Triallyl Isocyanurate as an Efficient Electrolyte Additive for Layered Oxide Cathode Material-Based Lithium-Ion Batteries with Improved Stability under High-Voltage
Abstract
:1. Introduction
2. Experimental Details
2.1. Preparation of the Electrodes
2.2. Electrochemical Measurements
2.3. Characterization Methods
2.4. Computational Simulation
3. Results and Discussions
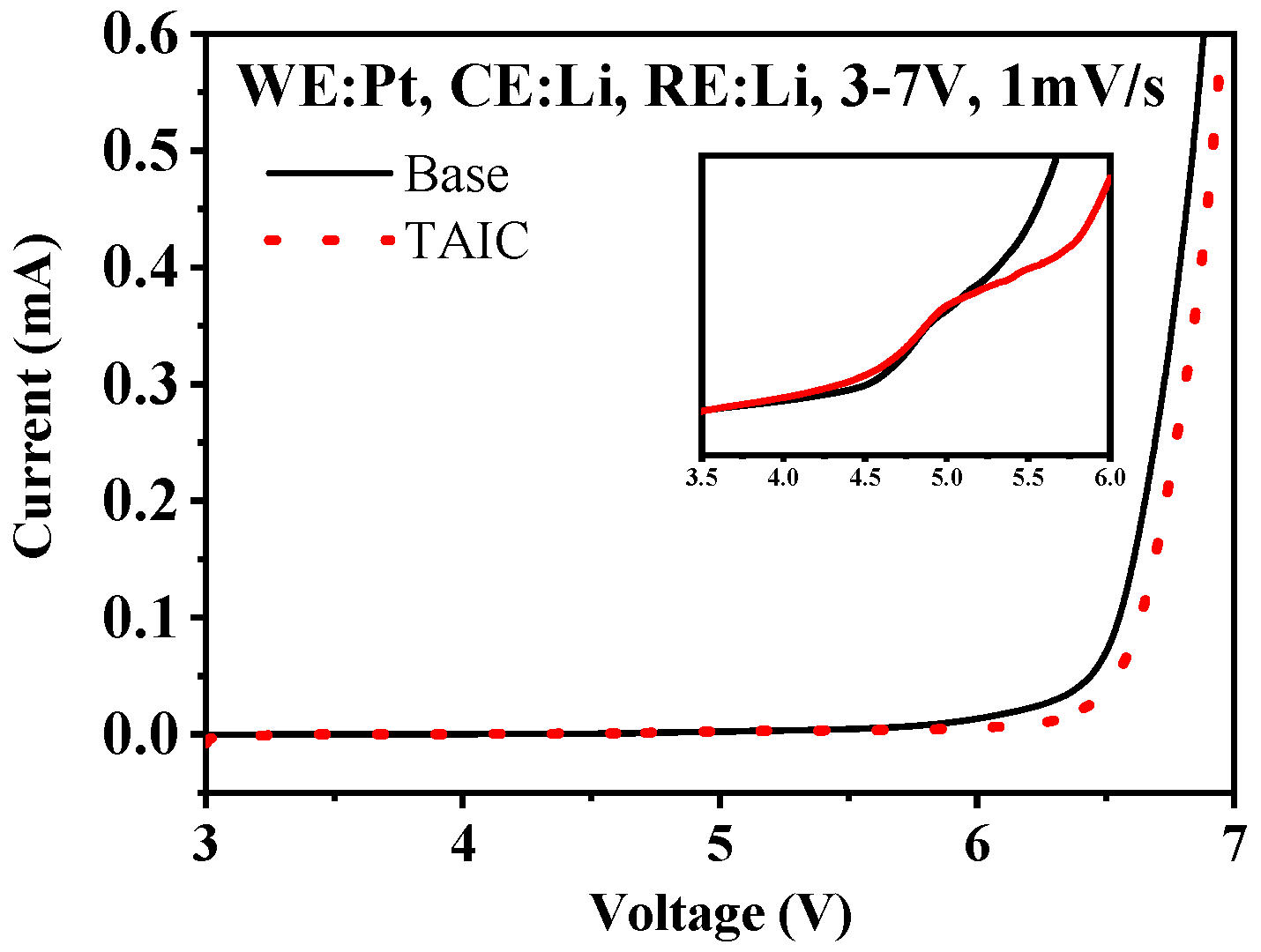
3.1. Oxidation and Reduction Performance
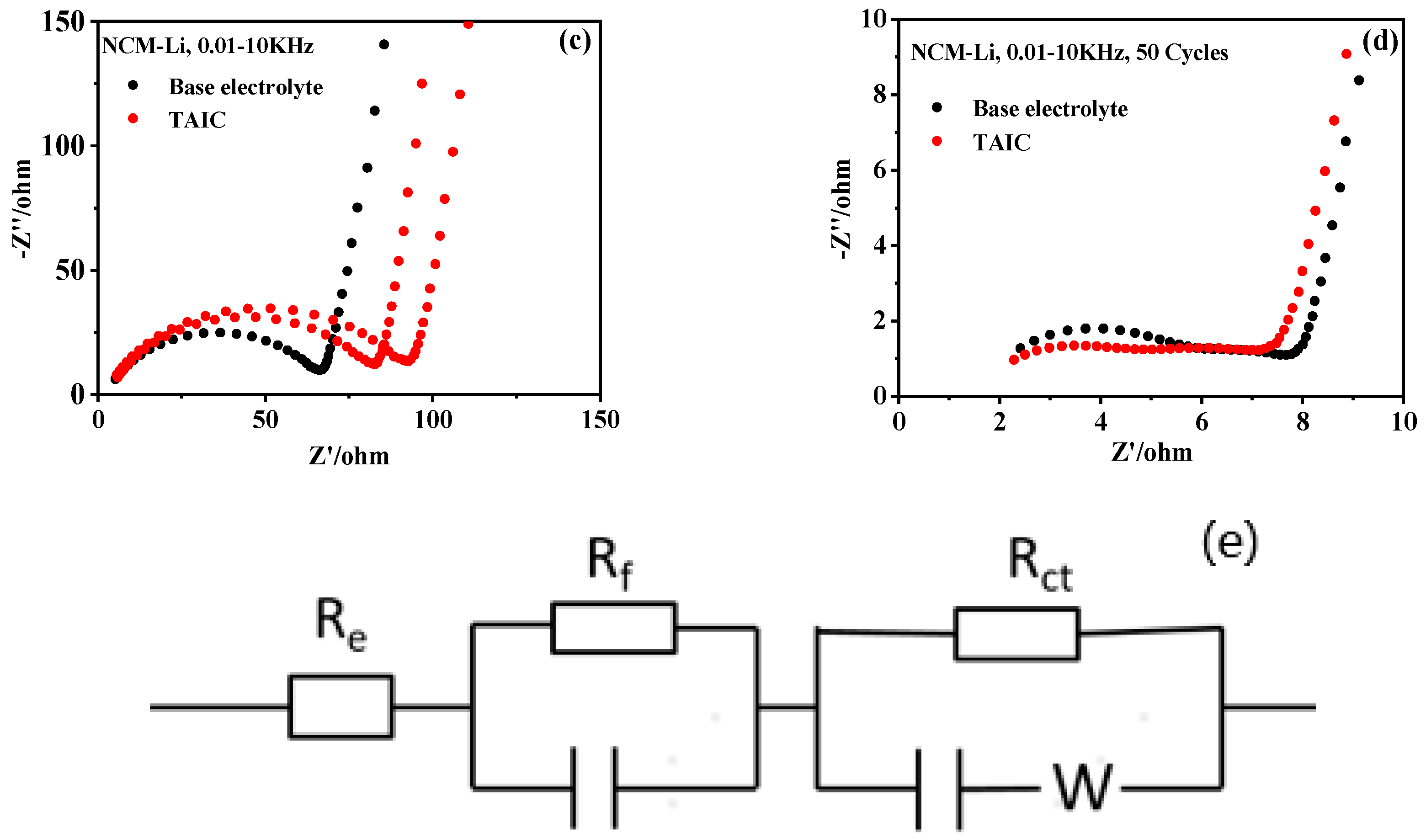
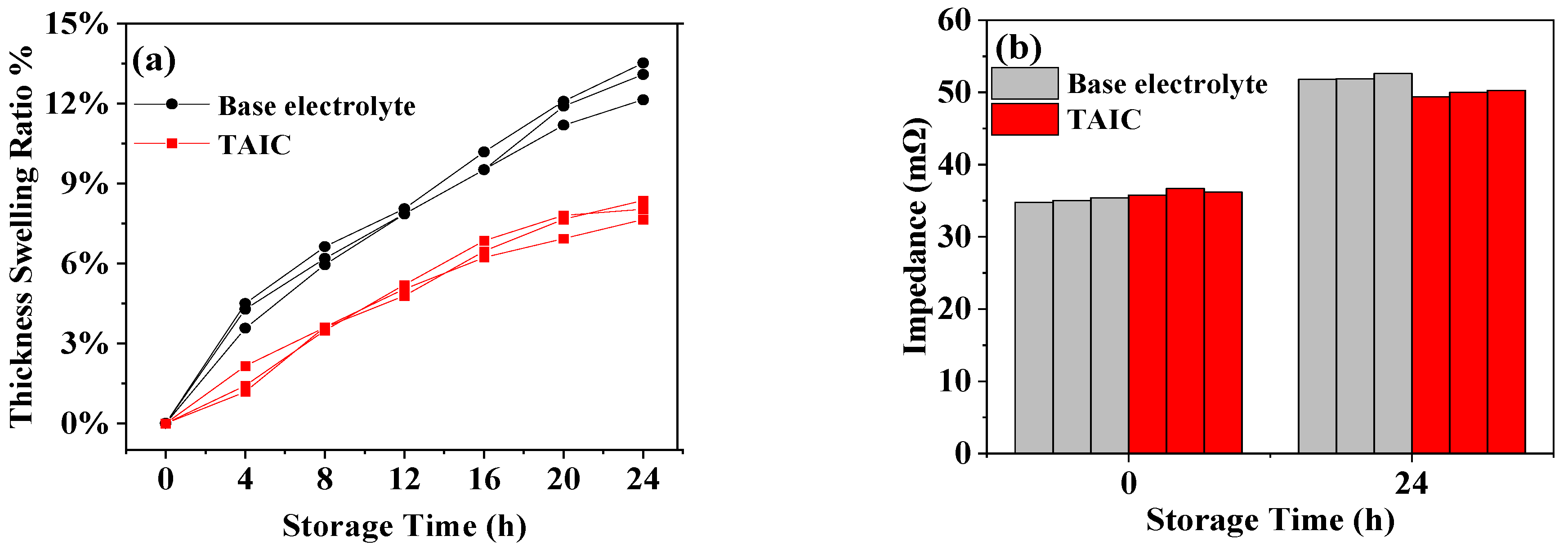
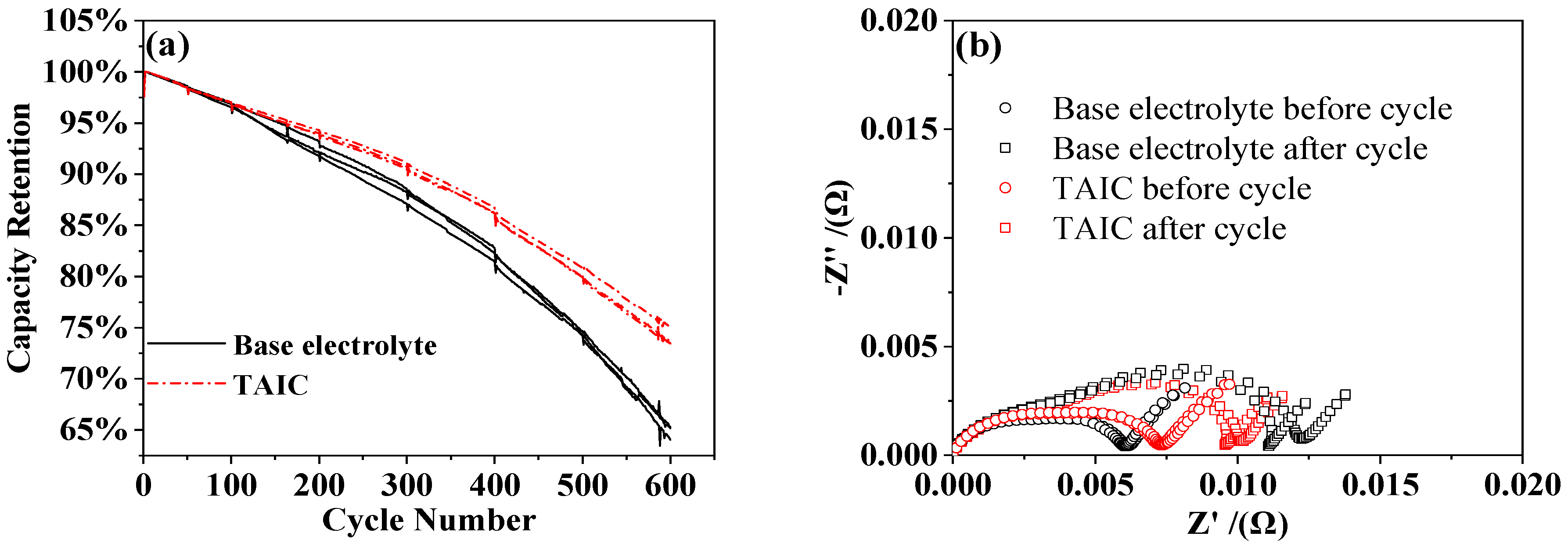
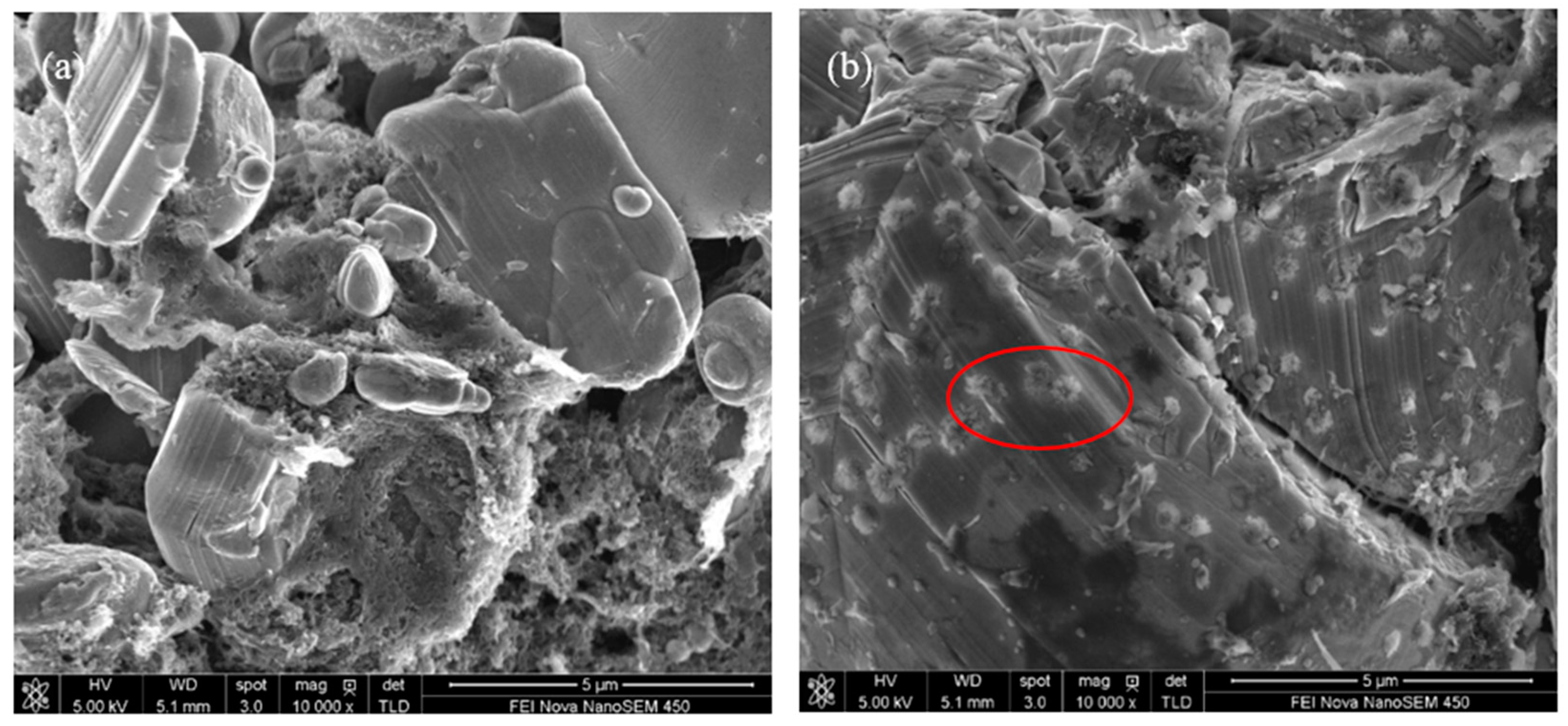
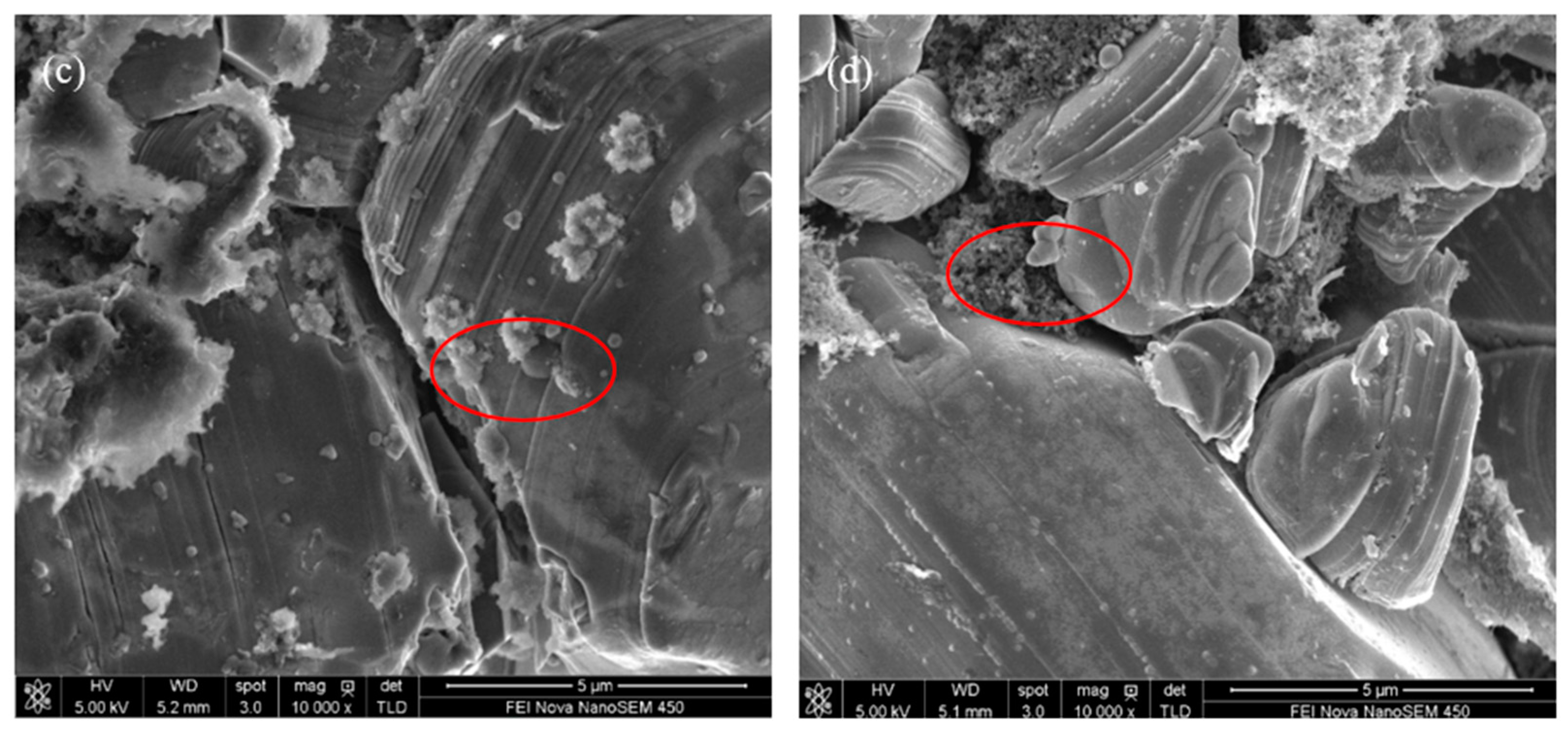
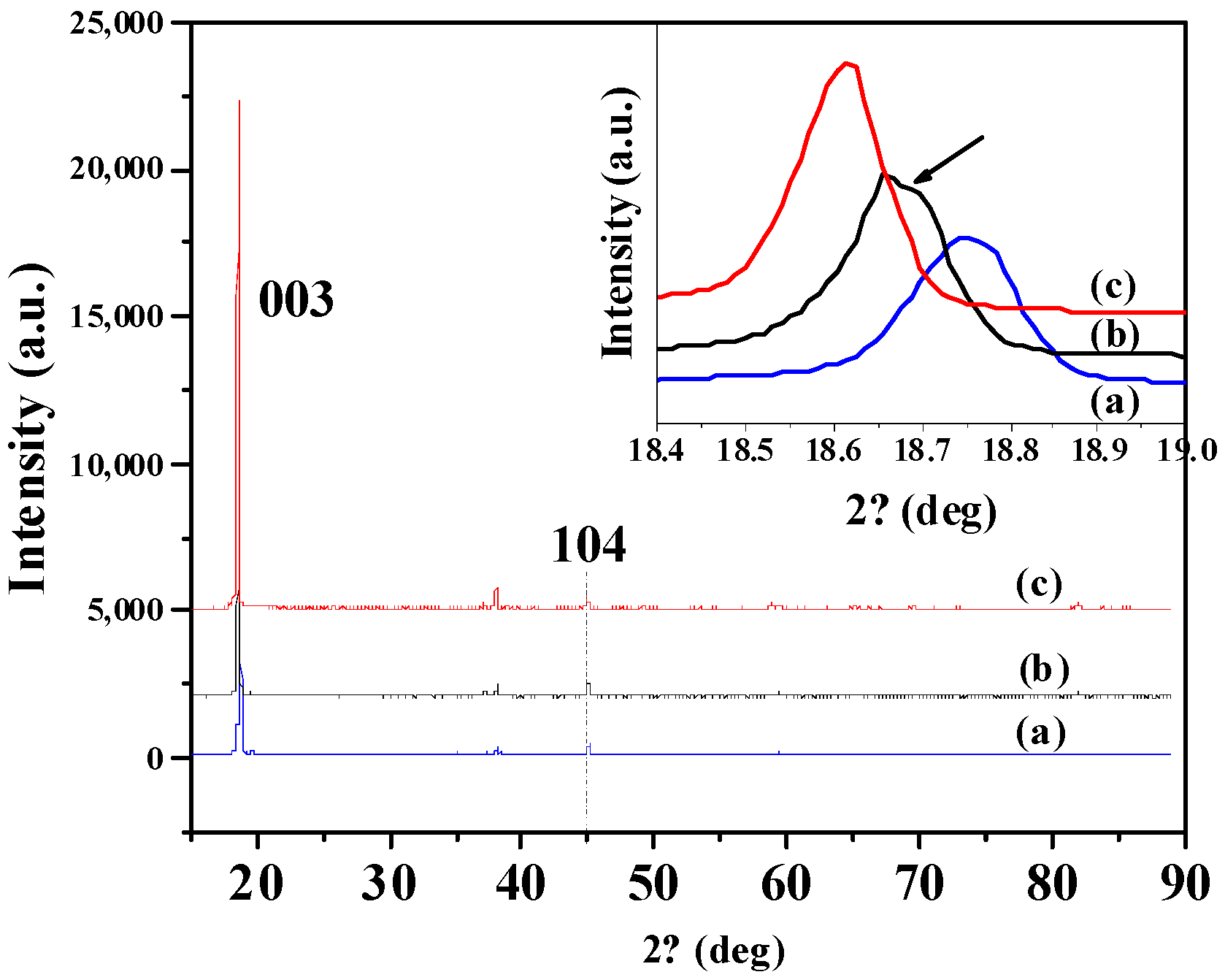
3.2. Influence of Additive TAIC on Electrochemical Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Megahed, S.; Scrosati, B. Lithium-ion rechargeable batteries. J. Power Sources 1994, 51, 79–104. [Google Scholar] [CrossRef]
- Ping, H.; Yu, H.; Li, D.; Zhou, H. Layered lithium transition metal oxide cathodes towards high energy lithium-ion batteries. J. Mater. Chem. 2012, 22, 3680–3695. [Google Scholar]
