Stabilizing the Exotic Carbonic Acid by Bisulfate Ion
Abstract
1. Introduction
2. Theoretical Methods
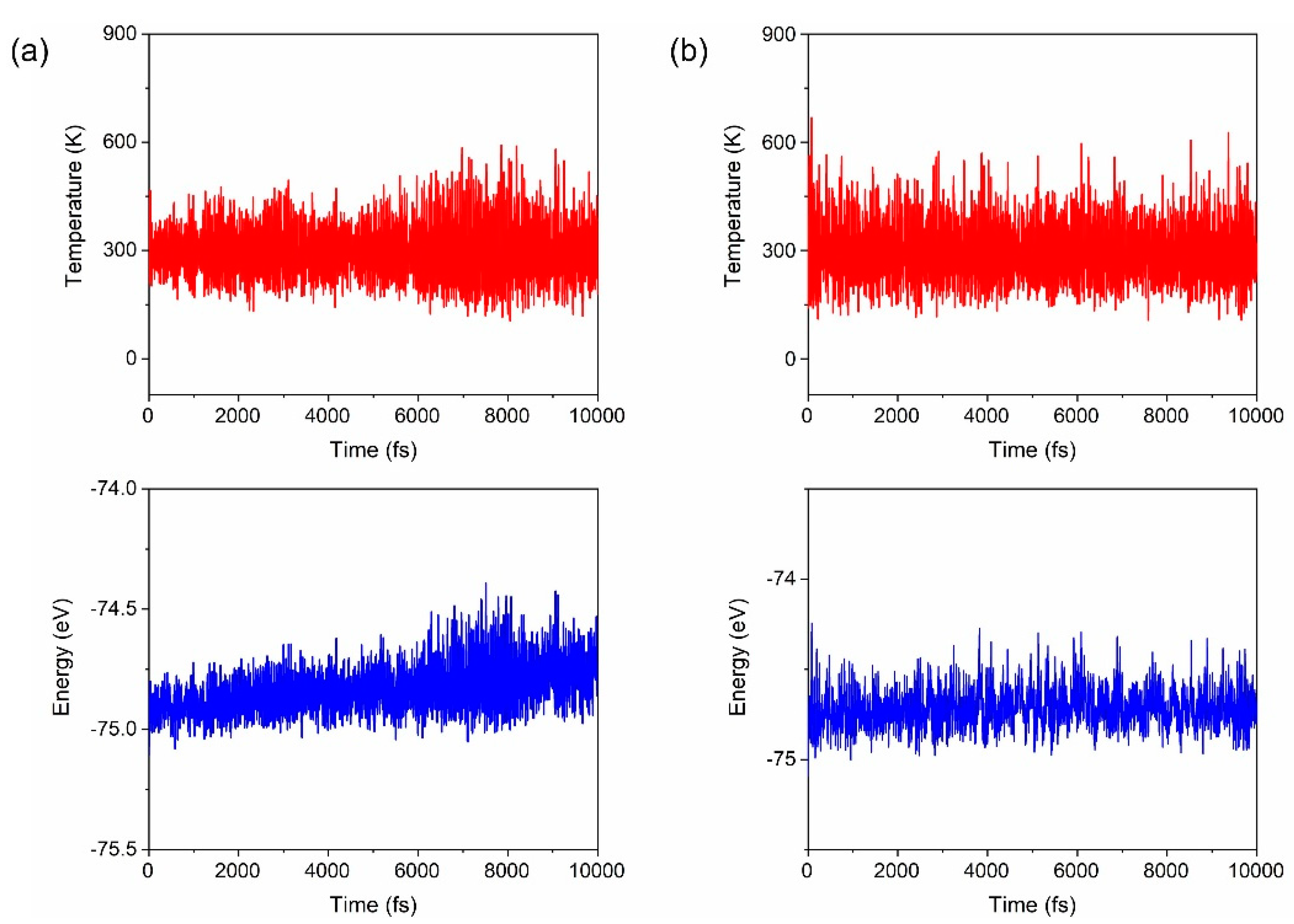
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Terlouw, J.K.; Lebrilla, C.B.; Schwarz, H. Thermolysis of NH4HCO3—A aimple route to the formation of free carbonic acid (H2CO3) in the gas phase. Angew. Chem. Int. Ed. 1987, 26, 354–355. [Google Scholar] [CrossRef]
- Andersson, A.J.; Mackenzie, F.T.; Lerman, A. Coastal ocean CO2-carbonic acid-carbonate sediment system of the Anthropocene. Glob. Biogeochem. Cycles 2006, 20, GB1S92. [Google Scholar] [CrossRef]
- Stolte, N.; Pan, D. Large presence of carbonic acid in CO2-rich aqueous fluids under earth’s mantle conditions. J. Phys. Chem. Lett. 2019, 10, 5135–5141. [Google Scholar] [CrossRef] [PubMed]
- Strazzulla, G.; Brucato, J.R.; Cimino, G.; Palumbo, M.E. Carbonic acid on Mars? Planet. Space Sci. 1996, 44, 1447–1450. [Google Scholar] [CrossRef]
- Hage, W.; Liedl Klaus, R.; Hallbrucker, A.; Mayer, E. Carbonic acid in the gas phase and its astrophysical relevance. Science 1998, 279, 1332–1335. [Google Scholar] [CrossRef]
- Delitsky, M.L.; Lane, A.L. Ice chemistry on the Galilean satellites. J. Geophys. Res. Planets 1998, 103, 31391–31403. [Google Scholar] [CrossRef]
- Aminov, D.; Pines, D.; Kiefer, P.M.; Daschakraborty, S.; Hynes, J.T.; Pines, E. Intact carbonic acid is a viable protonating agent for biological bases. Proc. Natl. Acad. Sci. USA 2019, 116, 20837. [Google Scholar] [CrossRef]
- Al-Hosney, H.A.; Grassian, V.H. Carbonic Acid: An important intermediate in the surface chemistry of calcium carbonate. J. Am. Chem. Soc. 2004, 126, 8068–8069. [Google Scholar] [CrossRef]
- Moore, M.H.; Hudson, R.L.; Gerakines, P.A. Mid- and far-infrared spectroscopic studies of the influence of temperature, ultraviolet photolysis and ion irradiation on cosmic-type ices. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2001, 57, 843–858. [Google Scholar] [CrossRef]
- Jönsson, B.; Karlström, G.; Wennerström, H.; Roos, B. Ab initio molecular orbital calculations on the water-carbon dioxide system: Carbonic acid. Chem. Phys. Lett. 1976, 41, 317–320. [Google Scholar] [CrossRef]
