Biotransformation of Phytosterols into Androstenedione—A Technological Prospecting Study
Abstract
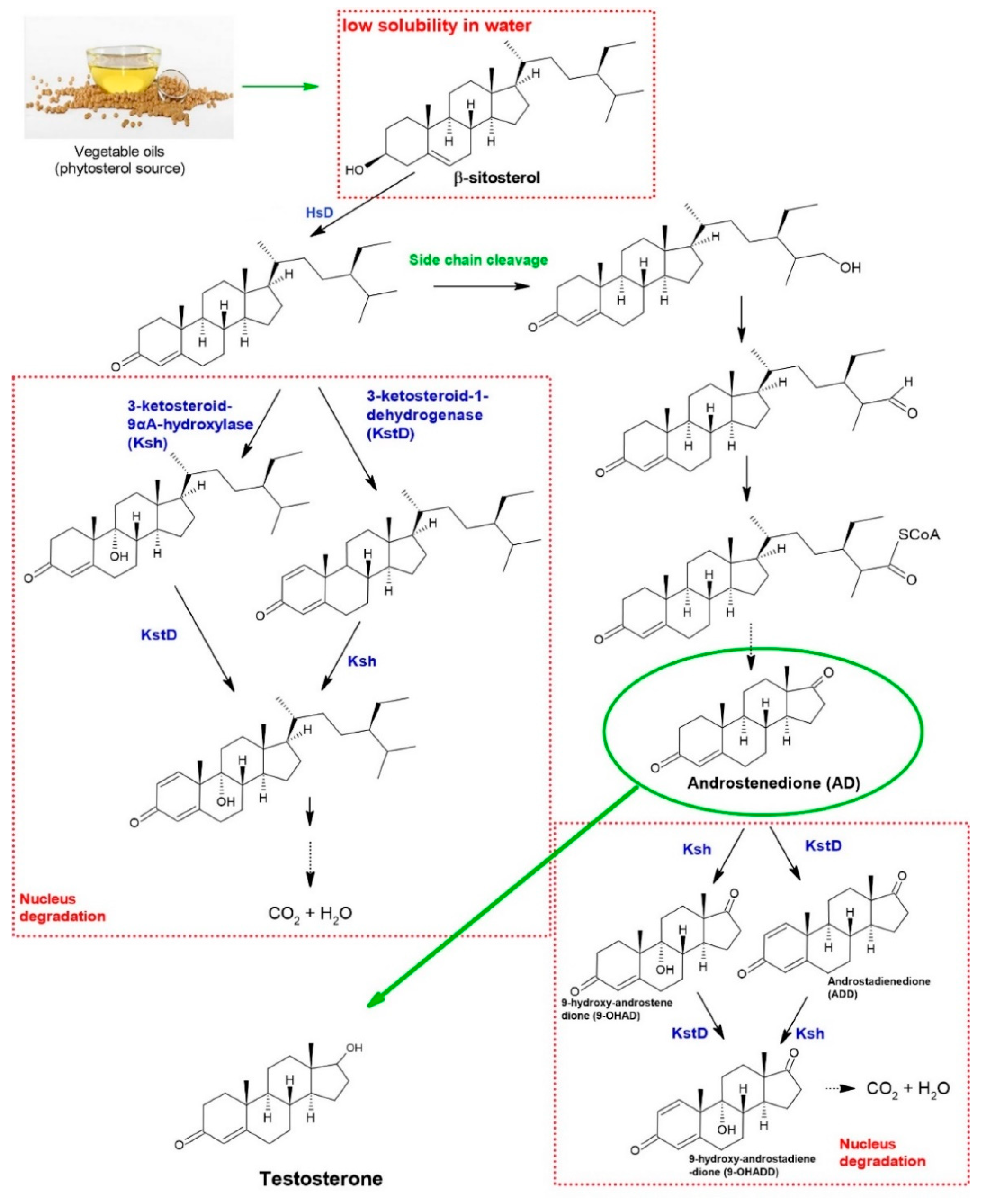
:1. Introduction
2. Materials and Methods
2.1. Systematic Search of Articles
2.2. Systematic Search of Patents
3. Results
3.1. Articles’ General Aspects—Macro Analysis
3.2. Categorizing the Articles by Groups—Meso Analysis
3.2.1. Scientific Articles Categorized as “Microorganism”—Micro Analysis
3.2.2. Scientific Articles Categorized as “Process Improvement”—Micro Analysis
3.2.3. Scientific Articles Categorized as “Metabolic Intermediates”—Micro Analysis
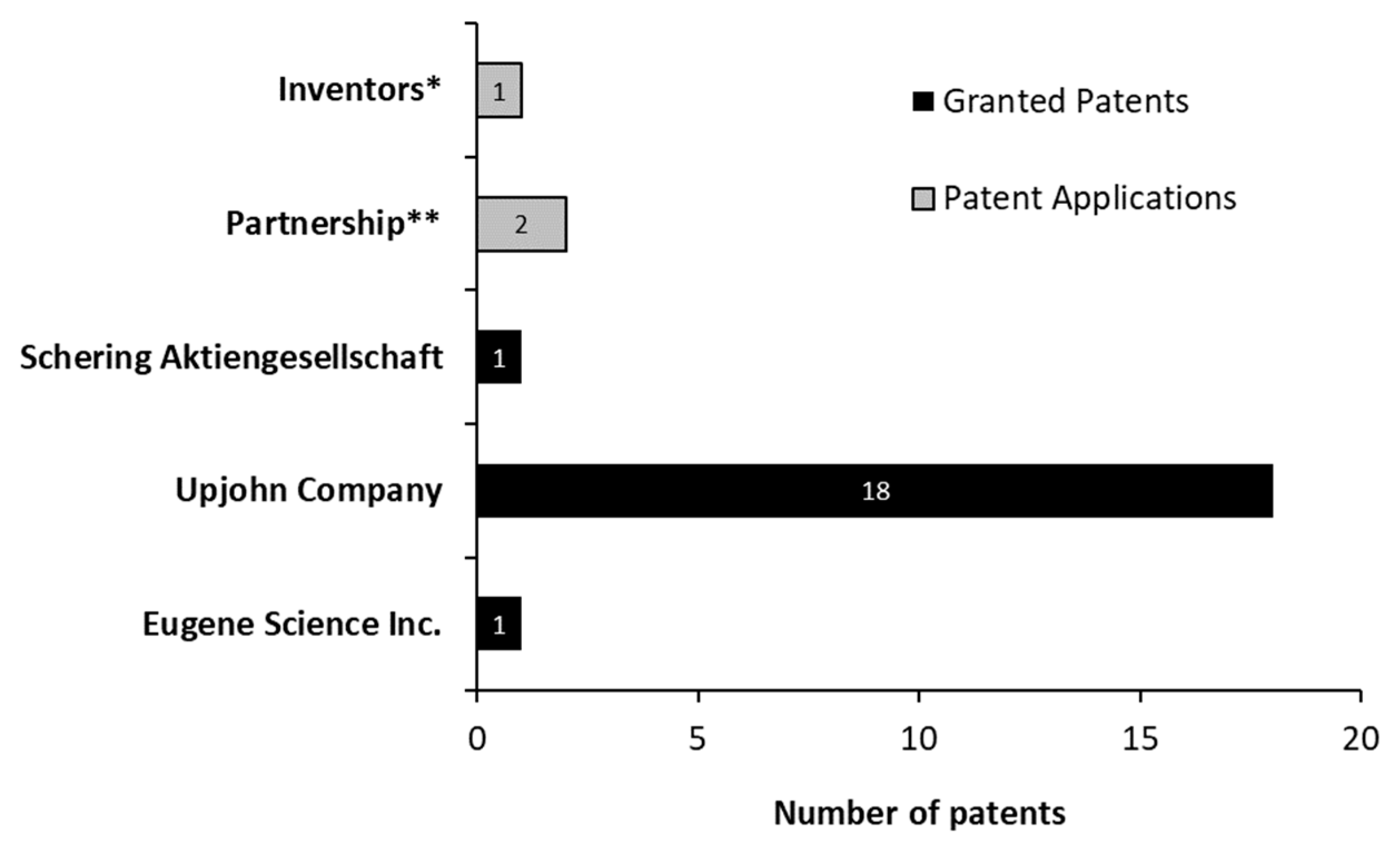
3.3. Patents General Aspects—Macro Analysis
3.4. Categorizing the Patents by Groups—Meso Analysis
3.5. Granted Patents Categorized as “Technology/Routes”—Micro Analysis
4. Challenges, Opportunities, and Development Efforts
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Rokade, R.; Ravindran, S.; Singh, P.; Suthar, J.K. Microbial Biotransformation for the Production of Steroid Medicament. In Secondary Metabolites-Sources and Applications; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Kreit, J. Microbial Catabolism of Sterols: Focus on the Enzymes That Transform the Sterol 3ß-Hydroxy-5-En into 3-Keto-4-En. FEMS Microbiol. Lett. 2017, 364, fnx007. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.-Q.; Yao, K.; Wei, D.-Z. From Soybean Phytosterols to Steroid Hormones. In Soybean and Health; IntechOpen: London, UK, 2011. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.L.; Chen, Y.R.; Liu, W.H. Production of Androstenones from Phytosterol by Mutants of Mycobacterium sp. Enzym. Microb. Technol. 2006, 39, 296–300. [Google Scholar] [CrossRef]