- Marom, R.; Amalraj, S.F.; Leifer, N.; Jacob, D.; Aurbach, D. A review of advanced and practical lithium battery materials. J. Mater. Chem. 2011, 21, 9938–9954. [Google Scholar] [CrossRef]
- Liang, L.W.; Sun, X.; Wu, C.; Hou, L.R.; Sun, J.F.; Zhang, X.G.; Yuan, C.Z. Nasicon-type surface functional modification in core shell LiNi0.5Mno3Co0.2O2@NaTi2(PO4)3 cathode enhances its high-voltage cycling stability and rate capacity toward Li-ion batteries. ACS Appl. Mater. Interfaces 2018, 10, 5498–5510. [Google Scholar] [CrossRef]
- Chen, J.N.; Peng, H.; Wang, X.P.; Shao, F.; Yuan, Z.D.; Han, H.Y. Graphene oxide exhibits broad-spectrum antimicrobial activity against bacterial phytopathogens and fungal conidia by intertwining and membrane perturbation. Nanoscale 2014, 6, 1879–1889. [Google Scholar] [CrossRef]
- Zuo, X.; Fan, C.; Liu, J.; Xin, X.; Wu, J.; Nan, J. Effect of tris(trimethylsilyl)borate on the high voltage capacity retention of LiNi0.5Co0.2Mn0.3O2/graphite cells. J. Power Sources 2013, 229, 308–312. [Google Scholar] [CrossRef]