- Kumar, P.P.; Kalinichev, A.G.; Kirkpatrick, R.J. Dissociation of carbonic acid: Gas phase energetics and mechanism from ab initio metadynamics simulations. J. Chem. Phys. 2007, 126, 204315. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Busch, D.H.; Subramaniam, B.; Thompson, W.H. Organic acids tunably catalyze carbonic acid decomposition. J. Phys. Chem. A 2014, 118, 5020–5028. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, B.; Biswas, P.; Kumar, P. Ammonia as an efficient catalyst for decomposition of carbonic acid: A quantum chemical investigation. Phys. Chem. Phys. Chem. 2016, 18, 15995–16004. [Google Scholar] [CrossRef] [PubMed]
- Mallick, S.; Sarkar, S.; Bandyopadhyay, B.; Kumar, P. Effect of ammonia-water complex on decomposition of carbonic acid in troposphere: A quantum chemical investigation. Comput. Theor. Chem. 2018, 1132, 50–58. [Google Scholar] [CrossRef]
- Ghoshal, S.; Hazra, M.K. H2CO3 → CO2 + H2O decomposition in the presence of H2O, HCOOH, CH3COOH, H2SO4 and HO2 radical: Instability of the gas-phase H2CO3 molecule in the troposphere and lower stratosphere. RSC Adv. 2015, 5, 17623–17635. [Google Scholar] [CrossRef]
- de Marothy, S.A. Autocatalytic decomposition of carbonic acid. Int. J. Quantum Chem. 2013, 113, 2306–2311. [Google Scholar] [CrossRef]
- Ghoshal, S.; Hazra, M.K. New mechanism for autocatalytic decomposition of H2CO3 in the vapor phase. J. Phys. Chem. A 2014, 118, 2385–2392. [Google Scholar] [CrossRef]
- Mori, T.; Suma, K.; Sumiyoshi, Y.; Endo, Y. Spectroscopic detection of isolated carbonic acid. J. Chem. Phys. 2009, 130, 204308. [Google Scholar] [CrossRef]
- Mori, T.; Suma, K.; Sumiyoshi, Y.; Endo, Y. Spectroscopic detection of the most stable carbonic acid, cis-cis H2CO3. J. Chem. Phys. 2011, 134, 044319. [Google Scholar] [CrossRef] [PubMed]
- Bernard, J.; Seidl, M.; Kohl, I.; Liedl, K.R.; Mayer, E.; Gálvez, Ó.; Grothe, H.; Loerting, T. Spectroscopic observation of matrix-isolated carbonic acid trapped from the gas phase. Angew. Chem. Int. Ed. 2011, 50, 1939–1943. [Google Scholar] [CrossRef]