- Malaviya, A.; Gomes, J. Androstenedione Production by Biotransformation of Phytosterols. Bioresour. Technol. 2008, 99, 6725–6737. [Google Scholar] [CrossRef] [PubMed]
- Galán, B.; Uhía, I.; García-Fernández, E.; Martínez, I.; Bahíllo, E.; de la Fuente, J.L.; Barredo, J.L.; Fernández-Cabezón, L.; García, J.L. Mycobacterium smegmatis Is a Suitable Cell Factory for the Production of Steroidic Synthons. Microb. Biotechnol. 2017, 10, 138–150. [Google Scholar] [CrossRef] [Green Version]
- Borschiver, S.; da Silva, A.L.R. Technology Roadmap–Planejamento Estratégico Para Alinhar Mercado-Produto-Tecnologia. In Interciência; Editora Interciência: Rio de janeiro, Brazil, 2016. [Google Scholar]
- Silva, R.G.C.; Ferreira, T.F.; Borges, É.R. Identification of Potential Technologies for 1, 4-Butanediol Production Using Prospecting Methodology. J. Chem. Technol. Biotechnol. 2020, 95, 3057–3070. [Google Scholar] [CrossRef]
- Zhao, A.; Zhang, X.; Li, Y.; Wang, Z.; Lv, Y.; Liu, J.; Alam, M.A.; Xiong, W.; Xu, J. Mycolicibacterium Cell Factory for the Production of Steroid-Based Drug Intermediates. Biotechnol. Adv. 2021, 53, 107860. [Google Scholar] [CrossRef]
- Shao, M.; Zhang, X.; Rao, Z.; Xu, M.; Yang, T.; Li, H.; Xu, Z.; Yang, S. A Mutant Form of 3-Ketosteroid-Δ1-Dehydrogenase Gives Altered Androst-1,4-Diene-3, 17-Dione/Androst-4-Ene-3,17-Dione Molar Ratios in Steroid Biotransformations by Mycobacterium neoaurum ST-095. J. Ind. Microbiol. Biotechnol. 