Low-Cost Potentiometric Sensor for Chloride Measurement in Continuous Industrial Process Control
Abstract
:1. Introduction
2. Materials and Methods
3. Results
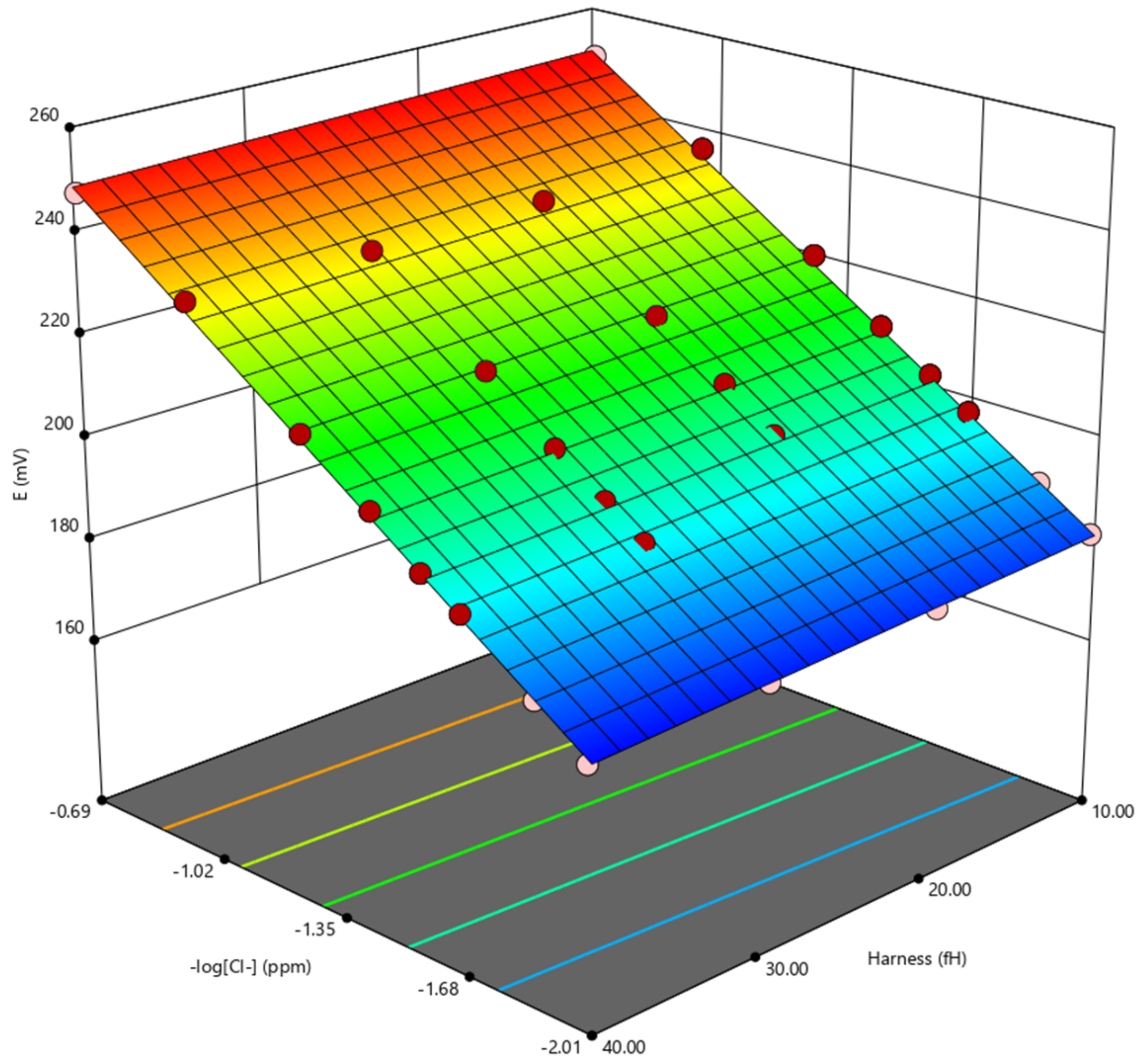
3.1. Study of the Sensor’s OCP Response in Solutions with Different Chloride Content and Water Hardness
3.2. OCP Signal’s Linearity in Solutions with Different Hardness Levels and Chloride Content
3.3. Reproducibility of the Chloride Sensor’s OCP Response
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sophocleous, M.; Atkinson, J.K. A Review of Screen-Printed Silver/Silver Chloride (Ag/AgCl) Reference Electrodes Potentially Suitable for Environmental Potentiometric Sensors. Sens. Actuators A Phys. 2017, 267, 106–120. [Google Scholar] [CrossRef] [Green Version]
- Soleimani, M.; Sophocleous, M.; Glanc, M.; Atkinson, J.; Wang, L.; Wood, R.J.K.; Taylor, R.I. Engine Oil Acidity Detection Using Solid State Ion Selective Electrodes. Tribol. Int. 2013, 65, 48–56. [Google Scholar] [CrossRef] [Green Version]
- Cranny, A.; Harris, N.; White, N. Screen Printed Potentiometric Chloride Sensors. Procedia Eng. 2014, 87, 220–223. [Google Scholar] [CrossRef] [Green Version]
- Cranny, A.; Harris, N.; Lewis, A.; Nie, M.; Wharton, J.; Wood, R.; Stokes, K. Screen-Printed Potentiometric Ag/AgCl Chloride Sensors: Lifetime Performance and Their Use in Soil Salt Measurements. Sens. Actuators A Phys. 2011, 169, 288–294. [Google Scholar] [CrossRef]