- Xing, L.D.; Li, W.S.; Wang, C.Y.; Gu, F.L.; Xu, M.Q.; Tan, C.L.; Yi, J. Theoretical investigations on oxidative stability of solvents and oxidative decomposition mechanism of ethylene carbonate for lithium ion battery use. J. Phys. Chem. B 2009, 113, 16596–16602. [Google Scholar] [CrossRef]
- Xu, K.; Ding, S.P.; Jow, T.R. Toward reliable values of electrochemical stability limits for electrolytes. J. Electrochem. Soc. 1999, 146, 4172–4178. [Google Scholar] [CrossRef]
- Santhanam, R.; Rambabu, B. Research progress in high voltage spinel LiNi0.5Mn1.5O4 material. J. Power Sources 2010, 195, 5442–5451. [Google Scholar] [CrossRef]
- Wang, Y.T.; Xing, L.D.; Li, W.S.; Bedrov, D. Why do sulfone-based electrolytes show stability at high voltages? Insight from density functional theory. J. Phys. Chem. Lett. 2013, 4, 3992–3999. [Google Scholar] [CrossRef]
- Pei, A.; Zheng, G.Y.; Shi, F.F.; Li, Y.Z.; Cui, Y. Nanoscale nucleation and growth of electrodeposited lithium metal. Nano Lett. 2017, 17, 1132–1139. [Google Scholar] [CrossRef] [PubMed]
