Bioactive Evaluation of Ursane-Type Pentacyclic Triterpenoids: β-Boswellic Acid Interferes with the Glycosylation and Transport of Intercellular Adhesion Molecule-1 in Human Lung Adenocarcinoma A549 Cells
Abstract
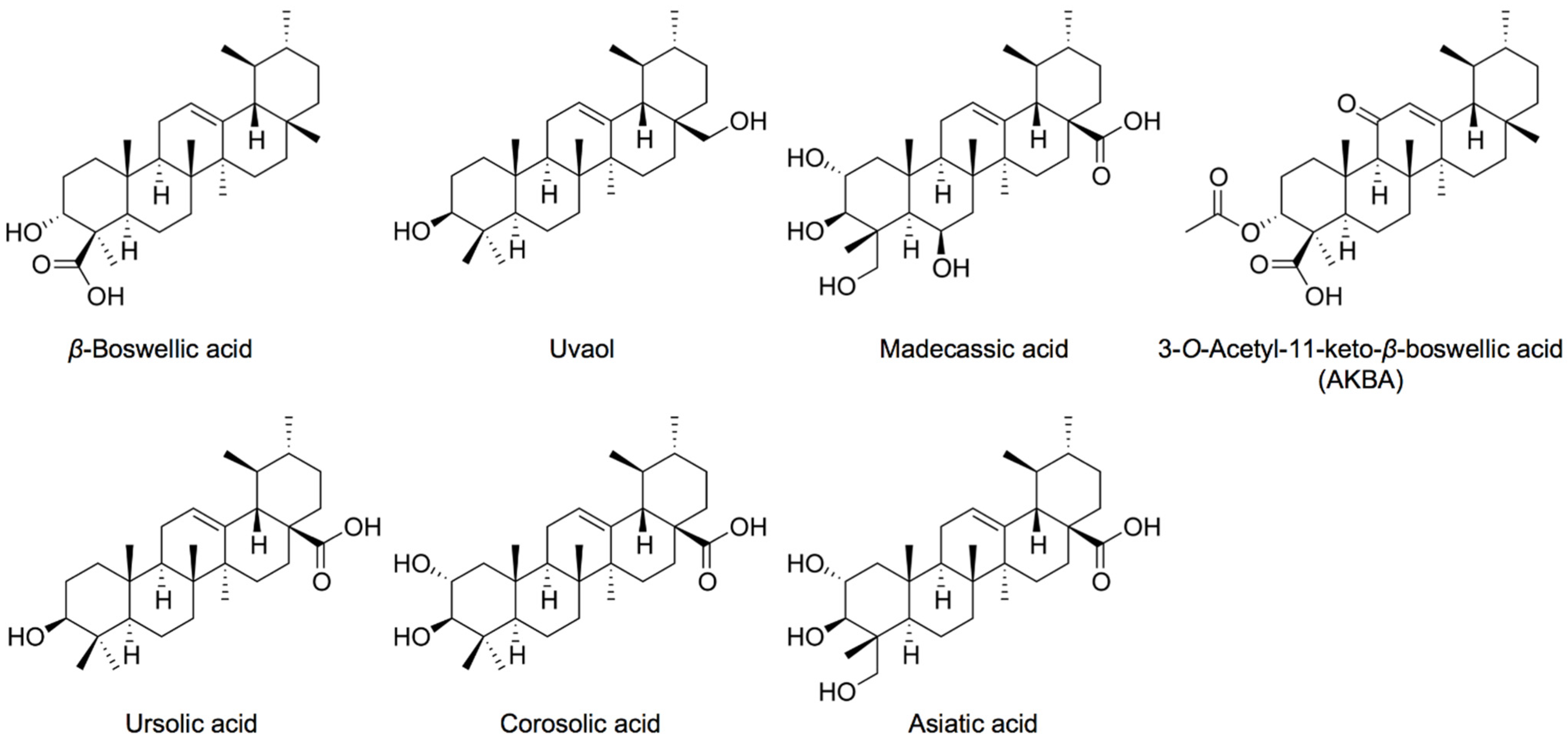
1. Introduction
2. Results and Discussion
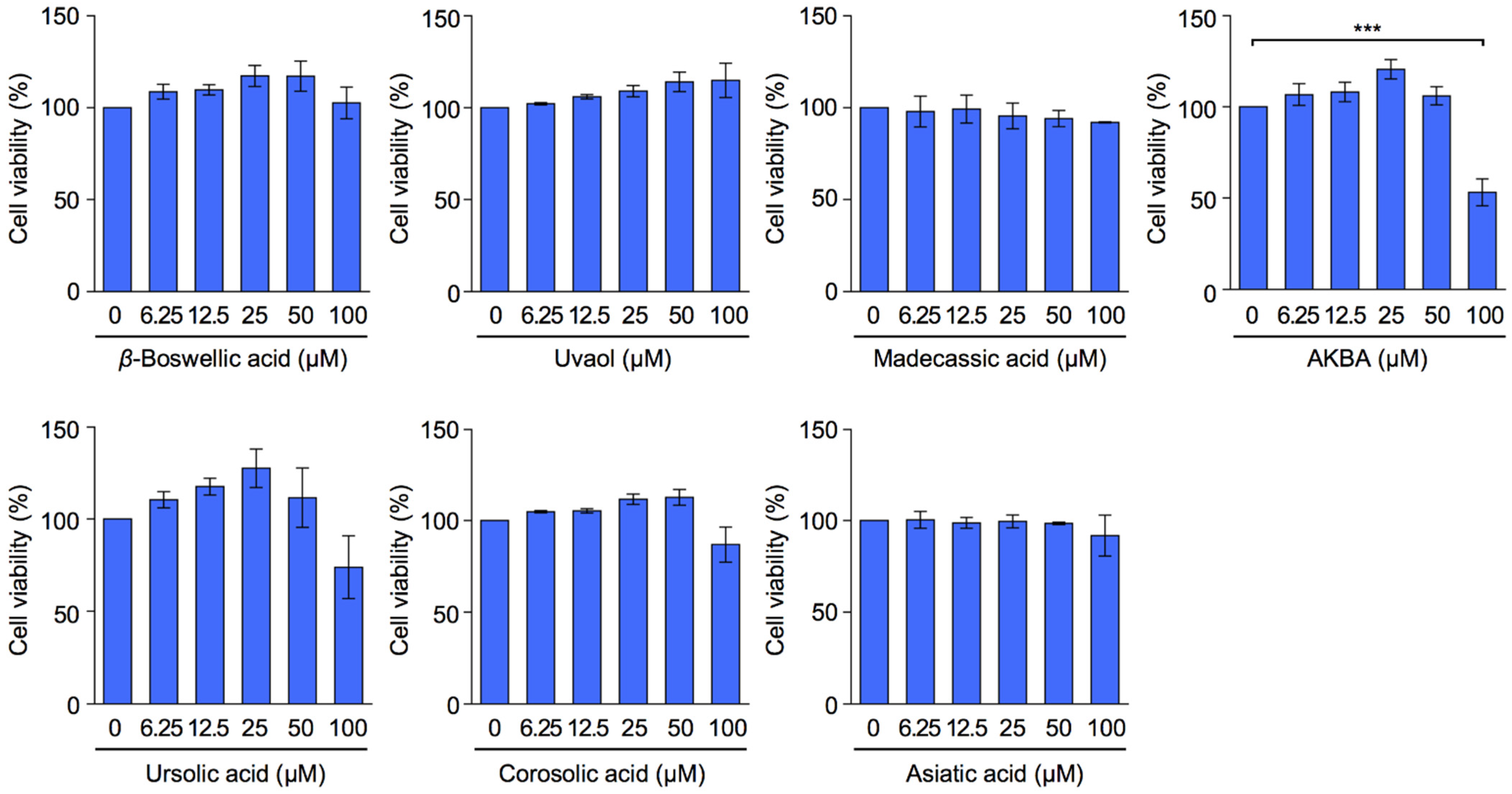
2.1. Bioactive Evaluation of Ursane-Type Pentacyclic Triterpenoids on the Viability of A549 Cells
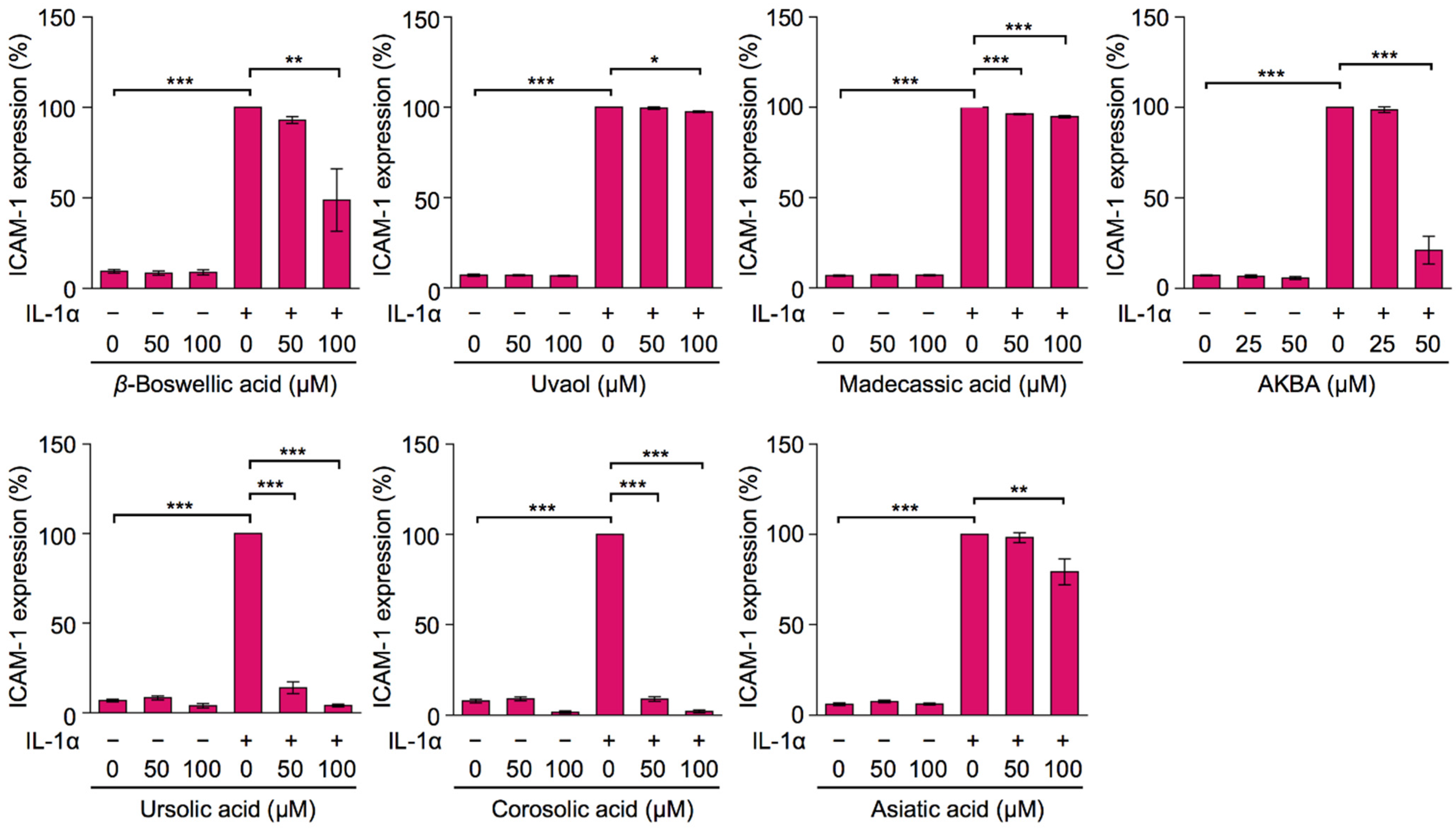
2.2. Bioactive Evaluation of Ursane-Type Pentacyclic Triterpenoids on Cell-Surface ICAM-1 Expression
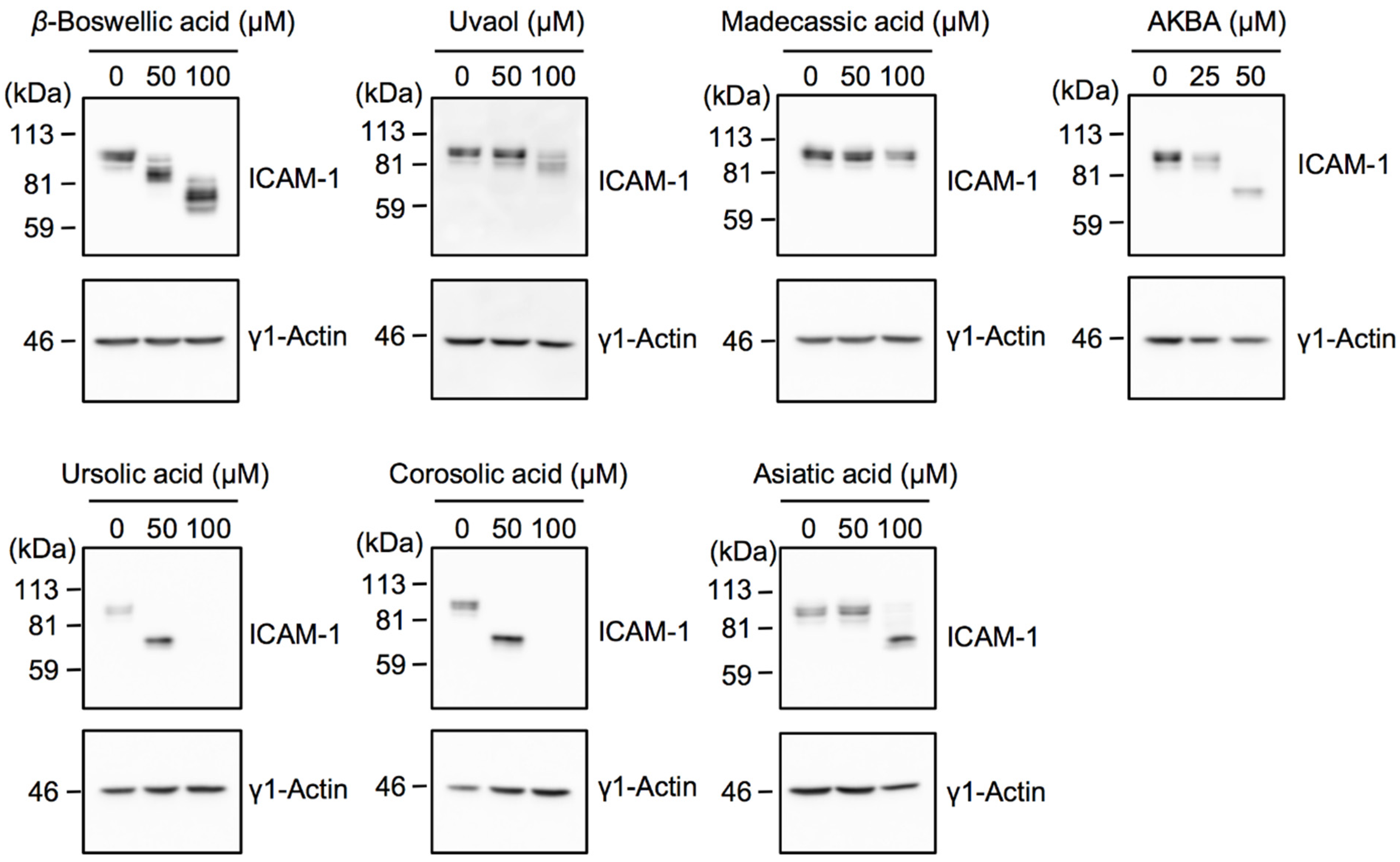
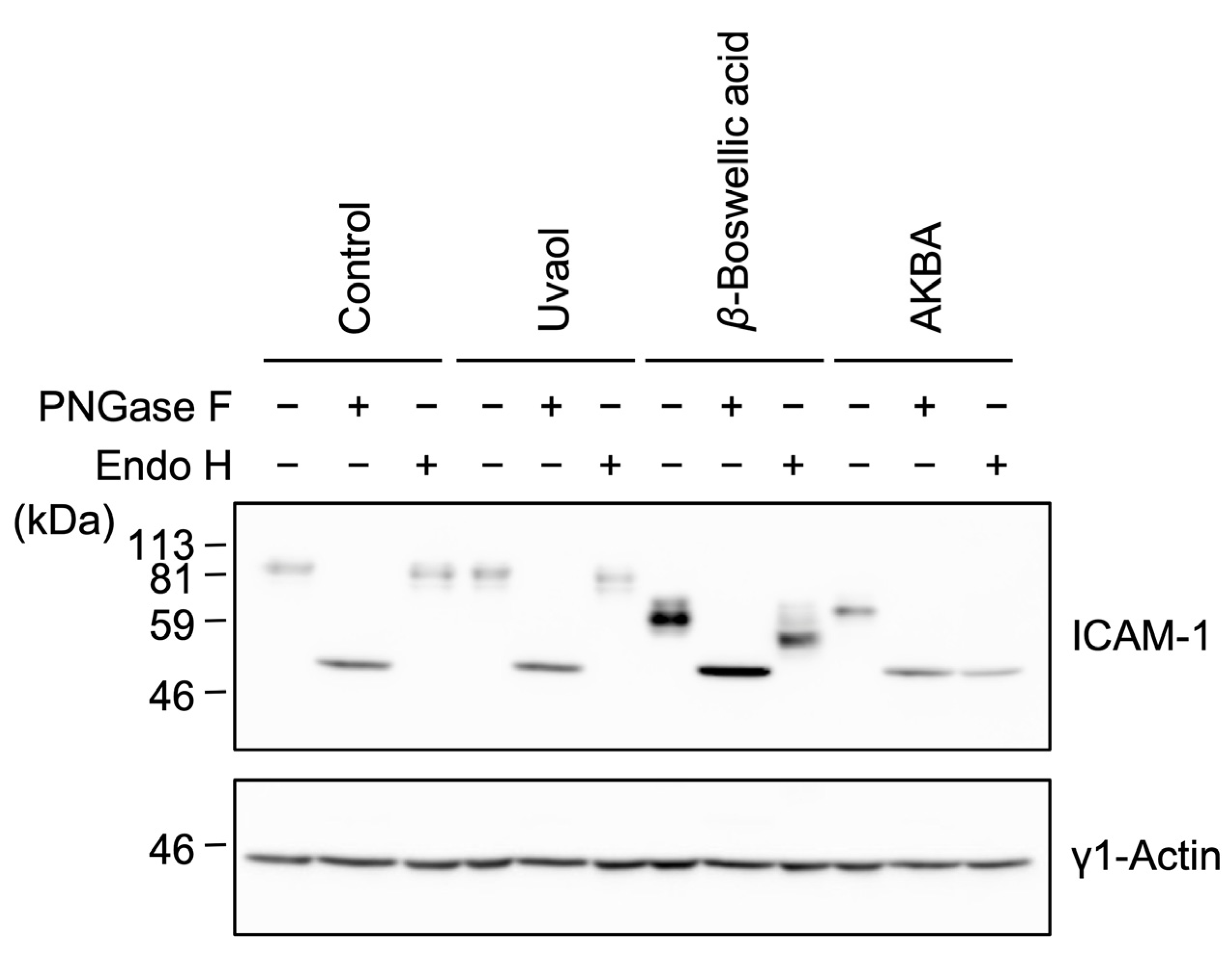
2.3. Bioactive Evaluation of Ursane-Type Pentacyclic Triterpenoids on ICAM-1 N-Glycosylation
2.4. Bioactive Evaluation of Ursane-Type Pentacyclic Triterpenoids on α-Glucosidase Activity
2.5. β-Boswellic Acid Altered ICAM-1 Glycosylation in a Different Manner from Other Ursane-Type Pentacyclic Triterpenoids
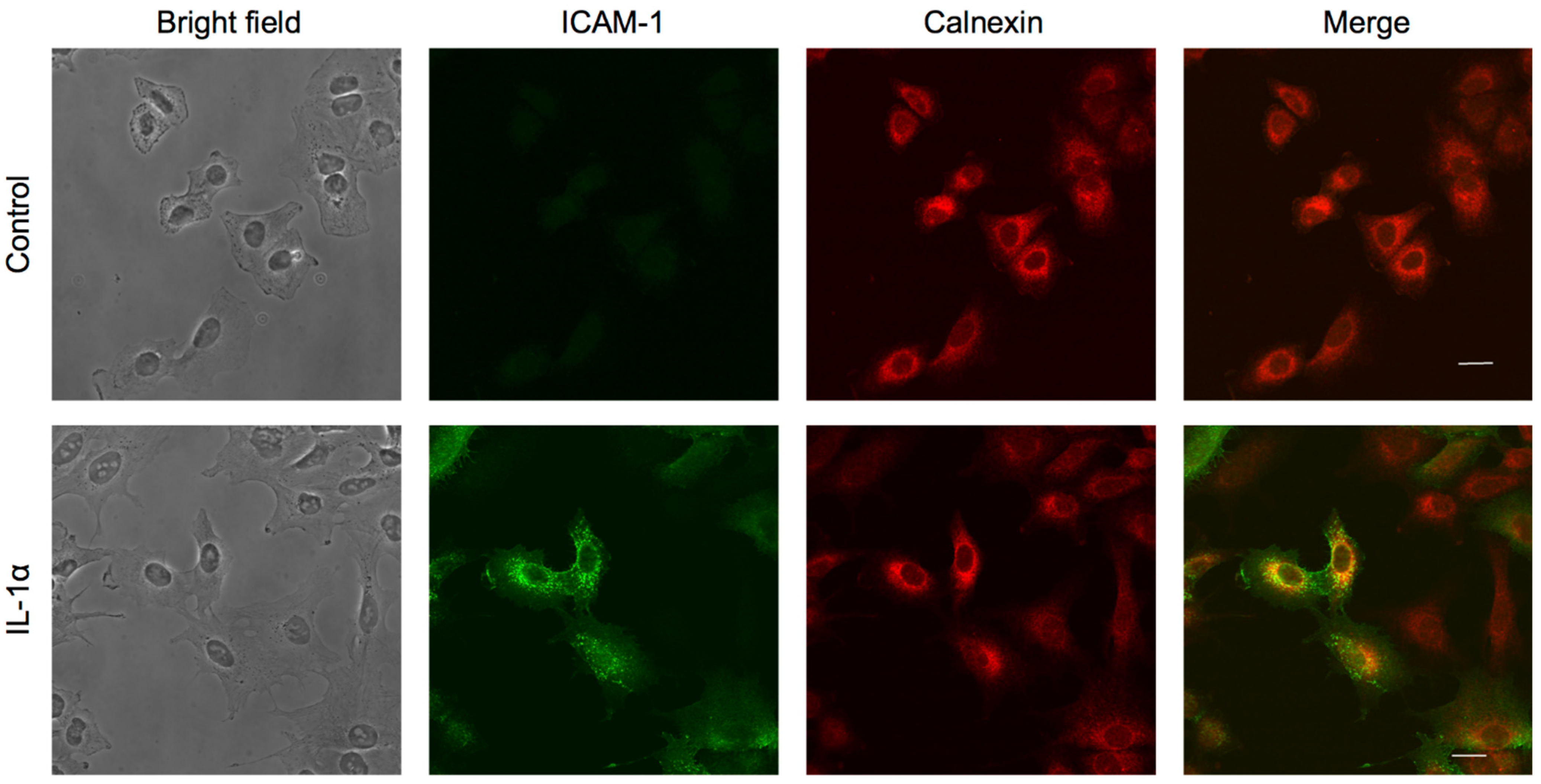
2.6. β-Boswellic Acid Induced the Localization of ICAM-1 at the Perinuclear Region
2.7. β-Boswellic Acid Interferes with ICAM-1 Glycosylation and Intracellular Transport in a Different Manner from Other Ursane-Type Pentacyclic Triterpenoids
3. Materials and Methods
3.1. Cell Culture
3.2. Reagents
3.3. Antibodies