2016, 43, 691–701. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, D.; Wang, X.; Wei, D. A Recycled Batch Biotransformation Strategy for 22-Hydroxy-23,24-Bisnorchol-4-Ene-3-One Production from High Concentration of Phytosterols by Mycobacterial Resting Cells. Biotechnol. Lett. 2020, 42, 2589–2594. [Google Scholar] [CrossRef]
- Wu, C.; Xu, J.; Xie, J.; Wang, Z. A Streamlined High Throughput Screening Method for the Mycobacterium neoaurum Mutants with Expected Yield of Biotransformation Derivatives from Sterols. Chin. Chem. Lett. 2018, 29, 1251–1253. [Google Scholar] [CrossRef]
- Chang, H.; Zhang, H.; Zhu, L.; Zhang, W.; You, S.; Qi, W.; Qian, J.; Su, R.; He, Z. A Combined Strategy of Metabolic Pathway Regulation and Two-Step Bioprocess for Improved 4-Androstene-3,17-Dione Production with an Engineered Mycobacterium neoaurum. Biochem. Eng. J. 2020, 164, 107789. [Google Scholar] [CrossRef]
- Tang, R.; Shen, Y.; Xia, M.; Tu, L.; Luo, J.; Geng, Y.; Gao, T.; Zhou, H.; Zhao, Y.; Wang, M. A Highly Efficient Step-Wise Biotransformation Strategy for Direct Conversion of Phytosterol to Boldenone. Bioresour. Technol. 2019, 283, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Chen, G.; Cheng, S.; Ge, F.; Qiong, W.; Li, W.; Li, J. Accumulation of 9α-Hydroxy-4-Androstene-3,17-Dione by Co-Expressing KshA and KshB Encoding Component of 3-Ketosteroid-9α-Hydroxylase in Mycobacterium sp. NRRL B-3805. Chin. J. Biotechnol. 2015, 31, 523–533. [Google Scholar]
