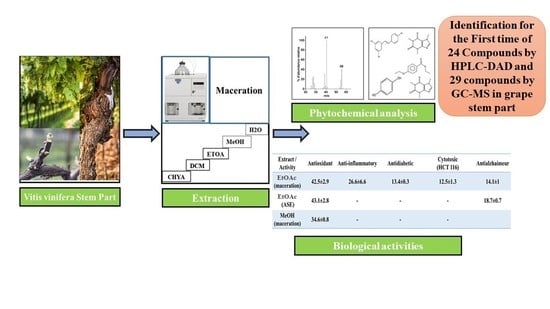
Study on the Chemical Composition and the Biological Activities of Vitis vinifera Stem Extracts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Extraction Yields
2.2. Total Polyphenol Content (TPC)
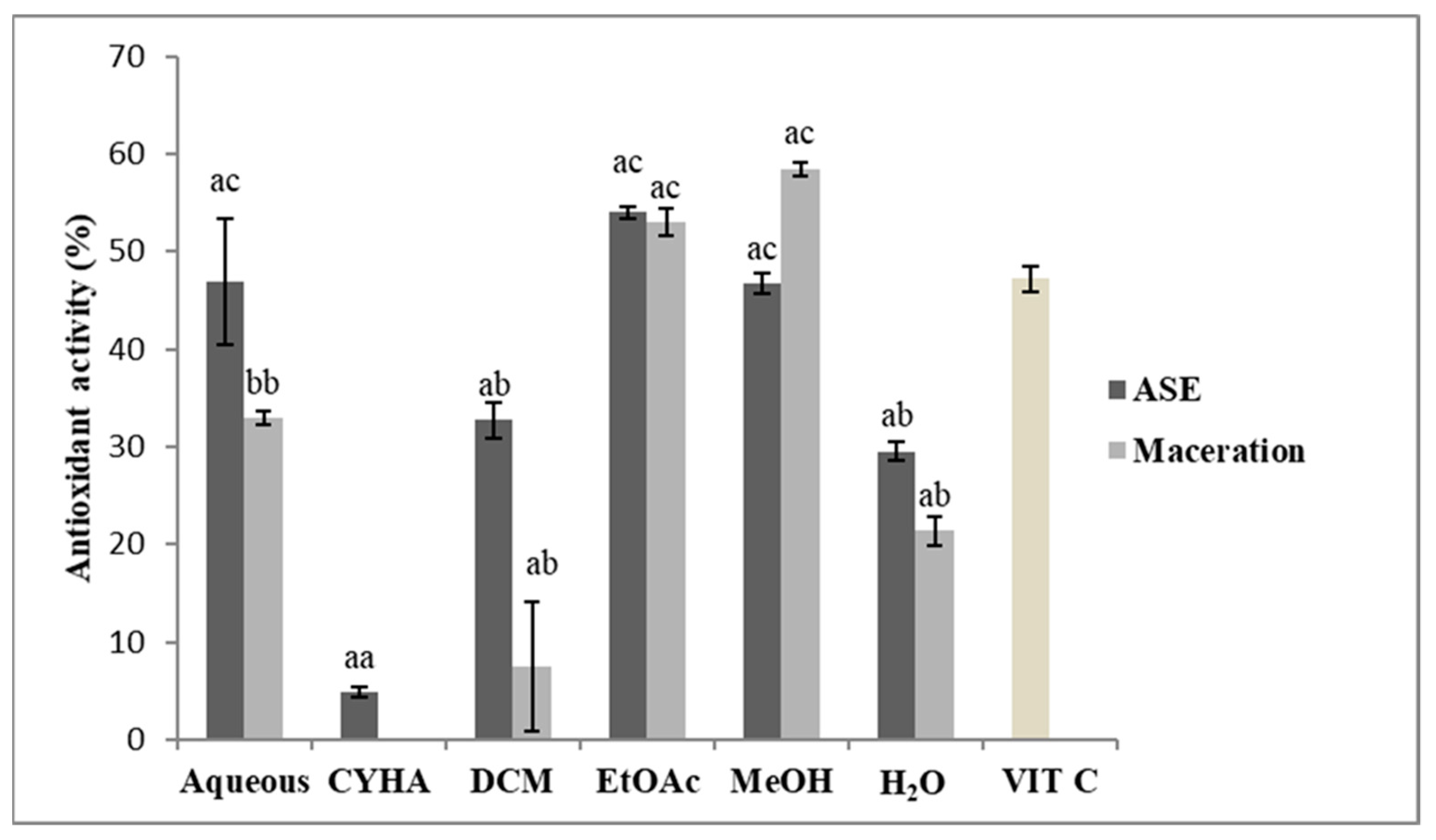
2.3. Antioxidant Activity (DPPH)
2.4. Chromatographic Analysis
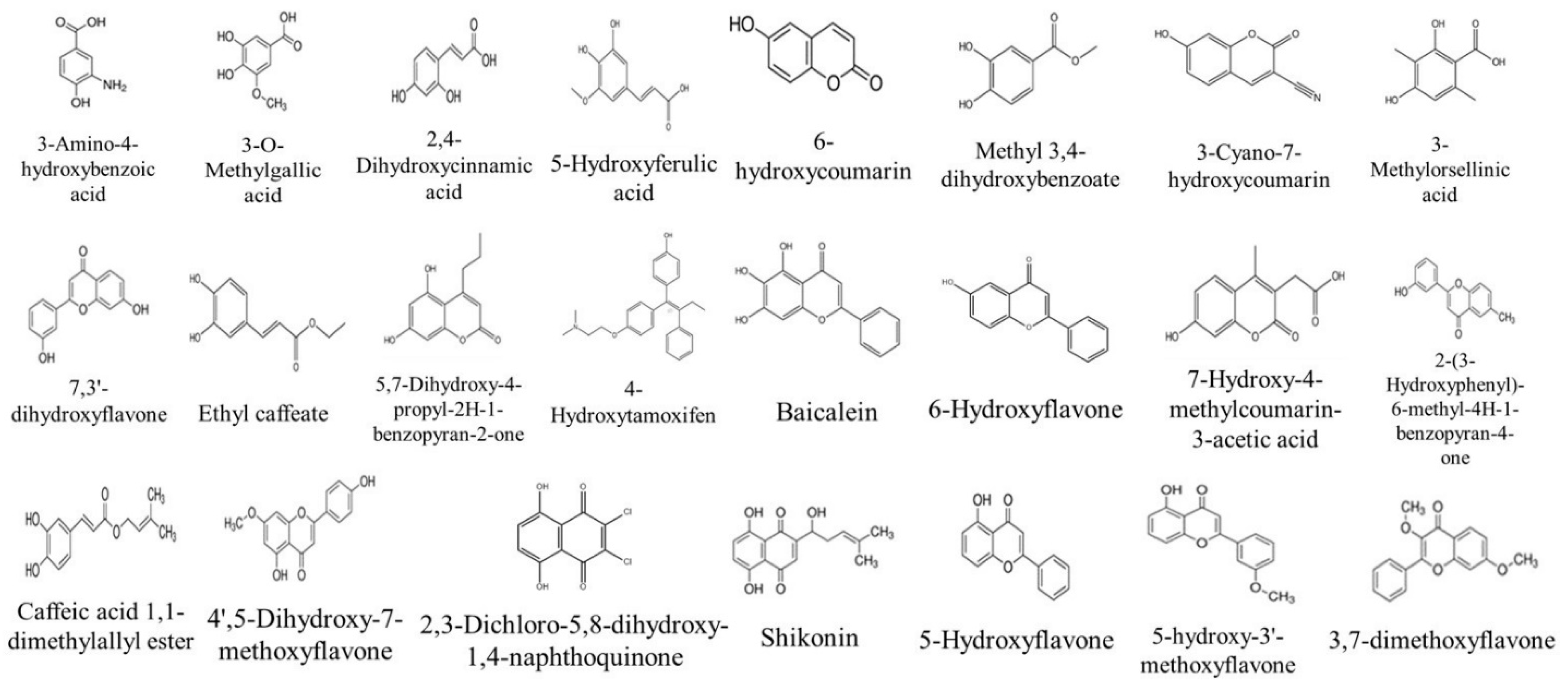
2.4.1. Identification of Compounds in V. vinifera Stem Extracts by HPLC-DAD
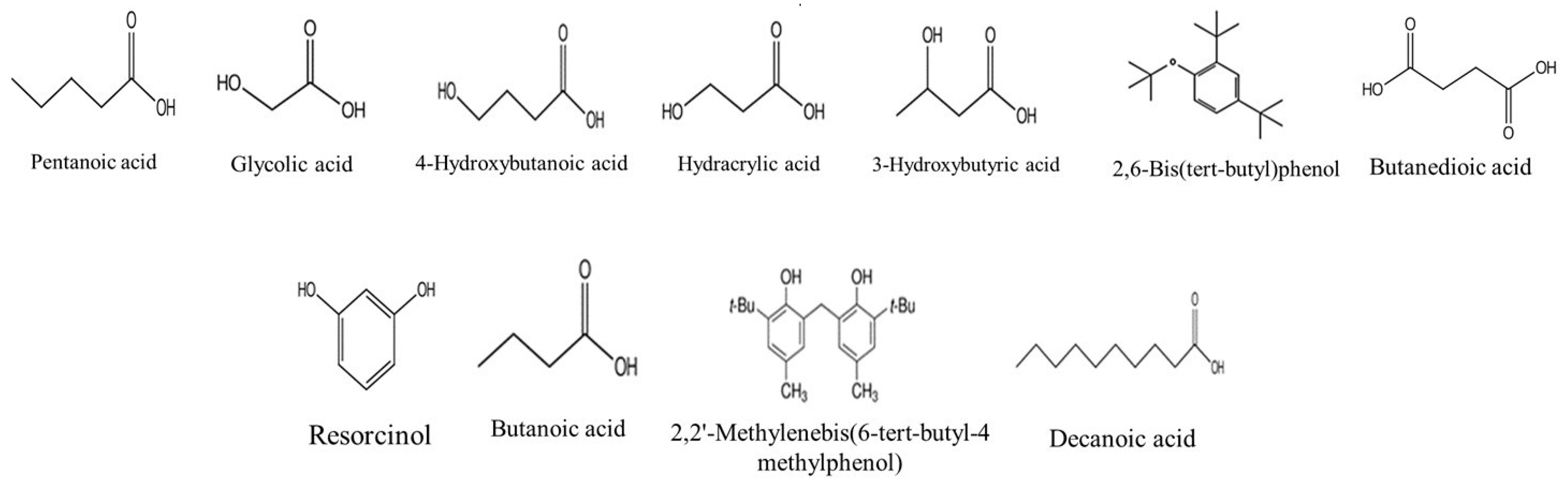
2.4.2. GC-MS Analysis of the V. vinifera Extracts before and after Derivatization
2.5. Biological Activities
2.5.1. Anti-Inflammatory Activity
2.5.2. Anti-Alzheimer Activity
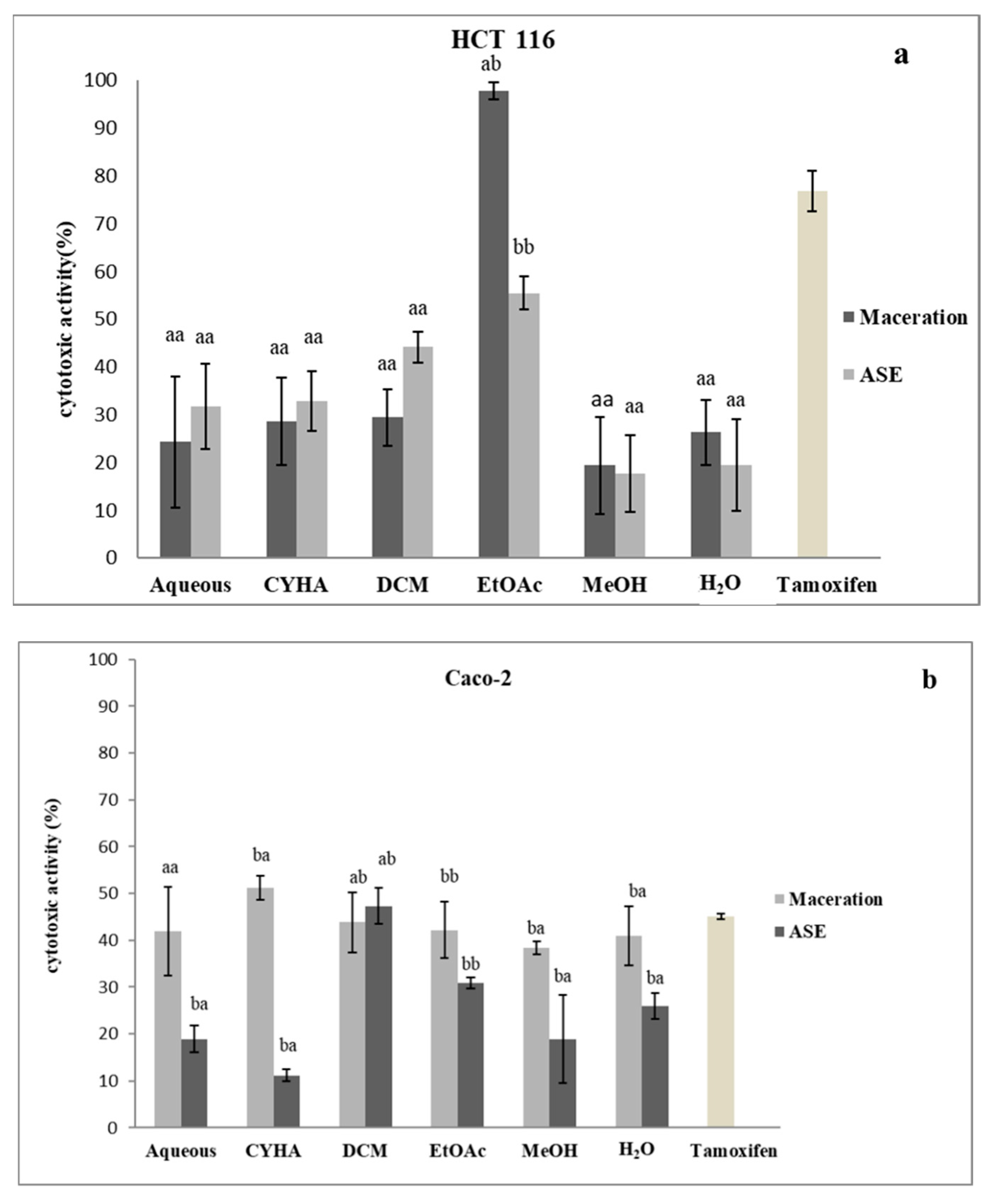
2.5.3. Cytotoxic Activity
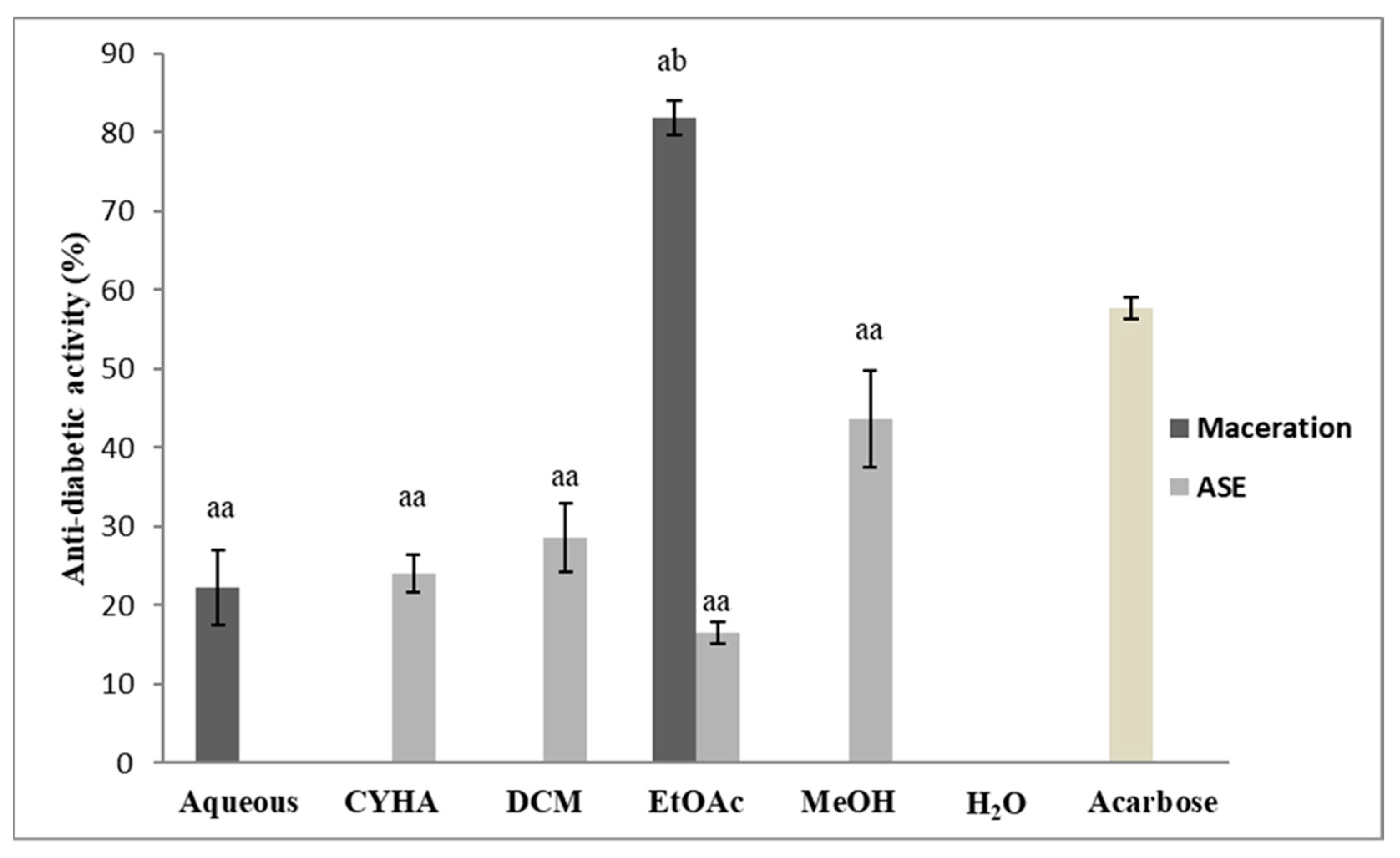
2.5.4. Antidiabetic Activity
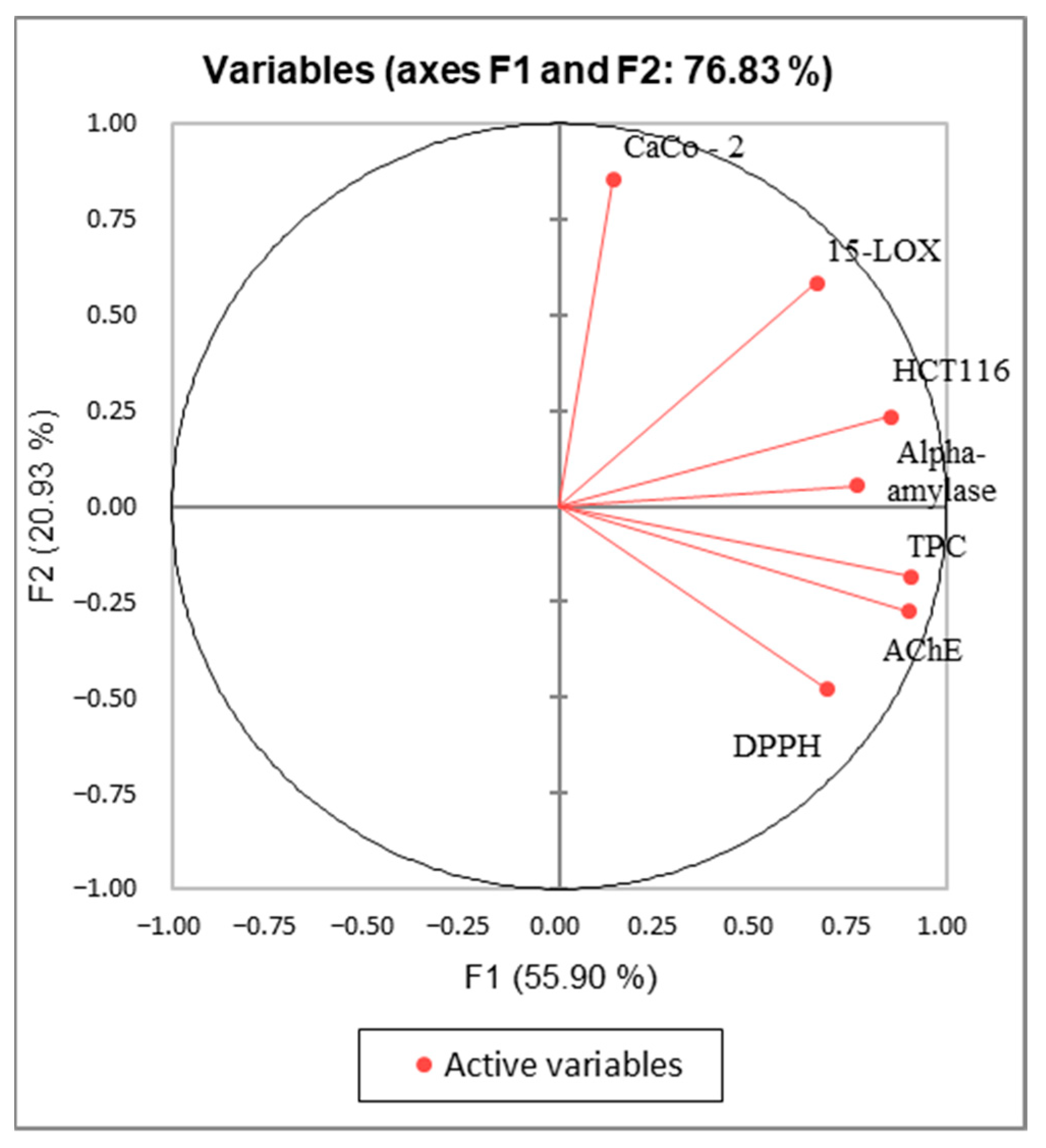
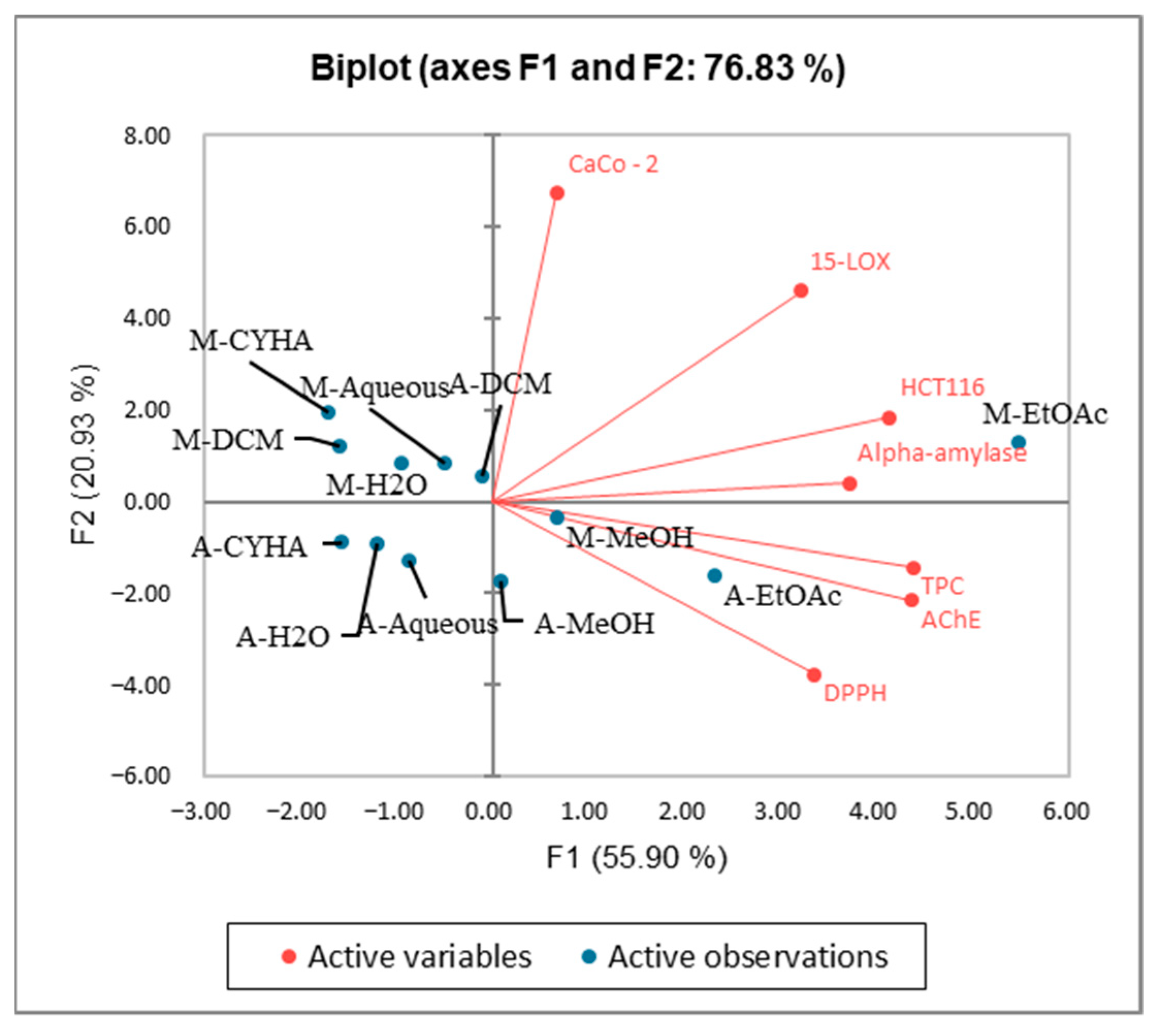
2.6. Principal Component Analysis (PCA)
3. Materials and Methods
3.1. Plant Material
3.2. Extraction
3.2.1. Maceration
3.2.2. Accelerated Solvent Extraction (ASE)
3.3. Total Phenolic Content (TPC)
3.4. Determination of DPPH Radical Scavenging Activity
3.5. Biological Activity
3.5.1. Anti-Inflammatory Activity
3.5.2. Anti-Cholinesterase Activity
3.5.3. Anti-α-Amylase Activity
3.5.4. Cytotoxic Activity
3.6. Chromatographic Analysis
3.6.1. High-Performance Liquid Chromatography Analysis (HPLC-DAD)
3.6.2. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
- Derivatization method:
- Compounds identification:
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gouvinhas, I.; Santos, R.A.; Queiroz, M.; Leal, C.; Saavedra, M.J.; Domínguez-Perles, R.; Rodrigues, M.; Barros, A.I. Monitoring the Antioxidant and Antimicrobial Power of Grape (Vitis vinifera L.) Stems Phenolics over Long-Term Storage. Ind. Crops Prod. 2018, 126, 83–91. [Google Scholar] [CrossRef]
- Gouvinhas, I.; Queiroz, M.; Rodrigues, M.; Barros, A.I. Evaluation of the Phytochemistry and Biological Activity of Grape (Vitis vinifera L.) Stems: Toward a Sustainable Winery Industry. Polyphen. Plant. 2019, 23, 381–394. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO). FAOSTAT Statistical Database of the United Nation Food and Agriculture Organization (FAO) Statistical Division; FAO: Rome, Italy, 2019. [Google Scholar]
- Kim, J.Y.; Jeong, H.Y.; Lee, H.K.; Kim, S.; Hwang, B.Y.; Bae, K.; Seong, Y.H. Neuroprotection of the Leaf and Stem of Vitis Amurensis and Their Active Compounds against Ischemic Brain Damage in Rats and Excitotoxicity in Cultured Neurons. Phytomedicine 2012, 19, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Lekakis, J.; Rallidis, L.S.; Andreadou, I.; Vamvakou, G.; Kazantzoglou, G.; Magiatis, P.; Skaltsounis, A.-L.; Kremastinos, D.T. Polyphenols Compounds from Red Grapes Acutely Improve Endothelial Function in Patients with Coronary Heart Disease. Eur. J. Prev. Cardiol. 2005, 12, 596–600. [Google Scholar] [CrossRef]
- Jayaprakasha, G.K.; Selvi, T.; Sakariah, K.K. Antibacterial and Antioxidant Activities of Grape (Vitis vinifera) Seed Extracts. Food Res. Int. 2003, 36, 117–122. [Google Scholar] [CrossRef]
- De Moura, R.S.; Viana, F.S.C.; Souza, M.A.V.; Kovary, K.; Guedes, D.C.; Oliveira, E.P.B.; Rubenich, L.M.S.; Carvalho, L.; Oliveira, R.M.; Tano, T. Antihypertensive, Vasodilator and Antioxidant Effects of a Vinifera Grape Skin Extract. J. Pharm. Pharmacol. 2002, 54, 1515–1520. [Google Scholar] [CrossRef]
- Cuevas, V.M.; Calzado, Y.R.; Guerra, Y.P.; Yera, A.O.; Despaigne, S.J.; Ferreiro, R.M.; Quintana, D.C. Effects of Grape Seed Extract, Vitamin C, and Vitamin E on Ethanol-and Aspirin-Induced Ulcers. Adv. Pharmacol. Sci. 2011, 2011, 740687. [Google Scholar] [CrossRef]
- Karvela, E.; Makris, D.P.; Kalogeropoulos, N.; Karathanos, V.T. Deployment of Response Surface Methodology to Optimise Recovery of Grape (Vitis vinifera) Stem Polyphenols. Talanta 2009, 79, 1311–1321. [Google Scholar] [CrossRef]