- Martínez-Máñez, R.; Soto, J.; García-Breijo, E.; Gil, L.; Ibáñez, J.; Gadea, E. A Multisensor in Thick-Film Technology for Water Quality Control. Sens. Actuators A Phys. 2005, 120, 589–595. [Google Scholar] [CrossRef]
- Ke, X. Micro-Fabricated Electrochemical Chloride Ion Sensors: From the Present to the Future. Talanta 2020, 211, 120734. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First Addendum, 4th ed.; World Health Organization: Geneva, Switzerland, 2017; ISBN 978-92-4-154995-0. [Google Scholar]
- Wharton, J.A.; Stokes, K.R. The Influence of Nickel-Aluminium Bronze Microstructure and Crevice Solution on the Initiation of Crevice Corrosion. Electrochim. Acta 2008, 53, 2463–2473. [Google Scholar] [CrossRef]
- White, S.P.; Weir, G.J.; Laycock, N.J. Calculating Chemical Concentrations during the Initiation of Crevice Corrosion. Corros. Sci. 2000, 42, 605–629. [Google Scholar] [CrossRef]
- Gandía-Romero, J.M.; Bataller, R.; Monzón, P.; Campos, I.; García-Breijo, E.; Valcuende, M.; Soto, J. Characterization of Embeddable Potentiometric Thick-Film Sensors for Monitoring Chloride Penetration in Concrete. Sens. Actuators B Chem. 2016, 222, 407–418. [Google Scholar] [CrossRef]
- Gregory, R. Galvanic Corrosion of Lead Solder in Copper Pipework. Water Environ. J. 1990, 4, 112–118. [Google Scholar] [CrossRef]
- Qian, Y.; Zhao, Y.; Wu, Q.l.; Yang, Y. Review of Salinity Measurement Technology Based on Optical Fiber Sensor. Sens. Actuators B Chem. 2018, 260, 86–105. [Google Scholar] [CrossRef]
