What Is the Trait d’Union between Retroactivity and Molecular Communication Performance Limits?
Abstract
:1. Introduction
2. Overview
3. Preliminaries
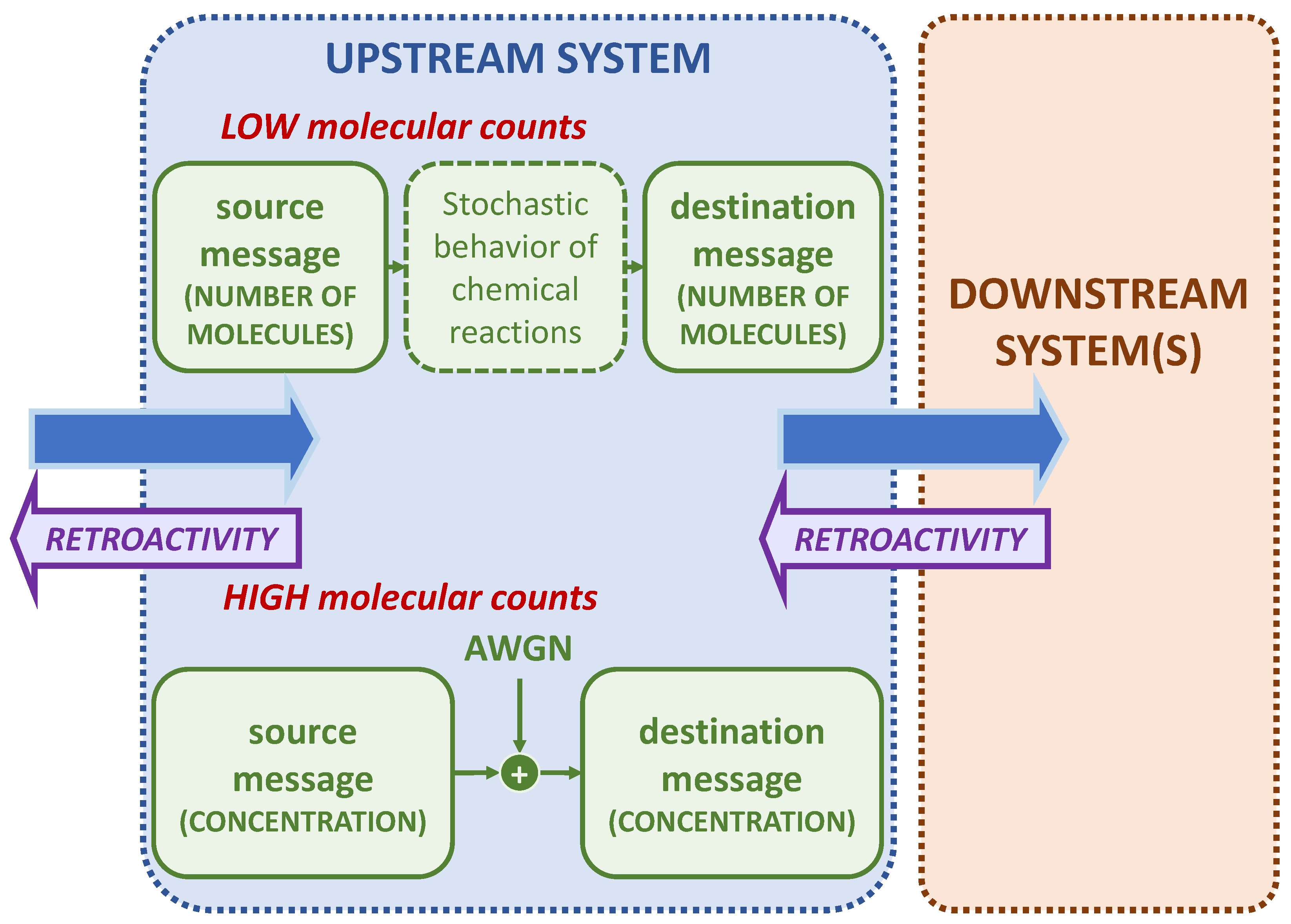
3.1. Retroactivity
3.2. Stochastic Models for Biochemical Systems
3.2.1. The Chemical Master Equation
3.2.2. The Linear Noise Approximation
4. System Model
5. Results: Communication Performance Evaluation
5.1. Low Molecular Counts
5.2. High Molecular Counts
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| System Model | State | E | D | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Isolated SISO | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | |
| SISO + downst. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | |
| Isolated MIMO | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | |
| 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | ||
| 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| MIMO with 1 downstream target | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | |
| 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | ||
| 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | ||
| Two isolated SISO with MAC | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | |
| 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | ||
| 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | ||
| 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| System Model | State | E | D | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Isolated SISO | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | |
| 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | ||
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| SISO with 1 downstream target | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | |
| 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | ||
| 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | ||
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | ||
| Isolated MIMO | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | |
| 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | ||
| 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | ||
| 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | ||
| 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | ||
| 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| MIMO with 1 downstream target | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | |
| 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | ||
| 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | ||
| 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | ||
| 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | ||
| 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | ||
| 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | ||
| 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | ||
| 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | ||
| 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | ||
| Two isolated SISO with MAC | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | |
| 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | ||
| 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | ||
| 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | ||