- Laufenberg, G.; Kunz, B.; Nystroem, M. Transformation of Vegetable Waste into Value Added Products::(A) the Upgrading Concept;(B) Practical Implementations. Bioresour. Technol. 2003, 87, 167–198. [Google Scholar] [CrossRef]
- Somkuwar, D.O.; Kamble, V.A. Phytochemical Screening of Ethanolic Extracts of Stem, Leaves, Flower and Seed Kernel of Mangifera indica L. Int. J. Pharm. Bio Sci. 2013, 4, 383–389. [Google Scholar]
- Barros, A.; Gironés-Vilaplana, A.; Teixeira, A.; Collado-González, J.; Moreno, D.A.; Gil-Izquierdo, A.; Rosa, E.; Domínguez-Perles, R. Evaluation of Grape (Vitis vinifera L.) Stems from Portuguese Varieties as a Resource of (Poly) Phenolic Compounds: A Comparative Study. Food Res. Int. 2014, 65, 375–384. [Google Scholar] [CrossRef]
- Domínguez-Perles, R.; Guedes, A.; Queiroz, M.; Silva, A.M.; Barros, A.I. Oxidative Stress Prevention and Anti-Apoptosis Activity of Grape (Vitis vinifera L.) Stems in Human Keratinocytes. Food Res. Int. 2016, 87, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Sahpazidou, D.; Geromichalos, G.D.; Stagos, D.; Apostolou, A.; Haroutounian, S.A.; Tsatsakis, A.M.; Tzanakakis, G.N.; Hayes, A.W.; Kouretas, D. Anticarcinogenic Activity of Polyphenolic Extracts from Grape Stems against Breast, Colon, Renal and Thyroid Cancer Cells. Toxicol. Lett. 2014, 230, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Anastasiadi, M.; Pratsinis, H.; Kletsas, D.; Skaltsounis, A.-L.; Haroutounian, S.A. Grape Stem Extracts: Polyphenolic Content and Assessment of Their in Vitro Antioxidant Properties. LWT-Food Sci. Technol. 2012, 48, 316–322. [Google Scholar] [CrossRef]
- Dias, C.; Domínguez-Perles, R.; Aires, A.; Teixeira, A.; Rosa, E.; Barros, A.; Saavedra, M.J. Phytochemistry and Activity against Digestive Pathogens of Grape (Vitis vinifera L.) Stem’s (Poly) Phenolic Extracts. LWT-Food Sci. Technol. 2015, 61, 25–32. [Google Scholar] [CrossRef]
- Kim, H.; Thuong, P.T.; Ngoc, T.M.; Lee, I.; Hung, N.D.; Bae, K. Antioxidant and Lipoxygenase Inhibitory Activity of Oligostilbenes from the Leaf and Stem of Vitis Amurensis. J. Ethnopharmacol. 2009, 125, 304–309. [Google Scholar]
- Jeong, H.Y.; Kim, J.Y.; Lee, H.K.; Ha, D.T.; Song, K.-S.; Bae, K.; Seong, Y.H. Leaf and Stem of Vitis Amurensis and Its Active Components Protect against Amyloid β Protein (25–35)-Induced Neurotoxicity. Arch. Pharm. Res. 2010, 33, 1655–1664. [Google Scholar] [CrossRef]
- Vázquez-Armenta, F.J.; Silva-Espinoza, B.A.; Cruz-Valenzuela, M.R.; González-Aguilar, G.A.; Nazzaro, F.; Fratianni, F.; Ayala-Zavala, J.F. Antibacterial and Antioxidant Properties of Grape Stem Extract Applied as Disinfectant in Fresh Leafy Vegetables. J. Food Sci. Technol. 2017, 54, 3192–3200. [Google Scholar] [CrossRef]
- Veskoukis, A.S.; Vassi, E.; Poulas, K.; Kokkinakis, M.; Asprodini, E.; Haroutounian, S.; Kouretas, D. Grape Stem Extracts from Three Native Greek Vine Varieties Exhibit Strong Antioxidant and Antimutagenic Properties. Anticancer Res. 2020, 40, 2025–2032. [Google Scholar] [CrossRef]
- Apostolou, A.; Stagos, D.; Galitsiou, E.; Spyrou, A.; Haroutounian, S.; Portesis, N.; Trizoglou, I.; Wallace Hayes, A.; Tsatsakis, A.M.; Kouretas, D. Assessment of Polyphenolic Content, Antioxidant Activity, Protection against ROS-Induced DNA Damage and Anticancer Activity of Vitis vinifera Stem Extracts. Food Chem. Toxicol. 2013, 61, 60–68. [Google Scholar] [CrossRef]
- Leal, C.; Gouvinhas, I.; Santos, R.A.; Rosa, E.; Silva, A.M.; Saavedra, M.J.; Barros, A.I. Potential Application of Grape (Vitis vinifera L.) Stem Extracts in the Cosmetic and Pharmaceutical Industries: Valorization of a by-Product. Ind. Crops Prod. 2020, 154, 112675. [Google Scholar] [CrossRef]