3.4. Cell Viability Assay
3.5. Cell-ELISA
3.6. Western Blotting
3.7. α-Glucosidase Assay
3.8. Glycosidase Digestion
3.9. Immunostaining
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, T.; He, C. Pro-inflammatory cytokines: The link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018, 44, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Karin, M.; Greten, F.R. NF-κB: Linking inflammation and immunity to cancer development and progression. Nat. Rev. Immunol. 2005, 5, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Baud, V.; Karin, M. Is NF-κB a good target for cancer therapy? Hopes and pitfalls. Nat. Rev. Drug Discov. 2009, 8, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Roebuck, K.A.; Finnegan, A. Regulation of intercellular adhesion molecule-1 (CD54) gene expression. J. Leukoc. Biol. 1999, 66, 876–888. [Google Scholar] [CrossRef]
- Singh, M.; Thakur, M.; Mishra, M.; Yadav, M.; Vibhuti, R.; Menon, A.M.; Nagda, G.; Dwivedi, V.P.; Dakal, T.C.; Yadav, V. Gene regulation of intracellular adhesion molecule-1 (ICAM-1): A molecule with multiple functions. Immunol. Lett. 2021, 240, 123–136. [Google Scholar] [CrossRef]
- Ramos, T.N.; Bullard, D.C.; Barnum, S.R. ICAM-1: Isoforms and phenotypes. J. Immunol. 2014, 192, 4464–4474. [Google Scholar] [CrossRef]