- Ding, L.; Li, Z.; Ding, Q.; Shen, X.; Yuan, Y.; Huang, J. Microstructured Optical Fiber Based Chloride Ion Sensing Method for Concrete Health Monitoring. Sens. Actuators B Chem. 2018, 260, 763–769. [Google Scholar] [CrossRef]
- Wang, J.N. A Microfluidic Long-Period Fiber Grating Sensor Platform for Chloride Ion Concentration Measurement. Sensors 2011, 11, 8550–8568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laxmeshwar, L.S.; Jadhav, M.S.; Akki, J.F.; Raikar, P.; Kumar, J.; Prakash, O.; Mahakud, R.; Raikar, U.S. Quantification of Chloride and Iron in Sugar Factory Effluent Using Long Period Fiber Grating Chemical Sensor. Sens. Actuators B Chem. 2018, 258, 850–856. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; de Ávila, B.E.F.; Wang, J. Wearable Biosensors for Healthcare Monitoring. Nat. Biotechnol. 2019, 37, 389–406. [Google Scholar] [CrossRef]
- Xu, G.; Cheng, C.; Yuan, W.; Liu, Z.; Zhu, L.; Li, X.; Lu, Y.; Chen, Z.; Liu, J.; Cui, Z.; et al. Smartphone-Based Battery-Free and Flexible Electrochemical Patch for Calcium and Chloride Ions Detections in Biofluids. Sens. Actuators B Chem. 2019, 297, 126743. [Google Scholar] [CrossRef]
- Cuartero, M.; Crespo, G.A. All-Solid-State Potentiometric Sensors: A New Wave for in Situ Aquatic Research. Curr. Opin. Electrochem. 2018, 10, 98–106. [Google Scholar] [CrossRef]
- Trnkova, L.; Adam, V.; Hubalek, J.; Babula, P.; Kizek, R. Amperometric Sensor for Detection of Chloride Ions. Sensors 2008, 8, 5619–5636. [Google Scholar] [CrossRef]