- Reisenauer, H.P.; Wagner, J.P.; Schreiner, P.R. Gas-phase preparation of carbonic acid and its monomethyl ester. Angew. Chem. Int. Ed. 2014, 53, 11766–11771. [Google Scholar] [CrossRef] [PubMed]
- Ioppolo, S.; Kaňuchová, Z.; James, R.L.; Dawes, A.; Ryabov, A.; Dezalay, J.; Jones, N.C.; Hoffmann, S.V.; Mason, N.J.; Strazzulla, G.; et al. Vacuum ultraviolet photoabsorption spectroscopy of space-related ices: Formation and destruction of solid carbonic acid upon 1 keV electron irradiation. Astron. Astrophys. 2021, 646, A172. [Google Scholar] [CrossRef]
- Wang, X.; Bgrgi, T. Observation of carbonic acid formation from interaction between carbon dioxide and ice by using in situ modulation excitation IR spectroscopy. Angew. Chem. Int. Ed. 2021, 60, 7860–7865. [Google Scholar] [CrossRef] [PubMed]
- Hou, G.-L.; Wang, X.-B. Molecular specificity and proton transfer mechanisms in aerosol prenucleation clusters relevant to new particle formation. Acc. Chem. Res. 2020, 53, 2816–2827. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Zhang, H.; Yuan, Q.; Zhou, X.; Kass, S.R.; Wang, X.-B. Observation of conformational simplification upon N-methylation on amino acid iodide clusters. J. Phys. Chem. Lett. 2021, 12, 2780–2787. [Google Scholar] [CrossRef]
- Wang, L.; Yuan, Q.; Cao, W.; Han, J.; Zhou, X.; Liu, S.; Wang, X.-B. Probing orientation-specific charge–dipole interactions between hexafluoroisopropanol and halides: A joint photoelectron spectroscopy and theoretical study. J. Phys. Chem. A 2020, 124, 2036–2045. [Google Scholar] [CrossRef]
- Hou, G.-L.; Lin, W.; Wang, X.-B. Direct observation of hierarchic molecular interactions critical to biogenic aerosol formation. Commun. Chem. 2018, 1, 37. [Google Scholar] [CrossRef]
- Wen, H.; Hou, G.-L.; Kathmann, S.M.; Valiev, M.; Wang, X.-B. Communication: Solute anisotropy effects in hydrated anion and neutral clusters. J. Chem. Phys. 2013, 138, 031101. [Google Scholar] [CrossRef]
- Hou, G.-L.; Zhang, J.; Valiev, M.; Wang, X.-B. Structures and energetics of hydrated deprotonated cis-pinonic acid anion clusters and their atmospheric relevance. Phys. Chem. Phys. Chem. 2017, 19, 10676–10684. [Google Scholar] [CrossRef]