- Bui, T.M.; Wiesolek, H.L.; Sumagin, R. ICAM-1: A master regulator of cellular responses in inflammation, injury resolution, and tumorigenesis. J. Leukoc. Biol. 2020, 108, 787–799. [Google Scholar] [CrossRef]
- Gerhardt, T.; Ley, K. Monocyte trafficking across the vessel wall. Cardiovasc. Res. 2015, 107, 321–330. [Google Scholar] [CrossRef]
- Vestweber, D. How leukocytes cross the vascular endothelium. Nat. Rev. Immunol. 2015, 15, 692–704. [Google Scholar] [CrossRef]
- He, P.; Srikrishna, G.; Freeze, H.H. N-glycosylation deficiency reduces ICAM-1 induction and impairs inflammatory response. Glycobiology 2014, 24, 392–398. [Google Scholar] [CrossRef]
- Mitsuda, S.; Yokomichi, T.; Yokoigawa, J.; Kataoka, T. Ursolic acid, a natural pentacyclic triterpenoid, inhibits intracellular trafficking of proteins and induces accumulation of intercellular adhesion molecule-1 linked to high-mannose-type glycans in the endoplasmic reticulum. FEBS Open Bio 2014, 4, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.W.; Dunn, T.S.; Ballestas, M.E.; Litovsky, S.H.; Patel, R.P. Identification of a high-mannose ICAM-1 glycoform: Effects of ICAM-1 hypoglycosylation on monocyte adhesion and outside in signaling. Am. J. Physiol. Cell Physiol. 2013, 305, C228–C237. [Google Scholar] [CrossRef] [PubMed]