- Thorn, P.; Mortensen, J. Simple Chloride Sensors for Continuous Groundwater Monitoring. Ground Water Monit. Remediat. 2012, 32, 40–47. [Google Scholar] [CrossRef]
- Bakker, E.; Diamond, D.; Lewenstam, A.; Pretsch, E. Ion Sensors: Current Limits and New Trends. Anal. Chim. Acta 1999, 393, 11–18. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, J.; Yang, Y.; Deng, H. Fabrication and Performance of All-Solid-State Chloride Sensors in Synthetic Concrete Pore Solutions. Sensors 2010, 10, 10226–10239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, V.K.; Goyal, R.N.; Sharma, R.A. Chloride Selective Potentiometric Sensor Based on a Newly Synthesized Hydrogen Bonding Anion Receptor. Electrochim. Acta 2009, 54, 4216–4222. [Google Scholar] [CrossRef]
- Mahajan, R.K.; Kaur, R.; Tabassum, S.; Arjmand, F.; Mathur, S. Cu(II) Complexes as Receptor Molecules for Development of New Chloride Sensors. Electrochim. Acta 2006, 52, 408–414. [Google Scholar] [CrossRef]
- Da Silva, I.S.; Richter, E.M.; do Lago, C.L.; Gutz, I.G.R.; Tanaka, A.A.; Angnes, L. FIA-Potentiometry in the Sub-Nernstian Response Region for Rapid and Direct Chloride Assays in Milk and in Coconut Water. Talanta 2005, 67, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Sjöberg-Eerola, P.; Nylund, J.; Bobacka, J.; Lewenstam, A.; Ivaska, A. Soluble Semiconducting Poly(3-Octylthiophene) as a Solid-Contact Material in All-Solid-State Chloride Sensors. Sens. Actuators B Chem. 2008, 134, 878–886. [Google Scholar] [CrossRef]
