renal Impairment. PLoS ONE 2014, 9, e101819. [Google Scholar] [CrossRef] [Green Version]
- Woziwodzka, K.; Vesole, D.H.; Małyszko, J.; Batko, K.; Jurczyszyn, A.; Koc-Żórawska, E.; Krzanowski, M.; Małyszko, J.; Żórawski, M.; Waszczuk-Gajda, A.; et al. New Markers of Renal Failure in Multiple Myeloma and Monoclonal Gammopathies. J. Clin. Med. 2020, 9, 1652. [Google Scholar] [CrossRef]
- Woziwodzka, K.; Małyszko, J.; Koc-Żórawska, E.; Żórawski, M.; Dumnicka, P.; Jurczyszyn, A.; Batko, K.; Mazur, P.; Banaszkiewicz, M.; Krzanowski, M.; et al. Renal Impairment Detectors: IGFBP-7 and NGAL as Tubular Injury Markers in Multiple Myeloma Patients. Medicina 2021, 57, 1348. [Google Scholar] [CrossRef]
- Herrera, G.A.; Joseph, L.; Gu, X.; Hough, A.; Barlogie, B. Renal Pathologic Spectrum in an Autopsy Series of Patients with Plasma Cell Dyscrasia. Arch. Pathol. Lab. Med. 2004, 128, 875–879. [Google Scholar] [CrossRef]
- Kim, H.-R.; Park, J.-S.; Karabulut, H.; Yasmin, F.; Jun, C.-D. Transgelin-2: A double-edged sword in immunity and cancer metastasis. Front. Cell Dev. Biol. 2021, 9, 606149. [Google Scholar] [CrossRef]
- Zatula, A.; Dikic, A.; Mulder, C.; Sharma, A.; Vågbø, C.B.; Sousa, M.M.L.; Waage, A.; Slupphaug, G. Proteome alterations associated with transformation of multiple myeloma to secondary plasma cell leukemia. Oncotarget 2016, 8, 19427–19442. [Google Scholar] [CrossRef] [Green Version]
- Meng, T.; Liu, L.; Hao, R.; Chen, S.; Dong, Y. Transgelin-2: A potential oncogenic factor. Tumor Biol. 2017, 39, 1010428317702650. [Google Scholar] [CrossRef] [Green Version]
- Shimada, S.; Hirose, T.; Takahashi, C.; Sato, E.; Kinugasa, S.; Ohsaki, Y.; Kisu, K.; Sato, H.; Ito, S.; Mori, T. Pathophysiological and molecular mechanisms involved in renal congestion in a novel rat model. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef]
- Gerolymos, M.; Karagianni, F.; Papasotiriou, M.; Kalliakmani, P.; Sotsiou, F.; Charonis, A.; Goumenos, D. Expression of Transgelin in Human Glomerulonephritis of Various Etiology. Nephron 2011, 119, c74–c82. [Google Scholar] [CrossRef]
- Hauser, P.V.; Perco, P.; Mühlberger, I.; Pippin, J.; Blonski, M.; Mayer, B.; Alpers, C.E.; Oberbauer, R.; Shankland, S.J. Microarray and Bioinformatics Analysis of Gene Expression in Experimental Membranous Nephropathy. Nephron 2009, 112, e43–e58. [Google Scholar] [CrossRef] [Green Version]
- Inomata, S.; Sakatsume, M.; Sakamaki, Y.; Wang, X.; Goto, S.; Yamamoto, T.; Gejyo, F.; Narita, I. Expression of SM22α (Transgelin) in Glomerular and Interstitial Renal Injury. Nephron 2011, 117, e104–e113. [Google Scholar] [CrossRef]
- Miao, J.; Fan, Q.; Cui, Q.; Zhang, H.; Chen, L.; Wang, S.; Guan, N.; Guan, Y.; Ding, J. Newly identified cytoskeletal components are associated with dynamic changes of podocyte foot processes. Nephrol. Dial. Transplant. 2009, 24, 3297–3305. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, A.; Sakatsume, M.; Wang, X.; Sakamaki, Y.; Tsubata, Y.; Alchi, B.; Kuroda, T.; Kawachi, H.; Narita, I.; Shimizu, F.; et al. SM22α: The Novel Phenotype Marker of Injured Glomerular Epithelial Cells in Anti-Glomerular Basement Membrane Nephritis. Nephron 2007, 106, e77–e87. [Google Scholar] [CrossRef]