- Regal-McDonald, K.; Xu, B.; Barnes, J.W.; Patel, R.P. High-mannose intercellular adhesion molecule-1 enhances CD16+ monocyte adhesion to the endothelium. Am. J. Physiol. Heart Circ. Physiol. 2019, 317, H1028–H1038. [Google Scholar] [CrossRef] [PubMed]
- Laszczyk, M. Pentacyclic triterpenes of the lupane, oleanane and ursane group as tools in cancer therapy. Planta Med. 2009, 75, 1549–1560. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.R.; Prasad, S.; Sung, B.; Kannappan, K.; Aggarwal, B.B. Targeting inflammatory pathways by triterpenoids for prevention and treatment of cancer. Toxins 2010, 2, 2428–2466. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Nguyen, A.H.; Kumar, A.P.; Tan, B.K.H.; Sethi, G. Targeted inhibition of tumor proliferation, survival, and metastasis by pentacyclic triterpenoids: Potential role in prevention and therapy of cancer. Cancer Lett. 2012, 320, 158–170. [Google Scholar] [CrossRef]
- Hiramatsu, R.; Fukuhara, S.; Mitsuda, S.; Yokomichi, T.; Kataoka, T. Betulinic acid and oleanolic acid, natural pentacyclic triterpenoids, interfere with N-linked glycan modifications to intercellular adhesion molecule-1, but not its intracellular transport to the cell surface. Eur. J. Pharmacol. 2015, 767, 126–134. [Google Scholar] [CrossRef]