- Sjöberg-Eerola, P.; Bobacka, J.; Lewenstam, A.; Ivaska, A. All-Solid-State Chloride Sensors Based on Electronically Conducting, Semiconducting and Insulating Polymer Membranes. Sens. Actuators B Chem. 2007, 127, 545–553. [Google Scholar] [CrossRef]
- Cranny, A.; Harris, N.R.; White, N.M. Screen-Printable Porous Glass: A New Material for Electrochemical Sensors. J. Mater. Sci. Mater. Electron. 2015, 26, 4557–4564. [Google Scholar] [CrossRef]
- Xiao, K.P.; Bühlmann, P.; Nishizawa, S.; Amemiya, S.; Umezawa, Y. A Chloride Ion-Selective Solvent Polymeric Membrane Electrode Based on a Hydrogen Bond Forming Ionophore. Anal. Chem. 1997, 69, 1038–1044. [Google Scholar] [CrossRef]
- Hwang, K.; Noguchi, T.; Tomosawa, F. Prediction Model of Compressive Strength Development of Fly-Ash Concrete. Cem. Concr. Res. 2004, 34, 2269–2276. [Google Scholar] [CrossRef]
- Chou, J.C.; Tseng, T.W.; Liao, Y.H.; Lai, C.H.; Yan, S.J.; Wu, Y.X.; Wu, C.Y.; Lin, S.H. Analysis of Chloride Ion Sensor Modified by Graphene Oxide under Microfluid Flow. IEEE Sens. J. 2019, 19, 3217–3223. [Google Scholar] [CrossRef]
- Bratov, A.; Abramova, N.; Domínguez, C. Investigation of Chloride Sensitive ISFETs with Different Membrane Compositions Suitable for Medical Applications. Anal. Chim. Acta 2004, 514, 99–106. [Google Scholar] [CrossRef]
- Zielińska, R.; Mulik, E.; Michalska, A.; Achmatowicz, S.; Maj-Zurawska, M. All-Solid-State Planar Miniature Ion-Selective Chloride Electrode. Anal. Chim. Acta 2002, 451, 243–249. [Google Scholar] [CrossRef]