- Sakamaki, Y.; Sakatsume, M.; Wang, X.; Inomata, S.; Yamamoto, T.; Gejyo, F.; Narita, I. Injured kidney cells express SM22α (transgelin): Unique features distinct from α-smooth muscle actin (αSMA). Nephrology 2011, 16, 211–218. [Google Scholar] [CrossRef]
- Karagianni, F.; Prakoura, N.; Kaltsa, G.; Politis, P.; Arvaniti, E.; Kaltezioti, V.; Psarras, S.; Pagakis, S.; Katsimpoulas, M.; Abed, A.; et al. Transgelin Up-Regulation in Obstructive Nephropathy. PLoS ONE 2013, 8, e66887. [Google Scholar] [CrossRef] [Green Version]
- Upadhyay, R.; Ying, W.-Z.; Nasrin, Z.; Safah, H.; Jaimes, E.A.; Feng, W.; Sanders, P.W.; Batuman, V. Free light chains injure proximal tubule cells through the STAT1/HMGB1/TLR axis. JCI Insight 2020, 5, 137191. [Google Scholar] [CrossRef]
- Ying, W.-Z.; Li, X.; Rangarajan, S.; Feng, W.; Curtis, L.M.; Sanders, P.W. Immunoglobulin light chains generate proinflammatory and profibrotic kidney injury. J. Clin. Investig. 2019, 129, 2792–2806. [Google Scholar] [CrossRef] [Green Version]
- Hutchison, C.A.; Cockwell, P.; Stringer, S.; Bradwell, A.; Cook, M.; Gertz, M.A.; Dispenzieri, A.; Winters, J.L.; Kumar, S.; Rajkumar, S.V.; et al. Early Reduction of Serum-Free Light Chains Associates with Renal Recovery in Myeloma Kidney. J. Am. Soc. Nephrol. 2011, 22, 1129–1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montseny, J.J.; Kleinknecht, D.; Meyrier, A.; Vanhille, P.; Simon, P.; Pruna, A.; Eladari, D. Long-term outcome according to renal histological lesions in 118 patients with monoclonal gammopathies. Nephrol. Dial. Transplant. 1998, 13, 1438–1445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Royal, V.; Leung, N.; Troyanov, S.; Nasr, S.H.; Écotière, L.; Leblanc, R.; Adam, B.; Angioi, A.; Alexander, M.P.; Asunis, A.M.; et al. Clinicopathologic predictors of renal outcomes in light chain cast nephropathy: A multicenter retrospective study. Blood 2020, 135, 1833–1846. [Google Scholar] [CrossRef] [PubMed]
- Eadon, M.T.; Schwantes-An, T.-H.; Phillips, C.L.; Roberts, A.R.; Greene, C.V.; Hallab, A.; Hart, K.J.; Lipp, S.; Perez-Ledezma, C.; Omar, K.O.; et al. Kidney Histopathology and Prediction of Kidney Failure: A Retrospective Cohort Study. Am. J. Kidney Dis. 2020, 76, 350–360. [Google Scholar] [CrossRef]
- Silliman, C.C.; Dzieciatkowska, M.; Moore, E.E.; Kelher, M.R.; Banerjee, A.; Liang, X.; Land, K.J.; Hansen, K.C. Proteomic analyses of human plasma: Venus versus Mars. Transfusion 2012, 52, 417–424. [Google Scholar] [CrossRef] [Green Version]
- Ortega, F.J.; Moreno-Navarrete, J.M.; Mercader, J.M.; Gómez-Serrano, M.; García-Santos, E.; Latorre, J.; Lluch, A.; Sabater, M.; Caballano-Infantes, E.; Guzmán, R.; et al. Cytoskeletal transgelin 2 contributes to gender-dependent adipose tissue expandability and immune function. FASEB J. 2019, 33, 9656–9671. [Google Scholar] [CrossRef] [Green Version]
- Zeidan, A.; Swärd, K.; Nordström, I.; Ekblad, E.; Zhang, J.C.; Parmacek, M.S.; Hellstrand, P. Ablation of SM22α decreases contractility and actin contents of mouse vascular smooth muscle. FEBS Lett. 2004, 562, 141–146. [Google Scholar] [CrossRef] [Green Version]
- Shapland, C.; Hsuan, J.J.; Totty, N.F.; Lawson, D. Purification and properties of transgelin: A transformation and shape change sensitive actin-gelling protein. J. Cell Biol. 1993, 121, 1065–1073. [Google Scholar] [CrossRef]