- Esparza, I.; Moler, J.A.; Arteta, M.; Jiménez-Moreno, N.; Ancín-Azpilicueta, C. Phenolic Composition of Grape Stems from Different Spanish Varieties and Vintages. Biomolecules 2021, 11, 1221. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Ohnishi, Y.; Furusho, Y.; Sakuda, S.; Horinouchi, S. Novel Benzene Ring Biosynthesis from C3 and C4 Primary Metabolites by Two Enzymes*♦. J. Biol. Chem. 2006, 281, 36944–36951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kammerer, D.; Claus, A.; Carle, R.; Schieber, A. Polyphenol Screening of Pomace from Red and White Grape Varieties (Vitis vinifera L.) by HPLC-DAD-MS/MS. J. Agric. Food Chem. 2004, 52, 4360–4367. [Google Scholar] [CrossRef]
- Zengin, G.; Diuzheva, A.; Jekő, J.; Cziáky, Z.; Bulut, G.; Dogan, A.; Haznedaroglu, M.Z.; Rengasamy, K.R.R.; Lobine, D.; Bahadori, M.B.; et al. HPLC–MS/MS-Based Metabolic Profiling and Pharmacological Properties of Extracts and Infusion Obtained from Amelanchier Parviflora Var. Dentata. Ind. Crops Prod. 2018, 124, 699–706. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, R.; Jiang, J.; Duan, W.; Fan, P.; Li, S.; Wang, L. Flavan-3-Ols in Vitis Seeds: Their Extraction and Analysis by HPLC-ESI-MS/MS. Food Res. Int. 2021, 139, 109911. [Google Scholar] [CrossRef]
- Gu, D.; Yang, Y.; Bakri, M.; Chen, Q.; Aisa, H.A. Biological Activity and LC-MS Profiling of Ethyl Acetate Extracts from Nitraria Sibirica (Pall.) Fruits. Nat. Prod. Res. 2018, 32, 2054–2057. [Google Scholar] [CrossRef]
- Lorini, A.; Damin, F.M.; de Oliveira, D.N.; Crizel, R.L.; Godoy, H.T.; Galli, V.; Meinhart, A.D. Characterization and Quantification of Bioactive Compounds from Ilex Paraguariensis Residue by HPLC-ESI-QTOF-MS from Plants Cultivated under Different Cultivation Systems. J. Food Sci. 2021, 86, 1599–1619. [Google Scholar] [CrossRef]
- Guex, C.G.; Reginato, F.Z.; de Jesus, P.R.; Brondani, J.C.; Lopes, G.H.H.; de Freitas Bauermann, L. Antidiabetic Effects of Olea europaea L. Leaves in Diabetic Rats Induced by High-Fat Diet and Low-Dose Streptozotocin. J. Ethnopharmacol. 2019, 235, 1–7. [Google Scholar] [CrossRef]
- Wu, J.-H.; Tung, Y.-T.; Chyu, C.-F.; Chien, S.-C.; Wang, S.-Y.; Chang, S.-T.; Kuo, Y.-H. Antioxidant Activity and Constituents of Extracts from the Root of Garcinia Multiflora. J. Wood Sci. 2008, 54, 383–389. [Google Scholar] [CrossRef]
- Somkuwar, R.G.; Bhange, M.A.; Oulkar, D.P.; Sharma, A.K.; Ahammed Shabeer, T.P. Estimation of Polyphenols by Using HPLC–DAD in Red and White Wine Grape Varieties Grown under Tropical Conditions of India. J. Food Sci. Technol. 2018, 55, 4994–5002. [Google Scholar] [CrossRef] [PubMed]
- Ekow Thomford, N.; Dzobo, K.; Adu, F.; Chirikure, S.; Wonkam, A.; Dandara, C. Bush Mint (Hyptis suaveolens) and Spreading Hogweed (Boerhavia diffusa) Medicinal Plant Extracts Differentially Affect Activities of CYP1A2, CYP2D6 and CYP3A4 Enzymes. J. Ethnopharmacol. 2018, 211, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Bernardo-Filho, M.; de Sá-Caputo, D.D.C.; Marin, P.J.; Chang, S. The Mechanism of Auriculotherapy: A Case Report Based on the Fractal Structure of Meridian System. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 30–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Figueiredo, M.J.; Grisi, C.V.B.; Santiago, Â.M.; de Andrade Vieira, E.; de Magalhães Cordeiro, A.M.T.; Vilela, A.F.; Viana, A.D.; de Sousa, S.; de Sousa Conrado, L. Characterization and Application of Croton Blanchetianus Baill Extract for Lamb Ribs Preservation. Food Chem. 2022, 373, 131404. [Google Scholar] [CrossRef]
- Shams Eldin, S.M.; Radwan, M.M.; Wanas, A.S.; Habib, A.-A.M.; Kassem, F.F.; Hammoda, H.M.; Khan, S.I.; Klein, M.L.; Elokely, K.M.; ElSohly, M.A. Bioactivity-Guided Isolation of Potential Antidiabetic and Antihyperlipidemic Compounds from Trigonella Stellata. J. Nat. Prod. 2018, 81, 1154–1161. [Google Scholar] [CrossRef]