- Seguí Femenias, Y.; Angst, U.; Caruso, F.; Elsener, B. Ag/AgCl Ion-Selective Electrodes in Neutral and Alkaline Environments Containing Interfering Ions. Mater. Struct./Mater. Et Constr. 2016, 49, 2637–2651. [Google Scholar] [CrossRef] [Green Version]
- Pargar, F.; Koleva, D.A.; van Breugel, K. Determination of Chloride Content in Cementitious Materials: From Fundamental Aspects to Application of Ag/AgCl Chloride Sensors. Sensors 2017, 17, 2482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elakneswaran, Y.; Nawa, T.; Kurumisawa, K. Electrokinetic Potential of Hydrated Cement in Relation to Adsorption of Chlorides. Cem. Concr. Res. 2009, 39, 340–344. [Google Scholar] [CrossRef]
- Vonau, W.; Oelßner, W.; Guth, U.; Henze, J. An All-Solid-State Reference Electrode. Sens. Actuators B Chem. 2010, 144, 368–373. [Google Scholar] [CrossRef]
- Guth, U.; Gerlach, F.; Decker, M.; Oelßner, W.; Vonau, W. Solid-State Reference Electrodes for Potentiometric Sensors. J. Solid State Electrochem. 2009, 13, 27–39. [Google Scholar] [CrossRef]
- Hobby, P.C.; Moody, G.J.; Thomas, J.D.R. Calcium Ion-Selective Electrode Studies: Covalent Bonding of Organic Phosphates and Phosphonates to Polymer Matrices. Analyst 1983, 108, 581–590. [Google Scholar] [CrossRef]
- Bühlmann, P.; Pretsch, E.; Bakker, E. Carrier-Based Ion-Selective Electrodes and Bulk Optodes. 2. Ionophores for Potentiometric and Optical Sensors. Chem. Rev. 1998, 98, 1593–1687. [Google Scholar] [CrossRef]