| 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | ||
| 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | ||
| 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | ||
| 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | ||
| 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | ||
| 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | ||
| 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | ||
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | ||
| 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | ||
| 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | ||
| 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |

References
- Akyildiz, I.F.; Brunetti, F.; Blázquez, C. Nanonetworks: A new communication paradigm. Comput. Netw. 2008, 52, 2260–2279. [Google Scholar] [CrossRef]
- Akan, O.B.; Ramezani, H.; Khan, T.; Abbasi, N.A.; Kuscu, M. Fundamentals of molecular information and communication science. Proc. IEEE 2017, 105, 306–318. [Google Scholar] [CrossRef] [Green Version]
- Forney, G.D.; Ungerboeck, G. Modulation and coding for linear Gaussian channels. IEEE Trans. Inf. Theory 1998, 44, 2384–2415. [Google Scholar] [CrossRef]
- Tuccitto, N.; Li-Destri, G.; Messina, G.M.L.; Marletta, G. Reactive messengers for digital molecular communication with variable transmitter–receiver distance. Phys. Chem. Chem. Phys. 2018, 20, 30312–30320. [Google Scholar] [CrossRef]
- Giannoukos, S.; McGuiness, D.T.; Marshall, A.; Smith, J.; Taylor, S. A chemical alphabet for macromolecular communications. Anal. Chem. 2018, 90, 7739–7746. [Google Scholar] [CrossRef]
- Llopis-Lorente, A.; Díez, P.; Sánchez, A.; Marcos, M.D.; Sancenón, F.; Martínez-Ruiz, P.; Villalonga, R.; Martínez-Máñez, R. Interactive models of communication at the nanoscale using nanoparticles that talk to one another. Nat. Commun. 2017, 8, 15511. [Google Scholar] [CrossRef] [Green Version]
- Wada, T.; Hironaka, K.I.; Kuroda, S. Cell-to-cell variability serves as information not noise. Curr. Opin. Syst. Biol. 2021, 27, 100339. [Google Scholar] [CrossRef]
- Gresho, P.M. Advection-diffusion. In Von Karman Inst. of Fluid Dynamics Computational Fluid Dynamics; Brussels CEST: Brussels, Belgium, 1985. [Google Scholar]
- Chang, R. Physical Chemistry for the Biosciences; University Science Books: Sausalito, CA, USA, 2005. [Google Scholar]
- Hundsdorfer, W.; Verwer, J.G. Numerical Solution of Time-Dependent Advection-Diffusion-Reaction Equations; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; Volume 33. [Google Scholar]
- Llatser, I.; Cabellos-Aparicio, A.; Pierobon, M.; Alarcón, E. Detection techniques for diffusion-based molecular communication. IEEE J. Sel. Areas Commun. 2013, 31, 726–734. [Google Scholar] [CrossRef]
- Mosayebi, R.; Arjmandi, H.; Gohari, A.; Nasiri-Kenari, M.; Mitra, U. Receivers for diffusion-based molecular communication: Exploiting memory and sampling rate. IEEE J. Sel. Areas Commun. 2014, 32, 2368–2380. [Google Scholar] [CrossRef] [Green Version]
- Ruhi, N.A.; Bogdan, P. Multiscale modeling of biological communication. In Proceedings of the 2015 IEEE International Conference on Communications (ICC), London, UK, 8–12 June 2015; pp. 1140–1145. [Google Scholar]