- Del-Castillo-Alonso, M.Á.; Monforte, L.; Tomás-Las-Heras, R.; Martínez-Abaigar, J.; Núñez-Olivera, E. Phenolic Characteristics Acquired by Berry Skins of Vitis vinifera Cv. Tempranillo in Response to Close-to-Ambient Solar Ultraviolet Radiation Are Mostly Reflected in the Resulting Wines. J. Sci. Food Agric. 2020, 100, 401–409. [Google Scholar] [CrossRef]
- Ulubelen, A.; Kerr, R.R.; Mabry, T.J. Two New Neoflavonoids and C-Glycosylflavones from Passiflora serratodigitata. Phytochemistry 1982, 21, 1145–1147. [Google Scholar] [CrossRef]
- Dawra, M.; El Rayess, Y.; El Beyrouthy, M.; Nehme, N.; El Hage, R.; Taillandier, P.; Bouajila, J. Biological Activities and Chemical Characterization of the Lebanese Endemic Plant Origanum ehrenbergii Boiss. Flavour Fragr. J. 2021, 36, 339–351. [Google Scholar] [CrossRef]
- Do, H.T.T.; Nguyen, T.H.; Nghiem, T.D.; Nguyen, H.T.; Choi, G.J.; Ho, C.T.; Le Dang, Q. Phytochemical Constituents and Extracts of the Roots of Scutellaria baicalensis Exhibit in Vitro and in Vivo Control Efficacy against Various Phytopathogenic Microorganisms. S. Afr. J. Bot. 2021, 142, 1–11. [Google Scholar] [CrossRef]
- Yahyaoui, M.; Bouajila, J.; Cazaux, S.; Abderrabba, M. The Impact of Regional Locality on Chemical Composition, Anti-Oxidant and Biological Activities of Thymelaea hirsuta L. Extracts. Phytomedicine 2018, 41, 13–23. [Google Scholar] [CrossRef]
- Morsi, A.E.; Ahmed, O.H.; Abdel-Hady, H.; El-Sayed, M.; Shemis, A.M. GC-Analysis, and Antioxidant, Anti-Inflammatory, and Anticancer Activities of Some Extracts and Fractions of Linum Usitatissimum. Curr. Bioact. Compd. 2020, 16, 1306–1318. [Google Scholar] [CrossRef]
- Gupta, A.; Tripathi, A.; Gupta, P. Extraction, Isolation and Characterization of Bauhinia variegata Flower. J. Appl. Pharm. Sci. Res. 2020, 3, 9–12. [Google Scholar] [CrossRef]
- Saoudi, M.M.; Bouajila, J.; Rahmani, R.; Alouani, K. Phytochemical Composition, Antioxidant, Antiacetylcholinesterase, and Cytotoxic Activities of Rumex crispus L. Int. J. Anal. Chem. 2021, 2021, 6675436. [Google Scholar] [CrossRef] [PubMed]
- Luna-Vázquez, F.J.; Ibarra-Alvarado, C.; Camacho-Corona, M.D.; Rojas-Molina, A.; Rojas-Molina, J.I.; García, A.; Bah, M. Vasodilator Activity of Compounds Isolated from Plants Used in Mexican Traditional Medicine. Molecules 2018, 23, 1474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, N.; Yamakawa, D.; Yamauchi, N.; Hashimoto, Y.; Matsuoka, E.; Hara, M. Induction of the Heat Shock Response in Arabidopsis by Chlorinated 1,4-Naphthoquinones. Plant. Growth Regul. 2019, 87, 413–420. [Google Scholar] [CrossRef]
- Chen, Y.; Si, L.; Zhang, J.; Yu, H.; Liu, X.; Chen, Y.; Wu, Y. Uncovering the Antitumor Effects and Mechanisms of Shikonin against Colon Cancer on Comprehensive Analysis. Phytomedicine 2021, 82, 153460. [Google Scholar] [CrossRef] [PubMed]
- Matarese, F.; Cuzzola, A.; Scalabrelli, G.; D’Onofrio, C. Expression of Terpene Synthase Genes Associated with the Formation of Volatiles in Different Organs of Vitis vinifera. Phytochemistry 2014, 105, 12–24. [Google Scholar] [CrossRef]
- Ismail, N.Z.; Adebayo, I.A.; Mohamed, W.A.S.; Mohamad Zain, N.N.; Arsad, H. Christia Vespertilionis Extract Induced Antiproliferation and Apoptosis in Breast Cancer (MCF7) Cells. Mol. Biol. Rep. 2021, 48, 7361–7370. [Google Scholar] [CrossRef]
- Rath, D.; Panigrahi, S.K.; Kar, D.M.; Maharana, L. Identification of Bioactive Constituents from Different Fractions of Stems of Cuscuta Reflexa Roxb. Using GC-MS. Nat. Prod. Res. 2018, 32, 1977–1981. [Google Scholar] [CrossRef]
- Alijani, Z.; Amini, J.; Ashengroph, M.; Bahramnejad, B. Antifungal Activity of Volatile Compounds Produced by Staphylococcus sciuri Strain MarR44 and Its Potential for the Biocontrol of Colletotrichum nymphaeae, Causal Agent Strawberry Anthracnose. Int. J. Food Microbiol. 2019, 307, 108276. [Google Scholar] [CrossRef]
- Bukvicki, D.R.; Tyagi, A.K.; Gottardi, D.G.; Veljic, M.M.; Jankovic, S.M.; Guerzoni, M.E.; Marin, P.D. Assessment of the Chemical Composition and In Vitro Antimicrobial Potential of Extracts of the Liverwort Scapania aspera. Nat. Prod. Commun. 2013, 8, 1934578X1300800932. [Google Scholar] [CrossRef] [Green Version]