- Jiao, S.H.; Ren, X.D.; Cao, R.G.; Engelhard, M.H.; Liu, Y.Z.; Hu, D.H.; Mei, D.H.; Zheng, J.M.; Zhao, W.G.; Li, Q.Y.; et al. Stable cycling of high-voltage lithium metal batteries in ether electrolytes. Nat. Energy 2018, 3, 739–746. [Google Scholar] [CrossRef]
- Yoshida, K.; Nakamura, M.; Kazue, Y.; Tachikawa, N.; Tsuzuki, S.; Seki, S.; Dokko, K.; Watanabe, M. Oxidative-stability enhancement and charge transport mechanism in glyme-lithium salt equimolar complexes. J. Am. Chem. Soc. 2011, 133, 13121–13129. [Google Scholar] [CrossRef] [PubMed]
- Amanchukwu, C.V.; Yu, Z.; Kong, X.; Qin, J.; Cui, Y.; Bao, Z.N. A new class of ionically conducting fluorinated ether electrolytes with high electrochemical stability. J. Am. Chem. Soc. 2020, 142, 7393–7403. [Google Scholar] [CrossRef]
- Xie, H.; Liao, Y.; Sun, P.; Chen, T.; Rao, M.; Li, W. Investigation on polyethylene-supported and nano-SiO2 doped poly(methyl methacrylate-co-butyl acrylate) based gel polymer electrolyte for high voltage lithium ion battery. Electrochim. Acta 2014, 127, 327–333. [Google Scholar] [CrossRef]