- Abbaszadeh, M.; Huang, Y.; Thomas, P.J.; Wen, M.; Ji, F.; Guo, W. Kolmogorov Turbulence and Information Dissipation in Molecular Communication. IEEE Trans. Mol. Biol. Multi-Scale Commun. 2021, 7, 262–270. [Google Scholar] [CrossRef]
- Koo, B.H.; Lee, C.; Pusane, A.E.; Tugcu, T.; Chae, C.B. MIMO Operations in Molecular Communications: Theory, Prototypes, and Open Challenges. IEEE Commun. Mag. 2021, 59, 98–104. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, K. Channel capacity analysis of a diffusion-based molecular communication system with ligand receptors. Int. J. Commun. Syst. 2015, 28, 1508–1520. [Google Scholar] [CrossRef]
- Pierobon, M.; Akyildiz, I.F. Capacity of a diffusion-based molecular communication system with channel memory and molecular noise. IEEE Trans. Inf. Theory 2013, 59, 942–954. [Google Scholar] [CrossRef]
- Awan, H.; Chou, C.T. Improving the capacity of molecular communication using enzymatic reaction cycles. IEEE Trans. Nanobiosci. 2017, 16, 744–754. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhu, Y.; Chi, K.; Li, Y.; Xia, M. Capacity analysis for diffusive molecular communication with ISI channel. Nano Commun. Netw. 2017, 13, 43–50. [Google Scholar] [CrossRef]
- Lu, Y.; Higgins, M.D.; Noel, A.; Leeson, M.S.; Chen, Y. The effect of two receivers on broadcast molecular communication systems. IEEE Trans. Nanobiosci. 2016, 15, 891–900. [Google Scholar] [CrossRef]
- Atakan, B.; Akan, O.B. On molecular multiple-access, broadcast, and relay channels in nanonetworks. In Proceedings of the 3rd International Conference on Bio-Inspired Models of Network, Information and Computing Systems, Hyogo, Japan, 25–28 November 2008; pp. 1–8. [Google Scholar]
- Liu, Q.; Yang, K. Multiple-access channel capacity of diffusion and ligand-based molecular communication. In Proceedings of the 16th ACM International Conference on Modeling, Analysis & Simulation of Wireless and Mobile Systems, Barcelona, Spain, 3–8 November 2013; pp. 151–158. [Google Scholar]
- Rouzegar, S.M.; Spagnolini, U. Channel estimation for diffusive MIMO molecular communications. In Proceedings of the 2017 European Conference on Networks and Communications (EuCNC), Oulu, Finland, 12–15 June 2017; pp. 1–5. [Google Scholar]
- Koo, B.H.; Lee, C.; Yilmaz, H.B.; Farsad, N.; Eckford, A.; Chae, C.B. Molecular MIMO: From theory to prototype. IEEE J. Sel. Areas Commun. 2016, 34, 600–614. [Google Scholar] [CrossRef] [Green Version]
- Meng, L.S.; Yeh, P.C.; Chen, K.C.; Akyildiz, I.F. MIMO communications based on molecular diffusion. In Proceedings of the 2012 IEEE Global Communications Conference (GLOBECOM), Anaheim, CA, USA, 3–7 December 2012; pp. 5380–5385. [Google Scholar]
- Del Vecchio, D.; Ninfa, A.J.; Sontag, E.D. Modular cell biology: Retroactivity and insulation. Mol. Syst. Biol. 2008, 4, 161. [Google Scholar] [CrossRef]
- Saez-Rodriguez, J.; Gayer, S.; Ginkel, M.; Gilles, E.D. Automatic decomposition of kinetic models of signaling networks minimizing the retroactivity among modules. Bioinformatics 2008, 24, i213–i219. [Google Scholar] [CrossRef]
- Del Vecchio, D.; Sontag, E.D. Engineering principles in bio-molecular systems: From retroactivity to modularity. In Proceedings of the 2009 European Control Conference (ECC), Budapest, Hungary, 23–26 August 2009; pp. 658–664. [Google Scholar]