- Sutter, J.; Radu, A.; Peper, S.; Bakker, E.; Pretsch, E. Solid-Contact Polymeric Membrane Electrodes with Detection Limits in the Subnanomolar Range. Anal. Chim. Acta 2004, 523, 53–59. [Google Scholar] [CrossRef]
- Parra, V.; Arrieta, Á.A.; Fernández-Escudero, J.A.; Rodríguez-Méndez, M.L.; de Saja, J.A. Electronic Tongue Based on Chemically Modified Electrodes and Voltammetry for the Detection of Adulterations in Wines. Sens. Actuators B Chem. 2006, 118, 448–453. [Google Scholar] [CrossRef]
- Noh, J.H.; Coetzee, P. Evaluation of the Potentiometric Determination of Trace Fluoride in Natural and Drinking Water with a Fluoride ISE. Water SA 2007, 33, 519–529. [Google Scholar]
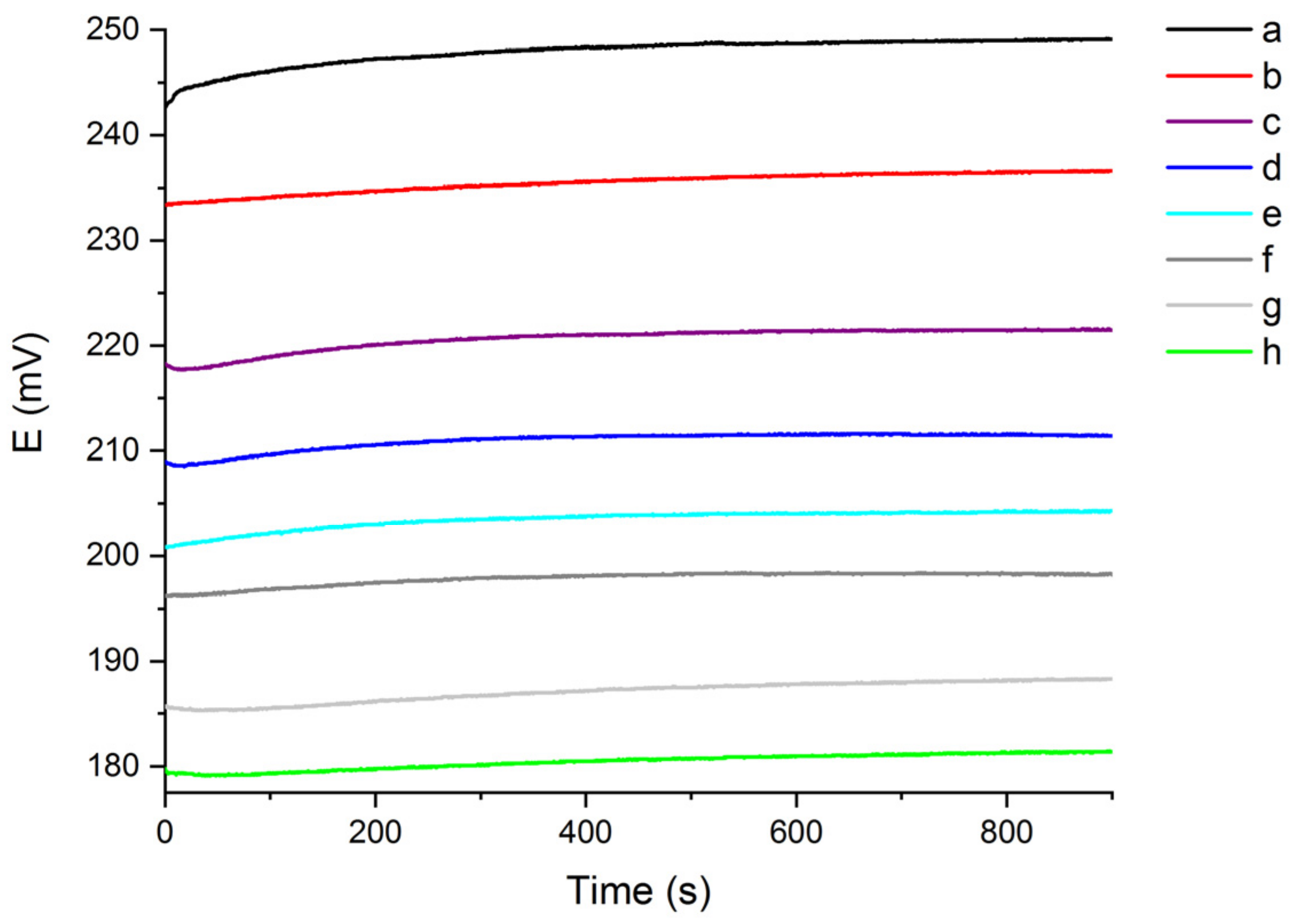
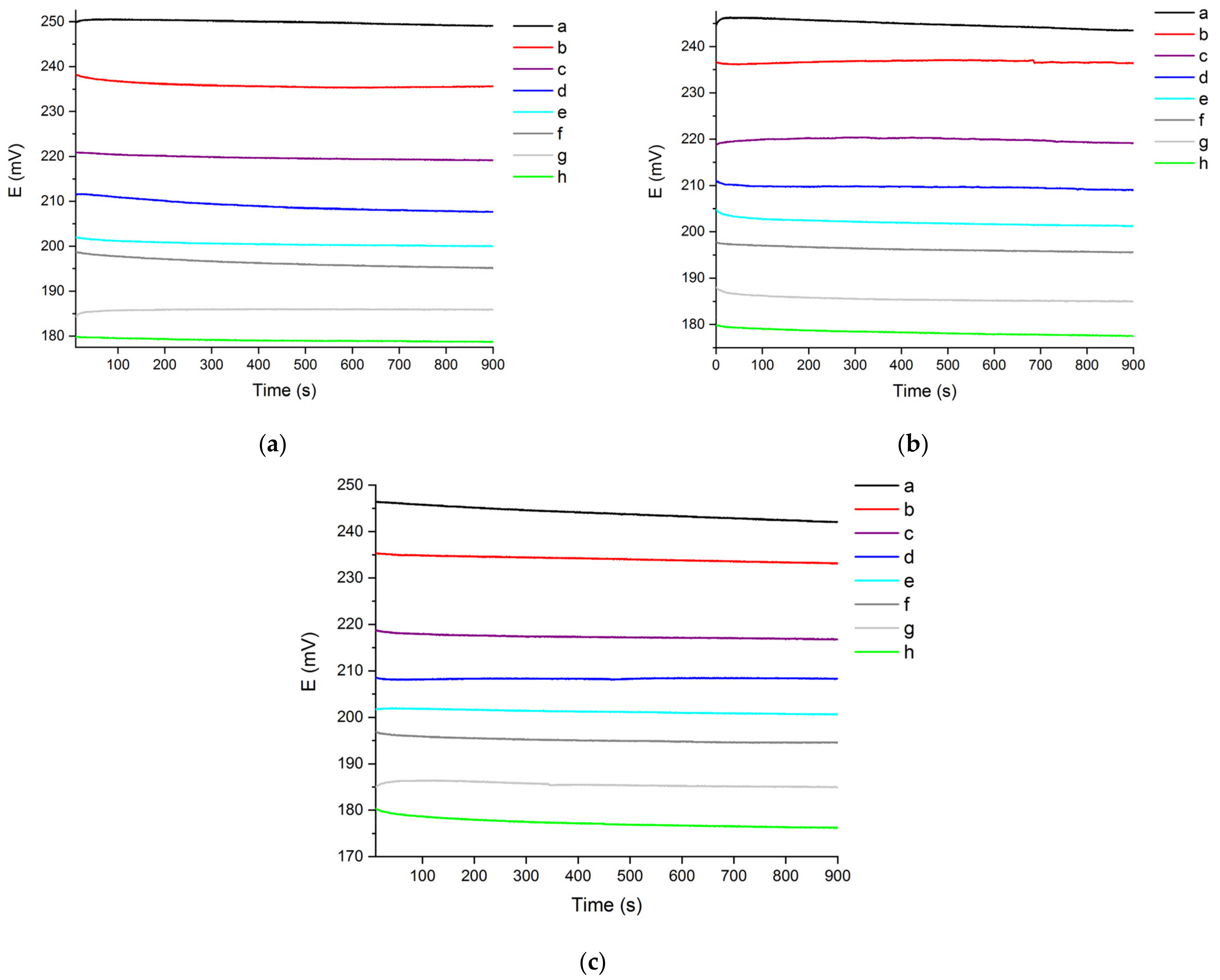
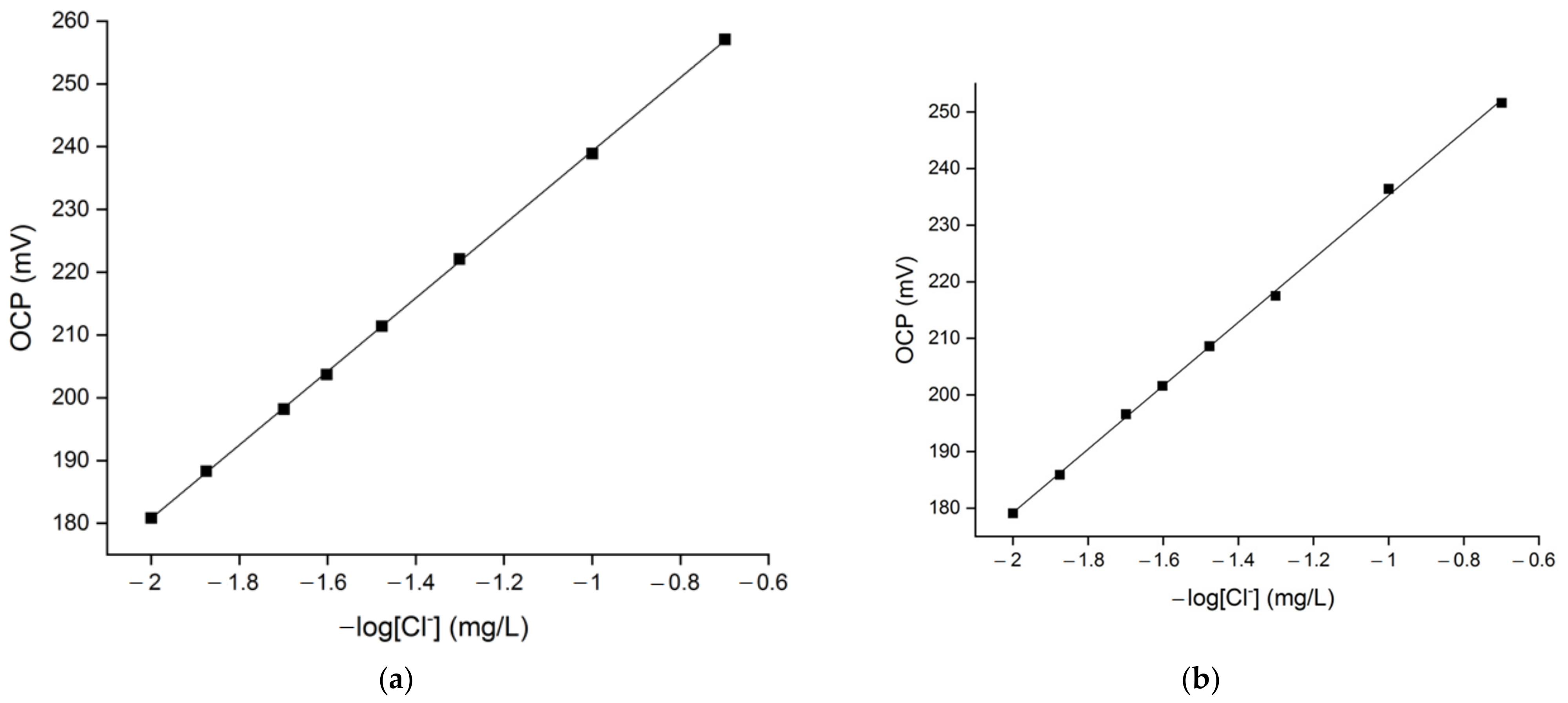
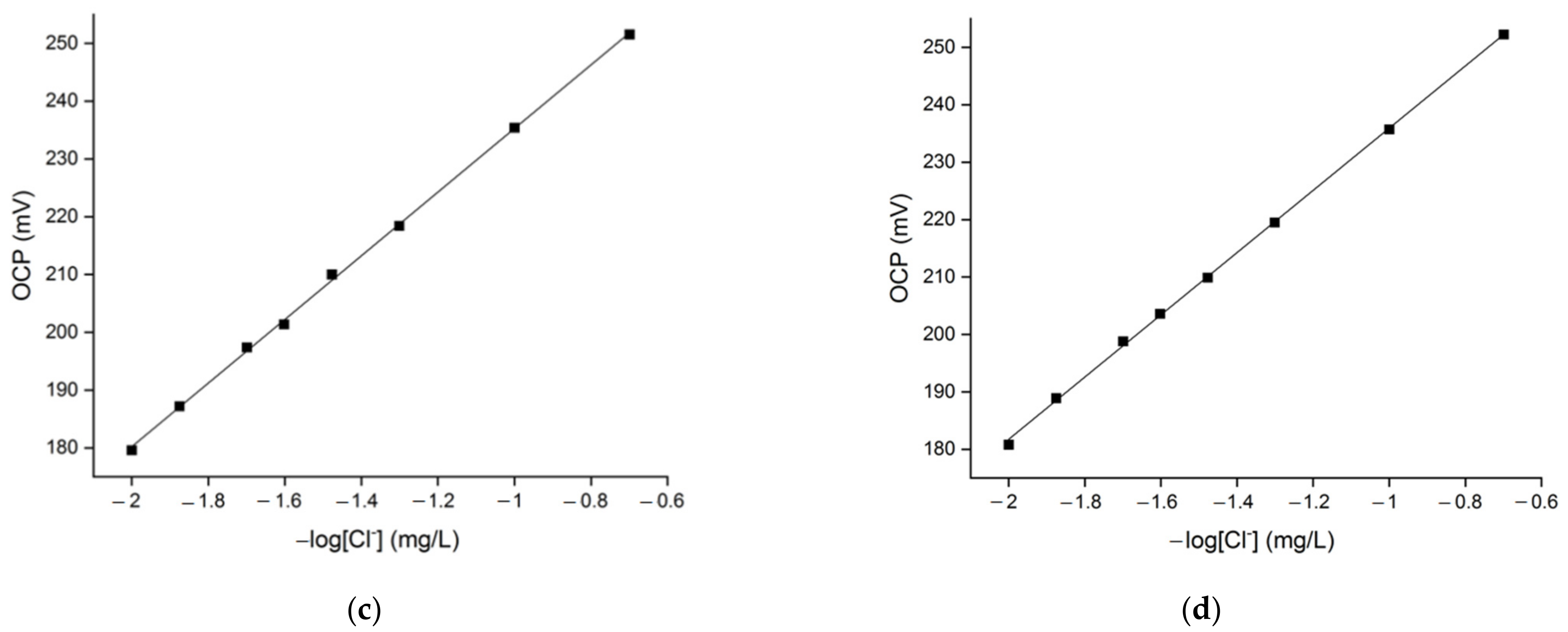
| Water Hardness (°fH) | Slope | Intercept | Coefficient of Determination (R2) |
|---|---|---|---|
| 10 | 58.5 | 297.8 | 0.9998 |
| 20 | 56.1 | 291.3 | 0.9992 |
| 30 | 55.1 | 290.3 | 0.9993 |
| 40 | 54.2 | 290.1 | 0.9995 |
| Water Hardness (°fH) | Chloride Content (mg/L) | OCP Arithmetical Average (mV) | Standard Deviation |
|---|---|---|---|
| 10 | 5 | 250.2 | 6.4 |
| 10 | 236.5 | 2.0 | |
| 20 | 220.7 | 1.6 | |
| 30 | 210.1 | 1.4 | |
| 40 | 203.1 | 1.1 | |
| 50 | 197.9 | 0.9 | |
| 75 | 188.1 | 1.3 | |
| 100 | 181.1 | 1.0 | |
| 20 | 5 | 247.2 | 2.6 |
| 10 | 234.6 | 1.8 | |
| 20 | 218.2 | 1.4 | |
| 30 | 208.9 | 1.4 | |
| 40 | 201.9 | 1.2 | |
| 50 | 196.2 | 1.6 | |
| 75 | 186.2 | 1.6 | |
| 100 | 178.8 | 1.6 | |
| 30 | 5 | 244.9 | 3.7 |
| 10 | 234.0 | 1.8 | |