- Baba, K.; Hiramatsu, R.; Suradej, B.; Tanigaki, R.; Koeda, S.; Waku, T.; Kataoka, T. Asiatic acid, corosolic acid, and maslinic acid interfere with intracellular trafficking and N-linked glycosylation of intercellular adhesion molecule-1. Biol. Pharm. Bull. 2018, 41, 1757–1768. [Google Scholar] [CrossRef]
- Malik, A.; Kanneganti, T.D. Function and regulation of IL-1α in inflammatory diseases and cancer. Immunol. Rev. 2018, 281, 124–137. [Google Scholar] [CrossRef]
- Cavalli, G.; Colafrancesco, S.; Emmi, G.; Imazio, M.; Lopalco, G.; Maggio, M.C.; Sota, J.; Dinarello, C.A. Interleukin 1α: A comprehensive review on the role of IL-1α in the pathogenesis and treatment of autoimmune and inflammatory diseases. Autoimmun. Rev. 2021, 20, 102763. [Google Scholar] [CrossRef]
- Lv, M.; Shao, S.; Zhang, Q.; Zhuang, X.; Qiao, T. Acetyl-11-keto-β-boswellic acid exerts the anti-cancer effects via cell cycle arrest, apoptosis induction and autophagy suppression in non-small cell lung cancer cells. OncoTargets Ther. 2020, 13, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; He, Q.; Xu, Y.; Lou, H.; Fan, P. Synthesis of 3-O-acetyl-11-keto-β-boswellic acid (AKBA)-derived amides and their mitochondria-targeted antitumor activities. ACS Omega 2022, 7, 9853–9866. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Block, T.M.; Guo, J.T. Antiviral therapies targeting host ER alpha-glucosidases: Current status and future directions. Antivir. Res. 2013, 99, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lu, Y.; Su, X.; Li, F.; She, Z.; He, X.; Li, Y. A norsesquiterpene lactone and a benzoic acid derivative from the leaves of Cyclocarya paliurus and their glucosidase and glycogen phosphorylase inhibiting activities. Planta Med. 2008, 74, 287–289. [Google Scholar] [CrossRef] [PubMed]
- Rehman, N.U.; Khan, A.; Al-Harrasi, A.; Hussain, H.; Wadood, A.; Riaz, M.; Al-Abri, Z. New α-glucosidase inhibitors from the resins of Boswellia species with structure-glucosidase activity and molecular docking studies. Bioorg. Chem. 2018, 79, 27–33. [Google Scholar] [CrossRef]
- Hou, W.; Li, Y.; Zhang, Q.; Wei, X.; Peng, A.; Cheng, L.; Wei, Y. Triterpene acids isolated from Lagerstroemia speciosa leaves as α-glucosidase inhibitors. Phytother. Res. 2009, 23, 614–618. [Google Scholar] [CrossRef]
- Zhang, B.-W.; Xing, Y.; Wen, C.; Yu, X.-X.; Sun, W.-L.; Xiu, Z.-L.; Dong, Y.-S. Pentacyclic triterpenes as α-glucosidase α-amylase inhibitors: Structure-activity relationships and the synergism with acarbose. Bioorg. Med. Chem. Lett. 2017, 27, 5065–5070. [Google Scholar] [CrossRef]
- Du, H.; Li, H.; Wu, P.; Wang, C.; Xue, J.; Wei, X. α-Glucosidase inhibitory pentacyclic triterpenoids from the leaves of Cleistocalyx consperipunctatus. Phytochem. Lett. 2021, 4, 109–113. [Google Scholar] [CrossRef]