- Jayanthi, S.; Del Vecchio, D. Retroactivity attenuation in bio-molecular systems based on timescale separation. IEEE Trans. Autom. Control 2010, 56, 748–761. [Google Scholar] [CrossRef] [Green Version]
- Ossareh, H.R.; Ventura, A.C.; Merajver, S.D.; Del Vecchio, D. Long signaling cascades tend to attenuate retroactivity. Biophys. J. 2011, 100, 1617–1626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, J.; Chang, Y.C.; Papachristodoulou, A. Model decomposition and reduction tools for large-scale networks in systems biology. Automatica 2011, 47, 1165–1174. [Google Scholar] [CrossRef]
- Del Vecchio, D. A control theoretic framework for modular analysis and design of biomolecular networks. Annu. Rev. Control 2013, 37, 333–345. [Google Scholar] [CrossRef] [Green Version]
- Sivakumar, H.; Hespanha, J.P. Towards modularity in biological networks while avoiding retroactivity. In Proceedings of the 2013 American Control Conference, Washington, DC, USA, 17–19 June 2013; pp. 4550–4556. [Google Scholar]
- Pantoja-Hernández, L.; Martínez-García, J.C. Retroactivity in the context of modularly structured biomolecular systems. Front. Bioeng. Biotechnol. 2015, 3, 85. [Google Scholar] [CrossRef] [Green Version]
- Bhaskaran, S.; Nair, A.S. Effect of retroactivity in the dynamics of mixed feedback loop. In Proceedings of the 2016 International Conference on Emerging Technological Trends (ICETT), Kollam, India, 21–22 October 2016; pp. 1–6. [Google Scholar]
- Awan, H. Effect of retroactivity on the performance of molecular communication networks. In Proceedings of the 2016 Australian Communications Theory Workshop (AusCTW), Melbourne, Australia, 20–22 January 2016; pp. 35–40. [Google Scholar]
- McBride, C.; Shah, R.; Del Vecchio, D. The effect of loads in molecular communications. Proc. IEEE 2019, 107, 1369–1386. [Google Scholar] [CrossRef]
- Ratti, F.; Magarini, M.; Del Vecchio, D. The impact of retroactivity on information exchange in molecular communications. In Proceedings of the 7th ACM International Conference on Nanoscale Computing and Communication, Virtual Conference, 23–25 September 2020; pp. 1–2. [Google Scholar]
- Chahibi, Y. Molecular communication for drug delivery systems: A survey. Nano Commun. Netw. 2017, 11, 90–102. [Google Scholar] [CrossRef]
- Veletić, M.; Barros, M.T.; Balasingham, I.; Balasubramaniam, S. A molecular communication model of exosome-mediated brain drug delivery. In Proceedings of the 6th Annual ACM International Conference on Nanoscale Computing and Communication, Dublin, Ireland, 25–27 September 2019; pp. 1–7. [Google Scholar]
- Van Bueren, E.L.; Backes, G.; De Vriend, H.; Østergård, H. The role of molecular markers and marker assisted selection in breeding for organic agriculture. Euphytica 2010, 175, 51–64. [Google Scholar] [CrossRef] [Green Version]
- Farsad, N.; Pan, D.; Goldsmith, A. A novel experimental platform for in-vessel multi-chemical molecular communications. In Proceedings of the GLOBECOM 2017–2017 IEEE Global Communications Conference, Singapore, 4–8 December 2017; pp. 1–6. [Google Scholar]