| 20 | 217.7 | 1.9 | |
| 30 | 207.3 | 2.7 | |
| 40 | 200.6 | 2.2 | |
| 50 | 195.3 | 2.6 | |
| 75 | 184.3 | 2.3 | |
| 100 | 177.7 | 1.9 | |
| 40 | 5 | 247.9 | 2.2 |
| 10 | 234.0 | 1.3 | |
| 20 | 216.7 | 1.7 | |
| 30 | 207.1 | 1.2 | |
| 40 | 199.2 | 1.5 | |
| 50 | 194.5 | 1.3 | |
| 75 | 184.1 | 1.1 | |
| 100 | 176.5 | 1.1 |
| Chloride Content (mg/L) | Arithmetical Average of OCP Recorded in 10 °fH–40 °fH Solutions | Standard Deviation |
|---|---|---|
| 5 | 247.5 | 3.9 |
| 10 | 234.8 | 1.9 |
| 20 | 218.3 | 2.1 |
| 30 | 208.3 | 2.2 |
| 40 | 201.2 | 1.9 |
| 50 | 196.0 | 2.1 |
| 75 | 185.7 | 2.3 |
| 100 | 178.5 | 2.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vizza, M.; Marcantelli, P.; Giovani, C.; Giurlani, W.; Giusti, P.; Fontanesi, C.; Innocenti, M. Low-Cost Potentiometric Sensor for Chloride Measurement in Continuous Industrial Process Control. Molecules 2022, 27, 3087. https://doi.org/10.3390/molecules27103087
Vizza M, Marcantelli P, Giovani C, Giurlani W, Giusti P, Fontanesi C, Innocenti M. Low-Cost Potentiometric Sensor for Chloride Measurement in Continuous Industrial Process Control. Molecules. 2022; 27(10):3087. https://doi.org/10.3390/molecules27103087
Chicago/Turabian StyleVizza, Martina, Patrick Marcantelli, Claudia Giovani, Walter Giurlani, Paolo Giusti, Claudio Fontanesi, and Massimo Innocenti. 2022. "Low-Cost Potentiometric Sensor for Chloride Measurement in Continuous Industrial Process Control" Molecules 27, no. 10: 3087. https://doi.org/10.3390/molecules27103087