- Hou, G.-L.; Liu, C.-W.; Li, R.-Z.; Xu, H.-G.; Gao, Y.Q.; Zheng, W.-J. Emergence of solvent-separated Na+–Cl– ion pair in salt water: Photoelectron spectroscopy and theoretical calculations. J. Phys. Chem. Lett. 2017, 8, 13–20. [Google Scholar] [CrossRef]
- Wen, H.; Hou, G.-L.; Liu, Y.-R.; Wang, X.-B.; Huang, W. Examining the structural evolution of bicarbonate–water clusters: Insights from photoelectron spectroscopy, basin-hopping structural search, and comparison with available IR spectral studies. Phys. Chem. Phys. Chem. 2016, 18, 17470–17482. [Google Scholar] [CrossRef]
- Hou, G.-L.; Valiev, M.; Wang, X.-B. Deprotonated dicarboxylic acid homodimers: Hydrogen bonds and atmospheric implications. J. Phys. Chem. A 2016, 120, 2342–2349. [Google Scholar] [CrossRef]
- Ferguson, S.J. Proton transfer: It’s a stringent process. Curr. Biol. 2000, 10, R627–R630. [Google Scholar] [CrossRef][Green Version]
- Blake, R.S.; Monks, P.S.; Ellis, A.M. Proton-transfer reaction mass spectrometry. Chem. Rev. 2009, 109, 861–896. [Google Scholar] [CrossRef] [PubMed]
- Hou, G.-L.; Lin, W.; Deng, S.H.M.; Zhang, J.; Zheng, W.-J.; Paesani, F.; Wang, X.-B. Negative ion photoelectron spectroscopy reveals thermodynamic advantage of organic acids in facilitating formation of bisulfate ion clusters: Atmospheric implications. J. Phys. Chem. Lett. 2013, 4, 779–785. [Google Scholar] [CrossRef]
- Hou, G.-L.; Wang, X.-B.; Valiev, M. Formation of (HCOO–)(H2SO4) anion clusters: Violation of gas-phase acidity predictions. J. Am. Chem. Soc. 2017, 139, 11321–11324. [Google Scholar] [CrossRef]
- Hou, G.-L.; Valiev, M.; Wang, X.-B. Sulfuric acid and aromatic carboxylate clusters H2SO4·ArCOO−: Structures, properties, and their relevance to the initial aerosol nucleation. Int. J. Mass Spectrom. 2019, 439, 27–33. [Google Scholar] [CrossRef]
- Hou, G.-L.; Wang, X.-B. Spectroscopic signature of proton location in proton bound HSO4–·H+·X– (X = F, Cl, Br, and I) clusters. J. Phys. Chem. Lett. 2019, 10, 6714–6719. [Google Scholar] [CrossRef]