- Ashmawy, A.M.; Ayoub, I.M.; Eldahshan, O.A. Chemical Composition, Cytotoxicity and Molecular Profiling of Cordia africana Lam. on Human Breast Cancer Cell Line. Nat. Prod. Res. 2021, 35, 4133–4138. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, L.-T.; Feng, Y.-X.; Zhang, D.; Guo, S.-S.; Pang, X.; Geng, Z.-F.; Xi, C.; Du, S.-S. Comparative Evaluation of the Chemical Composition and Bioactivities of Essential Oils from Four Spice Plants (Lauraceae) against Stored-Product Insects. Ind. Crops Prod. 2019, 140, 111640. [Google Scholar] [CrossRef]
- Mahnashi, M.H.; Alyami, B.A.; Alqahtani, Y.S.; Jan, M.S.; Rashid, U.; Sadiq, A.; Alqarni, A.O. Phytochemical Profiling of Bioactive Compounds, Anti-Inflammatory and Analgesic Potentials of Habenaria digitata Lindl.: Molecular Docking Based Synergistic Effect of the Identified Compounds. J. Ethnopharmacol. 2021, 273, 113976. [Google Scholar] [CrossRef]
- Duru, M.E.; Cakir, A.; Kordali, S.; Zengin, H.; Harmandar, M.; Izumi, S.; Hirata, T. Chemical Composition and Antifungal Properties of Essential Oils of Three Pistacia Species. Fitoterapia 2003, 74, 170–176. [Google Scholar] [CrossRef]
- Chandra, J.H.; Gunasekaran, H. Screening of Phytochemical, Antimicrobial and Antioxidant Activity of Glycyrrhiza Glabra Root Extract. J. Environ. Biol. 2017, 38, 161–165. [Google Scholar] [CrossRef]
- Fuchs, C.; Bakuradze, T.; Steinke, R.; Grewal, R.; Eckert, G.P.; Richling, E. Polyphenolic Composition of Extracts from Winery By-Products and Effects on Cellular Cytotoxicity and Mitochondrial Functions in HepG2 Cells. J. Funct. Foods 2020, 70, 103988. [Google Scholar] [CrossRef]
- Tasin, M.; Anfora, G.; Ioriatti, C.; Carlin, S.; de Cristofaro, A.; Schmidt, S.; Bengtsson, M.; Versini, G.; Witzgall, P. Antennal and Behavioral Responses of Grapevine Moth Lobesia Botrana Females to Volatiles from Grapevine. J. Chem. Ecol. 2005, 31, 77–87. [Google Scholar] [CrossRef]
- EL-Ghandour, H. Evaluation of Antibacterial Activity, Antioxidant Properties of Cynara Scolymus L. Aqueous Leaf and Pulp Extracts and Their Effect on Streptozotocin-Induced Diabetic Rats. JCBPS 2018, 8, 857–869. [Google Scholar]
- Goyal, M. Qualitative and Quantitative Evaluation of Phytochemicals in Leaf Extract of Alstonia scholaris (L.) R. Br.By GC-MS Technique. Int. J. Pharm. Biol. Sci. 2019, 9. [Google Scholar]
- Sermakkani, M.; Thangapandian, V. GC-MS Analysis of Cassia Italica Leaf Methanol Extract. Asian J. Pharm. Clin. Res. 2012, 5, 90–94. [Google Scholar]
- Bonatto Machado de Castilhos, M.; Luiz Del Bianchi, V.; Gómez-Alonso, S.; García-Romero, E.; Hermosín-Gutiérrez, I. Sensory Descriptive and Comprehensive GC-MS as Suitable Tools to Characterize the Effects of Alternative Winemaking Procedures on Wine Aroma. Part II: BRS Rúbea and BRS Cora. Food Chem. 2020, 311, 126025. [Google Scholar] [CrossRef] [PubMed]
- Osuntokun, O. Assessment of Antimicrobial, Phytochemical Screening and Gas Chromatography-Mass Spectrophotometric Profile of Crude chrysophyllum Albidum Essential Oil. Chem. Res. J. 2017, 2, 68–85. [Google Scholar]
- Beltagy, A. Phytochemical Attributes and Antimicrobial Activity of Green Tea and Rosemary Leaves: A Comparative Study. World J. Pharm. Res. 2016, 5, 1882–1896. [Google Scholar] [CrossRef]
- Yetayih, M. Extraction and GC-MS Analysis of the Essential Oil from the Peel of Solanum Incanum and Its Antibacterial Activity Studies. Asian J. Chem. 2020, 32, 2001–2006. [Google Scholar] [CrossRef]
- Batovska, D.I.; Todorova, I.T.; Nedelcheva, D.V.; Parushev, S.P.; Atanassov, A.I.; Hvarleva, T.D.; Djakova, G.J.; Bankova, V.S.; Popov, S.S. Preliminary Study on Biomarkers for the Fungal Resistance in Vitis vinifera Leaves. J. Plant. Physiol. 2008, 165, 791–795. [Google Scholar] [CrossRef]