- Morocho, V.; Valle, A.; García, J.; Gilardoni, G.; Cartuche, L.; Suárez, A.I. α-Glucosidase inhibition and antibacterial activity of secondary metabolites from the Ecuadorian species Clinopodium taxifolium (Kunth) govaerts. Molecules 2018, 23, 146. [Google Scholar] [CrossRef]
- Nyemb, J.N.; Tchuenguem, R.T.; Venditti, A.; Tchinda, A.T.; Henoumont, C.; Talla, E.; Laurent, S.; Iqbal, J. Antimicrobial and α-glucosidase inhibitory activities of chemical constituents from Gardenia aqualla (Rubiacease). Nat. Prod. Res. 2022. [Google Scholar] [CrossRef]
- Roy, N.K.; Parama, D.; Banik, K.; Bordoloi, D.; Devi, A.K.; Thakur, K.K.; Padmavathi, G.; Shakibaei, M.; Fan, L.; Sethi, G.; et al. An update on pharmacological potential of boswellic acids against chronic diseases. Int. J. Mol. Sci. 2019, 20, 4101. [Google Scholar] [CrossRef] [PubMed]
- Sharma, T.; Jana, S. Boswellic acids as natural anticancer medicine: Precious gift to humankind. J. Herb. Med. 2020, 20, 100313. [Google Scholar] [CrossRef]
- Siddiqui, A.; Shah, Z.; Jahan, R.N.; Othman, I.; Kumari, Y. Mechanistic role of boswellic acids in Alzheimer’s disease: Emphasis on anti-inflammatory properties. Biomed. Pharmacother. 2021, 144, 112250. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakano, K.; Sasaki, S.; Kataoka, T. Bioactive Evaluation of Ursane-Type Pentacyclic Triterpenoids: β-Boswellic Acid Interferes with the Glycosylation and Transport of Intercellular Adhesion Molecule-1 in Human Lung Adenocarcinoma A549 Cells. Molecules 2022, 27, 3073. https://doi.org/10.3390/molecules27103073
Nakano K, Sasaki S, Kataoka T. Bioactive Evaluation of Ursane-Type Pentacyclic Triterpenoids: β-Boswellic Acid Interferes with the Glycosylation and Transport of Intercellular Adhesion Molecule-1 in Human Lung Adenocarcinoma A549 Cells. Molecules. 2022; 27(10):3073. https://doi.org/10.3390/molecules27103073
Chicago/Turabian StyleNakano, Kaori, Saki Sasaki, and Takao Kataoka. 2022. "Bioactive Evaluation of Ursane-Type Pentacyclic Triterpenoids: β-Boswellic Acid Interferes with the Glycosylation and Transport of Intercellular Adhesion Molecule-1 in Human Lung Adenocarcinoma A549 Cells" Molecules 27, no. 10: 3073. https://doi.org/10.3390/molecules27103073
APA StyleNakano, K., Sasaki, S., & Kataoka, T. (2022). Bioactive Evaluation of Ursane-Type Pentacyclic Triterpenoids: β-Boswellic Acid Interferes with the Glycosylation and Transport of Intercellular Adhesion Molecule-1 in Human Lung Adenocarcinoma A549 Cells. Molecules, 27(10), 3073. https://doi.org/10.3390/molecules27103073