- Yamamura, H.; Masuda, H.; Ikeda, W.; Tokuyama, T.; Takagi, M.; Shibata, N.; Tatsuta, M.; Takahashi, K. Structure and Expression of the Human SM22 Gene, Assignment of the Gene to Chromosome 11, and Repression of the Promoter Activity by Cytosine DNA Methylation. J. Biochem. 1997, 122, 157–167. [Google Scholar] [CrossRef]
- Yin, L.-M.; Ulloa, L.; Yang, Y.-Q. Transgelin-2: Biochemical and Clinical Implications in Cancer and Asthma. Trends Biochem. Sci. 2019, 44, 885–896. [Google Scholar] [CrossRef]
- Field-Smith, A.; Morgan, G.; Davies, F. Bortezomib (Velcade?) in the treatment of multiple myeloma. Ther. Clin. Risk Manag. 2006, 2, 271–279. [Google Scholar] [CrossRef] [Green Version]
- Bolomsky, A.; Hübl, W.; Spada, S.; Müldür, E.; Schlangen, K.; Heintel, D.; Rocci, A.; Weißmann, A.; Fritz, V.; Willheim, M.; et al. IKAROS expression in distinct bone marrow cell populations as a candidate biomarker for outcome with lenalidomide-dexamethasone therapy in multiple myeloma. Am. J. Hematol. 2017, 92, 269–278. [Google Scholar] [CrossRef] [Green Version]
- Brunskill, E.W.; Sequeira-Lopez, M.L.S.; Pentz, E.S.; Lin, E.; Yu, J.; Aronow, B.J.; Potter, S.S.; Gomez, R.A. Genes that Confer the Identity of the Renin Cell. J. Am. Soc. Nephrol. 2011, 22, 2213–2225. [Google Scholar] [CrossRef]
- Kotla, V.; Goel, S.; Nischal, S.; Heuck, C.; Vivek, K.; Das, B.; Verma, A. Mechanism of action of lenalidomide in hematological malignancies. J. Hematol. Oncol. 2009, 2, 36. [Google Scholar] [CrossRef] [Green Version]
- Dai, X.; Thiagarajan, D.; Fang, J.; Shen, J.; Annam, N.P.; Yang, Z.; Jiang, H.; Ju, D.; Xie, Y.; Zhang, K.; et al. SM22α suppresses cytokine-induced inflammation and the transcription of NF-κB inducing kinase (Nik) by modulating SRF transcriptional activity in vascular smooth muscle cells. PLoS ONE 2017, 12, e0190191. [Google Scholar] [CrossRef]

| Characteristic | Results in the Studied MM Patients (n = 126) |
|---|---|
| Mean age (standard deviation), years | 67 (10) |
| Male sex, n (%) | 53 (42) |
| Median time since diagnosis (Q1; Q3), months | 30 (14; 63) |
| Smoldering MM, n (%) | 7 (6) |
| ISS: | |
| stage I, n (%) | 84 (67) |
| stage II, n (%) | 20 (16) |
| stage III, n (%) | 15 (12) |
| Immunofixation: | |
| IgG, n (%) | 92 (73) |
| IgM, n (%) | 2 (2) |
| IgA, n (%) | 23 (18) |
| κ, n (%) | 79 (63) |
| λ, n (%) | 44 (35) |
| free light chains, n (%) | 18 (14) |
| biclonal, n (%) | 5 (4) |
| non-secretory, n (%) | 5 (4) |
| Disease state: | |
| CR, n (%) | 41 (33) |
| PR, n (%) | 48 (38) |
| SD, n (%) | 9 (7) |
| PD, n (%) | 28 (22) |
| On maintenance treatment, n (%) | 61 (48) |
| Prior treatment: | |
| none, n (%) | 8 (6) |
| 1 line, n (%) | 35 (28) |
| 2 or more lines, n (%) | 83 (66) |
| History of auto-PBSCT, n (%) | 57 (45) |
| Anemia, n (%) | 25 (20) |
| Bone lesions, n (%) | 77 (61) |
| History of AKI, n (%) | 8 (6) |
| Laboratory Test | Results in MM Patients (n = 126) | Reference Range or Results in Healthy Controls (n = 32) |
|---|---|---|
| Creatinine, µmol/L | 78 (67; 98) | F: 44–80; M: 62–106 |
| eGFR (CKD-EPICr), mL/min/1.73 m2 | 73 (62; 91) | ≥90 |
| eGFR (CKD-EPICr) <60 mL/min/1.73 m2, n (%) | 29 (23) | - |