- Torras-Claveria, L.; Berkov, S.; Jáuregui, O.; Caujapé, J.; Viladomat, F.; Codina, C.; Bastida, J. Metabolic Profiling of Bioactive Pancratium Canariense Extracts by GC-MS. Phytochem. Anal. 2010, 21, 80–88. [Google Scholar] [CrossRef]
- Ananthi, T.; Subalakshmi, K. Ananthi GC-MS Analysis of Stem Bark Extracts of Senna alata (L.). J. Chem. Pharm. Res. 2016, 8, 280–283. [Google Scholar]
- Griesser, M.; Weingart, G.; Schoedl-Hummel, K.; Neumann, N.; Becker, M.; Varmuza, K.; Liebner, F.; Schuhmacher, R.; Forneck, A. Severe Drought Stress Is Affecting Selected Primary Metabolites, Polyphenols, and Volatile Metabolites in Grapevine Leaves (Vitis vinifera Cv. Pinot Noir). Plant. Physiol. Biochem. 2015, 88, 17–26. [Google Scholar] [CrossRef]
- Lutz, M.; Jorquera, K.; Cancino, B.; Ruby, R.; Henriquez, C. Phenolics and Antioxidant Capacity of Table Grape (Vitis vinifera L.) Cultivars Grown in Chile. J. Food Sci. 2011, 76, C1088–C1093. [Google Scholar] [CrossRef]
- de Campos, L.M.A.S.; Leimann, F.V.; Pedrosa, R.C.; Ferreira, S.R.S. Free Radical Scavenging of Grape Pomace Extracts from Cabernet Sauvingnon (Vitis vinifera). Bioresour. Technol. 2008, 99, 8413–8420. [Google Scholar] [CrossRef] [PubMed]
- Ekwy, O.C.; Ehiabhi, O.S.; Okechukwu, E.B. Phytochemical and GC-MS Analyses of the Bioactive Components of Securidaca Longepedunculata (Fresen) Roots for Anti-Breast Cancer Activity. World J. Pharm. Res. 2015, 4, 1503–1518. [Google Scholar]
- Tamborra, P.; Esti, M.; Minafra, M.; Sinesio, F. Phenolic compounds in red-berry skins of Uva di Troia and bombino nero grapes (Vitis vinifera L.). Ital. J. Food Sci. 2003, 15, 347–358. [Google Scholar]
- Yang, Q.-Q.; Zhang, D.; Farha, A.K.; Yang, X.; Li, H.-B.; Kong, K.-W.; Zhang, J.-R.; Chan, C.-L.; Lu, W.-Y.; Corke, H.; et al. Phytochemicals, Essential Oils, and Bioactivities of an Underutilized Wild Fruit Cili (Rosa Roxburghii). Ind. Crops Prod. 2020, 143, 111928. [Google Scholar] [CrossRef]
- Kumar, K.A.; Vijayalakshmi, K. GC-MS Analysis of Phytochemical Constituents in Ethanolic Extract of Punica Granatum Peel and Vitis vinifera Seeds. IJPBS 2011, 2, B461–B468. [Google Scholar]
- Da Porto, C.; Porretto, E.; Decorti, D. Comparison of Ultrasound-Assisted Extraction with Conventional Extraction Methods of Oil and Polyphenols from Grape (Vitis vinifera L.) Seeds. Ultrason. Sonochem. 2013, 20, 1076–1080. [Google Scholar] [CrossRef]
- Papastamoulis, Y.; Richard, T.; Nassra, M.; Badoc, A.; Krisa, S.; Harakat, D.; Monti, J.-P.; Mérillon, J.-M.; Waffo-Teguo, P. Viniphenol A, a Complex Resveratrol Hexamer from Vitis vinifera Stalks: Structural Elucidation and Protective Effects against Amyloid-β-Induced Toxicity in PC12 Cells. J. Nat. Prod. 2014, 77, 213–217. [Google Scholar] [CrossRef]
- Amico, V.; Barresi, V.; Chillemi, R.; Condorelli, D.F.; Sciuto, S.; Spatafora, C.; Tringali, C. Bioassay-Guided Isolation of Antiproliferative Compounds from Grape (Vitis vinifera) Stems. Nat. Prod. Commun. 2009, 4, 1934578X0900400108. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M.; Chavan, A.; Lakshmikantha, R.Y.; Satwadi, P.R.; Thimmappanahalli, K.B. Evaluation of Antidiabetic Activity of Vitis vinifera Stem Bark. J. Pharm. Res. 2012, 5, 5239–5252. [Google Scholar]
- Rahmani, R.; Bouajila, J.; Jouaidi, M.; Debouba, M. African Mustard (Brassica tournefortii) as Source of Nutrients and Nutraceuticals Properties. J. Food Sci. 2020, 85, 1856–1871. [Google Scholar] [CrossRef]
- Bekir, J.; Mars, M.; Souchard, J.P.; Bouajila, J. Assessment of Antioxidant, Anti-Inflammatory, Anti-Cholinesterase and Cytotoxic Activities of Pomegranate (Punica granatum) Leaves. Food Chem. Toxicol. 2013, 55, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Premakumara, G.A.S.; Abeysekera, W.K.S.M.; Ratnasooriya, W.D.; Chandrasekharan, N.V.; Bentota, A.P. Antioxidant, Anti-Amylase and Anti-Glycation Potential of Brans of Some Sri Lankan Traditional and Improved Rice (Oryza sativa L.) Varieties. J. Cereal Sci. 2013, 58, 451–456. [Google Scholar] [CrossRef]