- Kim, Y.A.; Przytycki, J.H.; Wuchty, S.; Przytycka, T.M. Modeling information flow in biological networks. Phys. Biol. 2011, 8, 035012. [Google Scholar] [CrossRef] [Green Version]
- Kadloor, S.; Adve, R.S.; Eckford, A.W. Molecular communication using Brownian motion with drift. IEEE Trans. Nanobiosci. 2012, 11, 89–99. [Google Scholar] [CrossRef] [Green Version]
- Alon, U. An introduction to Systems Biology: Design Principles of Biological Circuits; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Grunberg, T.W.; Del Vecchio, D. Modular analysis and design of biological circuits. Curr. Opin. Biotechnol. 2020, 63, 41–47. [Google Scholar] [CrossRef]
- Del Vecchio, D.; Murray, R.M. Biomolecular Feedback Systems; Princeton University Press: Princeton, NJ, USA, 2015. [Google Scholar]
- Van Kampen, N.G. Stochastic Processes in Physics and Chemistry; Elsevier: Amsterdam, The Netherlands, 1992; Volume 1. [Google Scholar]
- Cover, T.M. Elements of Information Theory; John Wiley & Sons: Hoboken, NJ, USA, 1999. [Google Scholar]
- Hougen, O.A.; Watson, K.M. Chemical Process Principles; John Wiley & Sons: Hoboken, NJ, USA, 1943; Volume 1. [Google Scholar]
- Gillespie, D.T. Exact stochastic simulation of coupled chemical reactions. J. Phys. Chem. 1977, 81, 2340–2361. [Google Scholar] [CrossRef]
- Risken, H. Fokker-Planck equation. In The Fokker–Planck Equation; Springer: Berlin/Heidelberg, Germany, 1996; pp. 63–95. [Google Scholar]
- Gillespie, D.T. The chemical Langevin equation. J. Chem. Phys. 2000, 113, 297–306. [Google Scholar] [CrossRef]
- Montgomery, D.C.; Runger, G.C. Applied Statistics and Probability for Engineers; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar]
- Kuran, M.S.; Yilmaz, H.B.; Tugcu, T.; Akyildiz, I.F. Modulation techniques for communication via diffusion in nanonetworks. In Proceedings of the 2011 IEEE International Conference on Communications (ICC), Kyoto, Japan, 5–9 June 2011; pp. 1–5. [Google Scholar]
- MacKay, D.J. Information Theory, Inference and Learning Algorithms; Cambridge University Press: Cambridge, MA, USA, 2003. [Google Scholar]
- Csiszar, I.; Körner, J. Information Theory: Coding Theorems for Discrete Memoryless Systems; Cambridge University Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Koorehdavoudi, H.; Bogdan, P.; Wei, G.; Marculescu, R.; Zhuang, J.; Carlsen, R.W.; Sitti, M. Multi-fractal characterization of bacterial swimming dynamics: A case study on real and simulated serratia marcescens. Proc. R. Soc. Math. Phys. Eng. Sci. 2017, 473, 20170154. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ratti, F.; Magarini, M.; Del Vecchio, D. What Is the Trait d’Union between Retroactivity and Molecular Communication Performance Limits? Molecules 2022, 27, 3130. https://doi.org/10.3390/molecules27103130
Ratti F, Magarini M, Del Vecchio D. What Is the Trait d’Union between Retroactivity and Molecular Communication Performance Limits? Molecules. 2022; 27(10):3130. https://doi.org/10.3390/molecules27103130
Chicago/Turabian StyleRatti, Francesca, Maurizio Magarini, and Domitilla Del Vecchio. 2022. "What Is the Trait d’Union between Retroactivity and Molecular Communication Performance Limits?" Molecules 27, no. 10: 3130. https://doi.org/10.3390/molecules27103130