- Thomas, D.A.; Mucha, E.; Lettow, M.; Meijer, G.; Rossi, M.; von Helden, G. Characterization of a trans–trans carbonic acid–fluoride complex by infrared action spectroscopy in helium nanodroplets. J. Am. Chem. Soc. 2019, 141, 5815–5823. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cao, W.; Yuan, Q.; Wang, L.; Zhou, X.; Liu, S.; Wang, X.-B. Spectroscopic evidence for intact carbonic acid stabilized by halide anions in the gas phase. Phys. Chem. Phys. Chem. 2020, 22, 19459–19467. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09 (Revision E.01); Gaussian Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Bader, R.F.W. A quantum theory of molecular structure and its applications. Chem. Rev. 1991, 91, 893–928. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Thomas, D.A.; Taccone, M.; Ober, K.; Mucha, E.; Meijer, G.; von Helden, G. Helium nanodroplet infrared action spectroscopy of the proton-bound dimer of hydrogen sulfate and formate: Examining nuclear quantum effects. J. Phys. Chem. A 2021, 125, 9279–9287. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhou, J.; Jena, P. Stability of B12(CN)122−: Implications for lithium and magnesium ion batteries. Angew. Chem. Int. Ed. 2016, 55, 3704–3708. [Google Scholar] [CrossRef]
- Xu, J.; Li, M.; Xu, S.; Pei, G.; Zhao, X.; Kong, C.; Yang, Z.; Yang, T.; Hou, G.-L. Stable noble gas compounds based on superelectrophilic anions [B12(BO)11]− and [B12(OBO)11]−. ChemPhysChem 2021, 22, 2240–2246. [Google Scholar] [CrossRef]
- Koch, U.; Popelier, P.L.A. Characterization of C–H–O hydrogen bonds on the basis of the charge density. J. Phys. Chem. 1995, 99, 9747–9754. [Google Scholar] [CrossRef]
- Emamian, S.; Lu, T.; Kruse, H.; Emamian, H. Exploring nature and predicting strength of hydrogen bonds: A correlation analysis between atoms-in-molecules descriptors, binding energies, and energy components of symmetry-adapted perturbation theory. J. Comput. Chem. 2019, 40, 2868–2881. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Duong, C.H.; Kelleher, P.J.; McCoy, A.B.; Johnson, M.A. Deconstructing water’s diffuse OH stretching vibrational spectrum with cold clusters. Science 2019, 364, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.J.; Johnson, M.A. Demystifying the diffuse vibrational spectrum of aqueous protons through cold cluster spectroscopy. Annu. Rev. Phys. Chem. 2021, 72, 667–691. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://cccbdb.nist.gov/vibscalejust.asp (accessed on 30 November 2021).


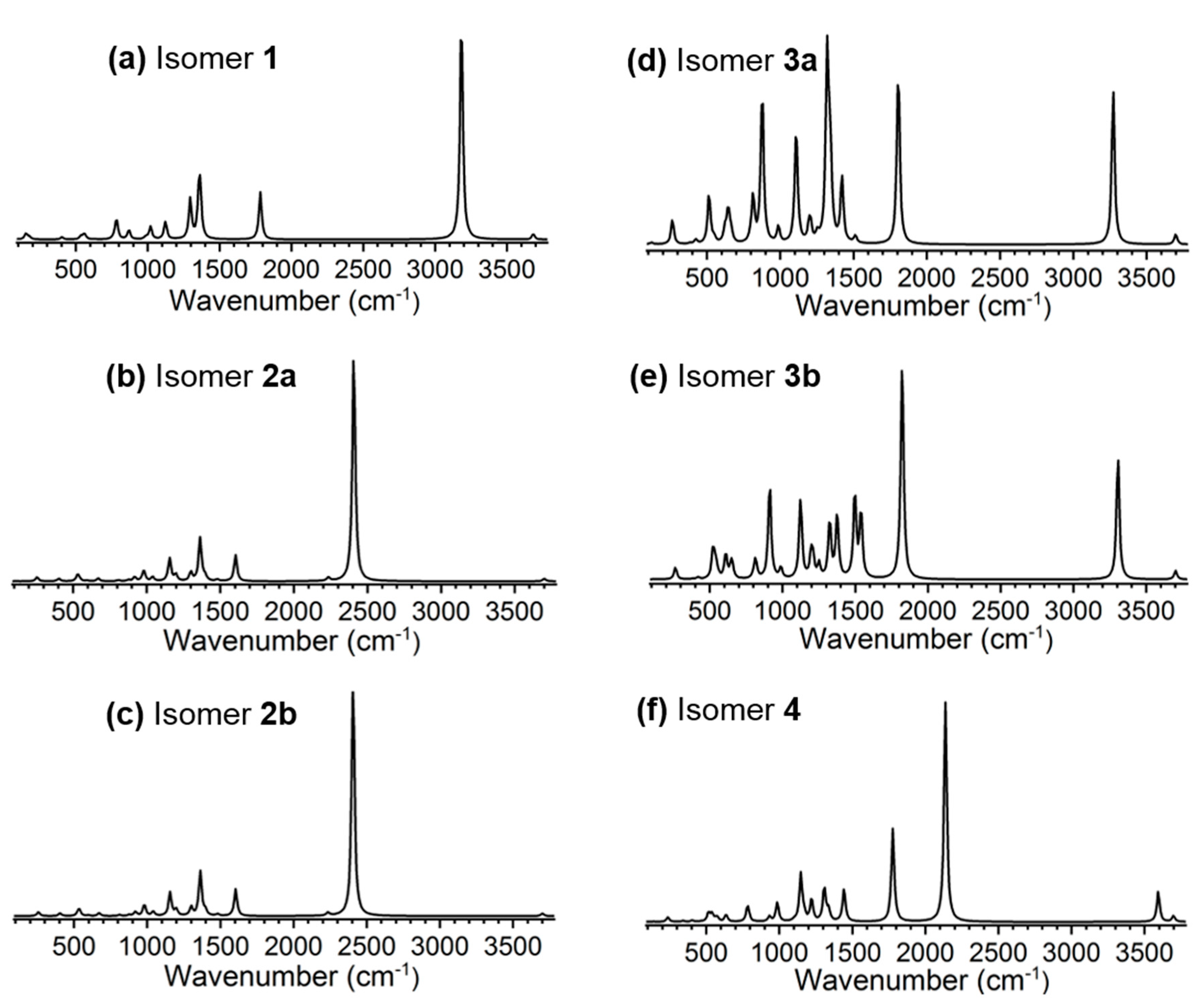
| [H2CO3·HSO4]− | ρ (10−2) a | ∇2ρ (10−2) | V(r) (10−2) | G(r) (10−2) | K(r) (10−2) | EHB | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I b | II | I | II | I | II | I | II | I | II | I | II | |
| Isomer 1 | 4.696 | 4.695 | 10.923 | 10.923 | −4.960 | −4.959 | 3.845 | 3.845 | 1.115 | 1.114 | −16.67 | −16.67 |
| Isomer 2a | 8.397 | 8.454 | 7.844 | 7.692 | −10.000 | −10.087 | 5.980 | 6.005 | 4.019 | 4.082 | −28.97 | −29.16 |
| Isomer 2b | 8.448 | 8.403 | 7.699 | 7.837 | −10.080 | −10.009 | 6.002 | 5.984 | 4.077 | 4.025 | −29.14 | −28.99 |
| Isomer 3a | 12.889 | 3.760 | −6.519 | 10.678 | −17.922 | −3.696 | 8.146 | 3.183 | 9.776 | 0.513 | −43.90 | −13.56 |
| Isomer 3b | 11.976 | 3.629 | −1.754 | 10.665 | −16.088 | −3.535 | 7.825 | 3.101 | 8.263 | 0.435 | −40.87 | −13.13 |
| Isomer 4 | 9.311 | 2.160 | 6.644 | 8.408 | −11.505 | −1.786 | 6.583 | 1.944 | 4.922 | −0.158 | −32.01 | −8.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, H.; Liu, S.-W.; Li, M.; Xu, B.; Zhao, L.; Yang, T.; Hou, G.-L. Stabilizing the Exotic Carbonic Acid by Bisulfate Ion. Molecules 2022, 27, 8. https://doi.org/10.3390/molecules27010008
Lu H, Liu S-W, Li M, Xu B, Zhao L, Yang T, Hou G-L. Stabilizing the Exotic Carbonic Acid by Bisulfate Ion. Molecules. 2022; 27(1):8. https://doi.org/10.3390/molecules27010008
Chicago/Turabian StyleLu, Huili, Shi-Wei Liu, Mengyang Li, Baocai Xu, Li Zhao, Tao Yang, and Gao-Lei Hou. 2022. "Stabilizing the Exotic Carbonic Acid by Bisulfate Ion" Molecules 27, no. 1: 8. https://doi.org/10.3390/molecules27010008
APA StyleLu, H., Liu, S.-W., Li, M., Xu, B., Zhao, L., Yang, T., & Hou, G.-L. (2022). Stabilizing the Exotic Carbonic Acid by Bisulfate Ion. Molecules, 27(1), 8. https://doi.org/10.3390/molecules27010008