- Rahmani, R.; Beaufort, S.; Villarreal-Soto, S.A.; Taillandier, P.; Bouajila, J.; Debouba, M. Kombucha Fermentation of African Mustard (Brassica tournefortii) Leaves: Chemical Composition and Bioactivity. Food Biosci. 2019, 30, 100414. [Google Scholar] [CrossRef] [Green Version]





| Fractional Extraction | ||||||
|---|---|---|---|---|---|---|
| Aqueous | CYHA | DCM | EtOAc | MeOH | H2O | |
| ASE | 5.08 | 0.11 | 0.20 | 0.31 | 2.24 | 2.91 |
| Maceration | 3.05 | 0.07 | 0.17 | 0.25 | 1.94 | 3.23 |
| Extract/Activity | Antioxidant | Anti-Inflammatory | Antidiabetic | Cytotoxic (HCT 116) | Anti-Alzheimer |
|---|---|---|---|---|---|
| EtOAc (Maceration) | 42.5 ± 2.9 | 26.6 ± 6.6 | 13.4 ± 0.3 | 12.5 ± 1.3 | 14.1 ± 1.0 |
| EtOAc (ASE) | 43.1 ± 2.8 | >50 | >50 | >50 | 18.7 ± 0.7 |
| MeOH (Maceration) | 34.6 ± 0.8 | >50 | >50 | >50 | >50 |
| % Area | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compounds | RT | Maceration | ASE | Ref. | ||||||||||
| Fractional Extraction | Fractional Extraction | |||||||||||||
| Aqueous | CYHA | DCM | EtOAc | MeOH | H2O | Aqueous | CYHA | DCM | EtOAc | MeOH | H2O | |||
| 3-Amino-4-hydroxybenzoic acid | 2.2 | 0.54 | 0.10 | 0.15 | 0.99 | 0.71 | [24] | |||||||
| Gallic acid | 3.5 | 1.34 | 0.81 | 0.59 | 0.73 | 0.56 | 0.58 | 0.28 | 0.46 | [25] | ||||
| 3-O-Methylgallic acid | 7.7 | 0.21 | 8.28 | [26] | ||||||||||
| (−)-Epicatechin | 11.9 | 0.19 | 0.02 | 0.02 | [27] | |||||||||
| 2,4-Dihydroxycinnamic acid | 14.5 | 6.63 | 0.19 | [28] | ||||||||||
| 5-Hydroxyferulic acid | 14.5 | 0.14 | 1.07 | 0.06 | [29] | |||||||||
| 6-hydroxycoumarin | 18.2 | 2.24 | [30] | |||||||||||
| Methyl 3,4-dihydroxybenzoate | 19.2 | 18.92 | 11.09 | [31] | ||||||||||
| Rutin hydrate | 22.7 | 0.25 | 6.92 | [32] | ||||||||||
| 3-Cyano-7-hydroxycoumarin | 30.3 | 0.09 | 3.97 | 3.14 | [33] | |||||||||
| 3-Methylorsellinic acid | 35.2 | 1.35 | 0.02 | [34] | ||||||||||
| Trans-Cinnamic acid | 41.5 | 0.25 | [35] | |||||||||||
| 7,3′-dihydroxyflavone | 41.9 | 1.07 | 0.15 | 0.04 | 4.32 | [36] | ||||||||
| Ethyl caffeate | 42 | 1.51 | 4.34 | [37] | ||||||||||
| 5,7-Dihydroxy-4-propyl-2H-1-benzopyran-2-one | 42.8 | 0.02 | [38] | |||||||||||
| 4-Hydroxytamoxifen | 43.1 | 1.71 | [39] | |||||||||||
| Baicalein | 44.5 | 0.47 | 903.45 | 121.22 | 7.73 | [40] | ||||||||
| 6-Hydroxyflavone | 45 | 40.87 | 5.27 | 2.37 | [41] | |||||||||
| 7-Hydroxy-4-methylcoumarin-3-acetic acid | 45.5 | 21.43 | [42] | |||||||||||
| 2-(3-Hydroxyphenyl)-6-methyl-4H-1-benzopyran-4-one | 45.7 | 0.03 | 26.86 | [43] | ||||||||||
| Caffeic acid 1,1-dimethylallyl ester | 46 | 8.55 | [41] | |||||||||||
| 4′,5-Dihydroxy-7-methoxyflavone | 46.3 | 0.05 | [44] | |||||||||||
| 3,7-dimethoxyflavone | 47.5 | 0.03 | [45] | |||||||||||
| 2,3-Dichloro-5,8-dihydroxy-1,4-naphthoquinone | 47.7 | 0.59 | [46] | |||||||||||
| Shikonin | 48 | 0.15 | 0.92 | [47] | ||||||||||
| 5-Hydroxyflavone | 48.5 | 0.36 | [44] | |||||||||||
| 5-Hydroxy-3′-methoxyflavone | 49.4 | 3.03 | 0.83 | 0.42 | 0.36 | 0.07 | [44] | |||||||
| 3′-Hydroxy-b-naphthoflavone | 49.8 | 0.083 | [44] | |||||||||||
| No. | RI | Compound | Maceration | ASE | Ref. | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fractional Extraction | Fractional Extraction | ||||||||||||||
| Aqueous | CYHA | DCM | EtOAc | MeOH | H2O | Aqueous | CYHA | DCM | EtOAc | MeOH | H2O | ||||
| 1 | 1019 | Cyclododecane | X | X | X | [49] | |||||||||
| 2 | 1044 | 2,4-Dihydroxy-2,5-dimethyl-3(2H)-furan-3-one | X | X | X | X | [50] | ||||||||
| 3 | 1075 | Decane, 3-methyl- | X | [51] | |||||||||||
| 4 | 1088 | 2,3-Dimethyldecane | X | [52] | |||||||||||
| 5 | 1100 | Undecane | X | X | [53] | ||||||||||
| 6 | 1119 | Cyclohexanone, 3,3,5trimethyl- | X | [50] | |||||||||||
| 7 | 1187 | Benzene, 1,2,4,5-tetramethyl- | X | [54] | |||||||||||