| eGFR (CKD-EPICr-CysC), mL/min/1.73 m2 | 76 (27) | ≥90 |
| Uric acid, µmol/L | 289 (81) | F: 143–340; M: 202–416 |
| Albumin, g/L | 42.0 (4.7) | 35–52 |
| β2-microglobulin, mg/L | 2.54 (2.10; 3.63) | 1.09–2.53 |
| Involved serum FLC, mg/L | 29.6 (16.3; 100.0) | κ: 6.7–22.4; λ: 8.3–27.0 |
| Involved urine LC, mg/L | 6.8 (6.3; 30.0) | κ: <7.09; λ: <3.89 |
| White blood cell count, ×103/µL | 6.12 (4.89; 7.25) | 4.0–10.0 |
| Hemoglobin, g/dL | 12.6 (1.7) | F: 11–15; M: 12–17 |
| Platelet count, ×103/µL | 172 (143; 213) | 125–340 |
| Lactate dehydrogenase, U/L | 355 (303; 404) | 240–480 |
| Interleukin 6, pg/mL a | 2.97 (1.61; 6.00) | 1.51 (1.07; 2.05) c,* |
| Ferritin, µg/L b | 164 (63; 414) | 13–400 |
| Hepcidin, ng/mL a | 28.8 (16.5; 44.6) | 27.1 (20.0; 37.2) c |
| Alanine aminotransferase, U/L | 21 (16; 29) | F: 5–33; M: 5–41 |
| Aspartate aminotransferase, U/L | 21 (17; 27) | F: 5–32; M: 5–40 |
| Transgelin, ng/mL | 84.1 (65.4; 116.4) | 69.3 (56.8–90.4) c,* |
| Periostin, pmol/L | 1133 (798; 1663) | 1176 (995–1455) c |
| Proteinuria, n (%) | 24 (19) | - |
| Urine IGFBP-7, ng/mL | 5.19 (2.24; 12.74) | 2.65 (1.36–5.75) c,* |
| Urine TIMP-2, ng/mL | 2.60 (0.48; 8.78) | 1.08 (0.15; 2.35) c,* |
| Urine cystatin C, ng/mL | 42.6 (16.3; 86.5) | 46.7 (26.5–64.3) c |
| Urine NGAL monomer, ng/mL | 9.23 (4.42; 26.8) | 9.06 (4.73–11.86) c |
| Independent Variable | Model 1 | Model 2 | ||
|---|---|---|---|---|
| Beta ± SE | p-Value | Beta ± SE | p-Value | |
| Baseline eGFR | 0.75 ± 0.07 | <0.001 | 0.71 ± 0.07 | <0.001 |
| log (baseline transgelin) | −0.14 ± 0.05 | 0.011 | not included | |
| Transgelin tertiles: | not included | |||
| lower | reference | - | ||
| middle | −0.07 ± 0.06 | 0.3 | ||
| upper | −0.20 ± 0.07 | 0.003 | ||
| log (urine NGAL monomer) | −0.05 ± 0.06 | 0.4 | −0.06 ± 0.06 | 0.4 |
| log (urine IGFBP-7) | −0.01 ± 0.06 | 0.9 | −0.01 ± 0.06 | 0.9 |
| Adjusted R2 for the model | 0.72 | <0.001 | 0.72 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woziwodzka, K.; Małyszko, J.; Koc-Żórawska, E.; Żórawski, M.; Dumnicka, P.; Jurczyszyn, A.; Batko, K.; Mazur, P.; Banaszkiewicz, M.; Krzanowski, M.; et al. Transgelin-2 in Multiple Myeloma: A New Marker of Renal Impairment? Molecules 2022, 27, 79. https://doi.org/10.3390/molecules27010079
Woziwodzka K, Małyszko J, Koc-Żórawska E, Żórawski M, Dumnicka P, Jurczyszyn A, Batko K, Mazur P, Banaszkiewicz M, Krzanowski M, et al. Transgelin-2 in Multiple Myeloma: A New Marker of Renal Impairment? Molecules. 2022; 27(1):79. https://doi.org/10.3390/molecules27010079
Chicago/Turabian StyleWoziwodzka, Karolina, Jolanta Małyszko, Ewa Koc-Żórawska, Marcin Żórawski, Paulina Dumnicka, Artur Jurczyszyn, Krzysztof Batko, Paulina Mazur, Małgorzata Banaszkiewicz, Marcin Krzanowski, and et al. 2022. "Transgelin-2 in Multiple Myeloma: A New Marker of Renal Impairment?" Molecules 27, no. 1: 79. https://doi.org/10.3390/molecules27010079
APA StyleWoziwodzka, K., Małyszko, J., Koc-Żórawska, E., Żórawski, M., Dumnicka, P., Jurczyszyn, A., Batko, K., Mazur, P., Banaszkiewicz, M., Krzanowski, M., Gołasa, P., Małyszko, J. A., Drożdż, R., & Krzanowska, K. (2022). Transgelin-2 in Multiple Myeloma: A New Marker of Renal Impairment? Molecules, 27(1), 79. https://doi.org/10.3390/molecules27010079