- Guerfi, A.; Dontigny, M.; Charest, P.; Petitclerc, M.; Lagace, M.; Vijh, A.; Zaghib, K. Improved electrolytes for Li-ion batteries: Mixtures of ionic liquid and organic electrolyte with enhanced safety and electrochemical performance. J. Power Sources 2010, 195, 845–852. [Google Scholar] [CrossRef]
- Shkrob, I.A.; Han, B.H.; Sahore, R.; Tornheim, A.P.; Zhang, L.; Abraham, D.P.; Dogan, F.; Zhang, Z.C.; Liao, C. Facile in situ syntheses of cathode protective electrolyte additives for high energy density Li-ion cells. Chem. Mater. 2019, 31, 2459–2468. [Google Scholar] [CrossRef]
- Kang, Y.S.; Yoon, T.; Lee, S.S.; Mun, J.; Oh, S.M. 1,3,5-Trihydroxybenzene as a film-forming additive for high-voltage positive electrode. Electrochem. Commun. 2013, 27, 26–28. [Google Scholar] [CrossRef]
- Lan, J.L.; Zheng, Q.F.; Zhou, H.B.; Li, J.H.; Xing, L.D.; Xu, K.; Fan, W.Z.; Yu, L.; Li, W.S. Stabilizing a high-voltage lithium-rich layered oxide cathode with a novel electrolyte additive. ACS Appl. Mater. Interfaces 2019, 11, 28841–28850. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, H.P.; Yang, L.C.; Qu, Q.T.; Wu, Y.P.; Gan, C.L.; Zhou, D.L. Improving electrochemical performance of graphitic carbon in PC-based electrolytes by using N-vinyl-2-pyrrolidone as an additive. Electrochem. Commun. 2008, 10, 1571–1574. [Google Scholar] [CrossRef]
- Chen, J.; Yuan, G.; Li, C.; Hui, Z.; Zhang, Q. Interface modification in high voltage spinel lithium-ion battery by using N-methylpyrrole as an electrolyte additive. Electrochim. Acta 2015, 178, 127–133. [Google Scholar] [CrossRef]
- He, M.; Su, C.; Peebles, C.; Feng, Z.; Connell, J.G.; Liao, C.; Wang, Y.; Shkrob, I.A.; Zhang, Z. Mechanistic insight in the function of phosphite additives for protection of LiNi0.5Co0.2Mn0.3O2 cathode in high voltage Li-ion cells. ACS Appl. Mater. Interfaces 2016, 18, 11450–11458. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Tan, J.; Gu, J.; Xue, Y.; Qiao, L.; Zhang, Q. Facile synthesis of imidazole microcapsules via thiol-click chemistry and their application as thermally latent curing agent for epoxy resins. Compos. Sci. Technol. 2017, 142, 198–206. [Google Scholar] [CrossRef]
- Tan, J.; Li, C.; Zhou, J.; Yin, C.; Zhang, B.; Gu, J.; Zhang, Q. Fast and facile fabrication of porous polymer particles via thiol–ene suspension photopolymerization. RSC Adv. 2014, 4, 13334. [Google Scholar] [CrossRef]
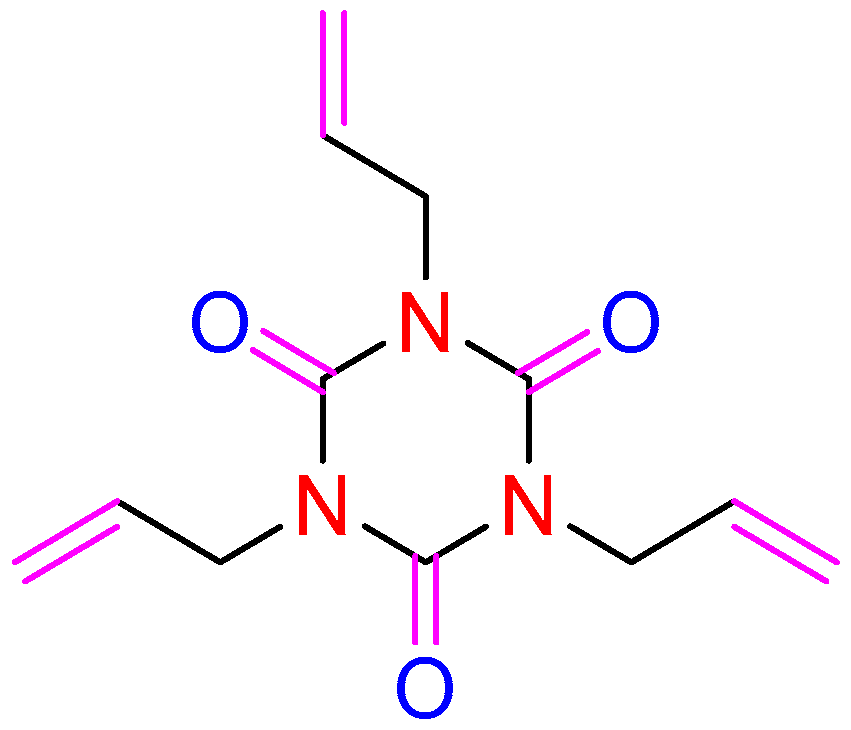


| Code | Solution | Additive/wt.% | Lithium Salt/mol L−1 | |||||
|---|---|---|---|---|---|---|---|---|
| EC | PC | DEC | PP | FEC | ADN | TAIC | LiPF6 | |
| Base-1# | 20 | 20 | 20 | 40 | 3 | 3 | 0 | 1.2 |
| TAIC-2# | 20 | 20 | 20 | 40 | 3 | 3 | 0.5 | 1.2 |
| Organic Molecules | TAIC | EC | DEC |
|---|---|---|---|
| HOMO (eV) | −7.86 | −8.47 | −8.02 |
| LUMO (eV) | −0.56 | −0.6 | −0.42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.-M.; Li, F.; Zhu, X.-Q.; Yu, J.-G. Triallyl Isocyanurate as an Efficient Electrolyte Additive for Layered Oxide Cathode Material-Based Lithium-Ion Batteries with Improved Stability under High-Voltage. Molecules 2022, 27, 3107. https://doi.org/10.3390/molecules27103107
Zhang C-M, Li F, Zhu X-Q, Yu J-G. Triallyl Isocyanurate as an Efficient Electrolyte Additive for Layered Oxide Cathode Material-Based Lithium-Ion Batteries with Improved Stability under High-Voltage. Molecules. 2022; 27(10):3107. https://doi.org/10.3390/molecules27103107
Chicago/Turabian StyleZhang, Chang-Ming, Feng Li, Xue-Quan Zhu, and Jin-Gang Yu. 2022. "Triallyl Isocyanurate as an Efficient Electrolyte Additive for Layered Oxide Cathode Material-Based Lithium-Ion Batteries with Improved Stability under High-Voltage" Molecules 27, no. 10: 3107. https://doi.org/10.3390/molecules27103107
APA StyleZhang, C.-M., Li, F., Zhu, X.-Q., & Yu, J.-G. (2022). Triallyl Isocyanurate as an Efficient Electrolyte Additive for Layered Oxide Cathode Material-Based Lithium-Ion Batteries with Improved Stability under High-Voltage. Molecules, 27(10), 3107. https://doi.org/10.3390/molecules27103107