| 8 | 1205 | Dodecane | X | [50] | |||||||||||
| 9 | 1249 | 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- | X | X | X | X | X | X | [55] | ||||||
| 10 | 1306 | Tridecane | X | X | [56] | ||||||||||
| 11 | 1312 | Nonanoic acid | X | [56] | |||||||||||
| 12 | 1404 | 1,1′-Bicyclohexyl | X | [57] | |||||||||||
| 13 | 1470 | Benzaldehyde, 4-hydroxy- | X | X | X | [58] | |||||||||
| 14 | 1533 | Pentadecane | X | [59] | |||||||||||
| 15 | 1599 | 2,5-di-tert-Butyl-1,4-benzoquinone | X | [60] | |||||||||||
| 16 | 1617 | 2,4-Di-tert-butylphenol | X | X | X | [61] | |||||||||
| 17 | 2161 | n-Hexadecanoic acid | X | X | X | X | [62] | ||||||||
| 18 | 2194 | Benzenepropanoic acid, 3,5-bis(1,1-dimethylethyl)-4-hydroxy- methyl ester | X | X | [63] | ||||||||||
| 19 | 2717 | Phthalic acid, di(2-propylpentyl) ester | X | [64] | |||||||||||
| No. | RI | Compound | Maceration | ASE | Ref. | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fractional Extraction | Fractional Extraction | ||||||||||||||
| Aqueous | CYHA | DCM | EtOAc | MeOH | H2O | Aqueous | CYHA | DCM | EtOAc | MeOH | H2O | ||||
| 1 | 1013 | Pentanoic acid | X | X | [65] | ||||||||||
| 2 | 1094 | Hexanoic acid | X | X | X | X | [66] | ||||||||
| 3 | 1112 | Glycolic acid | X | [66] | |||||||||||
| 4 | 1187 | Hydracrylic acid | X | [67] | |||||||||||
| 5 | 1199 | 3-Hydroxybutyric acid | X | [68] | |||||||||||
| 6 | 1283 | 4-Hydroxybutanoic acid | X | [69] | |||||||||||
| 7 | 1377 | Butanedioic acid | X | X | X | [70] | |||||||||
| 8 | 1436 | Resorcinol | X | X | X | [71] | |||||||||
| 9 | 1517 | Decanoic acid | X | [72] | |||||||||||
| 10 | 1636 | 2,6-Bis(tert-butyl)phenol | X | X | [73] | ||||||||||
| 11 | 1710 | Tartaric acid | X | X | X | [74] | |||||||||
| 12 | 1926 | 2,2′-Methylenebis(6-tert-butyl-4 methylphenol) | X | X | [75] | ||||||||||
| 13 | 2043 | Pentadecanoic acid | X | [76] | |||||||||||
| 14 | 2064 | Palmitic Acid | X | X | X | X | X | X | [76] | ||||||
| 15 | 2074 | Gallic acid | X | [21] | |||||||||||
| 16 | 2128 | Resveratrol | X | [21] | |||||||||||
| 17 | 2342 | 9,12-Octadecadienoic acid (Z,Z)- | X | [77] | |||||||||||
| Variables | DPPH | HCT116 | CaCo-2 | AChE | 15-LOX | Alpha-Amylase | TPC |
|---|---|---|---|---|---|---|---|
| DPPH | 1 | 0.316 | −0.153 | 0.671 | 0.247 | 0.366 | 0.792 |
| HCT116 | 0.316 | 1 | 0.212 | 0.763 | 0.624 | 0.744 | 0.627 |
| CaCo-2 | −0.153 | 0.212 | 1 | −0.074 | 0.472 | −0.028 | 0.113 |
| AChE | 0.671 | 0.763 | −0.074 | 1 | 0.369 | 0.594 | 0.912 |
| 15-LOX | 0.247 | 0.624 | 0.472 | 0.369 | 1 | 0.527 | 0.470 |
| Alpha-amylase | 0.366 | 0.744 | −0.028 | 0.594 | 0.527 | 1 | 0.517 |
| TPC | 0.792 | 0.627 | 0.113 | 0.912 | 0.470 | 0.517 | 1 |
| F1 | F2 | |
|---|---|---|
| DPPH | 0.694 | −0.480 |
| HCT116 | 0.857 | 0.234 |
| CaCo-2 | 0.138 | 0.853 |
| AChE | 0.906 | −0.274 |
| 15-LOX | 0.665 | 0.583 |
| Alpha-amylase | 0.771 | 0.053 |
| TPC | 0.906 | −0.183 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben Khadher, T.; Aydi, S.; Mars, M.; Bouajila, J. Study on the Chemical Composition and the Biological Activities of Vitis vinifera Stem Extracts. Molecules 2022, 27, 3109. https://doi.org/10.3390/molecules27103109
Ben Khadher T, Aydi S, Mars M, Bouajila J. Study on the Chemical Composition and the Biological Activities of Vitis vinifera Stem Extracts. Molecules. 2022; 27(10):3109. https://doi.org/10.3390/molecules27103109
Chicago/Turabian StyleBen Khadher, Talel, Samir Aydi, Mohamed Mars, and Jalloul Bouajila. 2022. "Study on the Chemical Composition and the Biological Activities of Vitis vinifera Stem Extracts" Molecules 27, no. 10: 3109. https://doi.org/10.3390/molecules27103109
APA StyleBen Khadher, T., Aydi, S., Mars, M., & Bouajila, J. (2022). Study on the Chemical Composition and the Biological Activities of Vitis vinifera Stem Extracts. Molecules, 27(10), 3109. https://doi.org/10.3390/molecules27103109