- García-Fernández, J.; Martínez, I.; Fernández-Cabezón, L.; Felpeto-Santero, C.; García, J.-L.; Galán, B. Bioconversion of Phytosterols into Androstadienedione by Mycobacterium smegmatis CECT 8331. In Methods in Molecular Biology; Barredo, J.L., Herráiz, I., Eds.; Humana Press: New York, NY, USA, 2017; Volume 1645, pp. 211–225. [Google Scholar]
- Josefsen, K.D.; Nordborg, A.; Sletta, H. Bioconversion of Phytosterols into Androstenedione by Mycobacterium. Methods Mol. Biol. 2017, 1645, 177–197. [Google Scholar] [CrossRef] [PubMed]
- Gulla, V.; Banerjee, T.; Patil, S. Bioconversion of Soysterols to Androstenedione by Mycobacterium fortuitum subsp. fortuitum NCIM 5239, a Mutant Derived from Total Sterol Degrader Strain. J. Chem. Technol. Biotechnol. 2010, 85, 1135–1141. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, F.; Chen, D.; Li, D. Biotransformation of Phytosterol to Produce Androsta-Diene-Dione by Resting Cells of Mycobacterium in Cloud Point System. Process Biochem. 2006, 41, 557–561. [Google Scholar] [CrossRef]
- Sarangthem, K.; Singh, T.N. Biosynthesis of Succulent Bamboo Shoots of Bambusa Balcooa into Phytosterols and Its Biotransformation into ADD. J. Integr. Plant Biol. 2003, 45, 114–117. [Google Scholar]
- Liu, X.; Zhang, R.; Bao, Z.; Yuan, C.; Cao, H.; Shi, J.; Sun, J.; Zhang, B. Biotransformation of Phytosterols to Androst-1,4-Diene-3,17-Dione by Mycobacterium sp. ZFZ Expressing 3-Ketosteroid-Δ1-Dehydrogenase. Catalysts 2020, 10, 663. [Google Scholar] [CrossRef]
- Sharma, P.; Slathia, P.S.; Somal, P.; Mehta, P. Biotransformation of Cholesterol to 1,4-Androstadiene-3,17-Dione (ADD) by Nocardia Species. Ann. Microbiol. 2012, 62, 1651–1659. [Google Scholar] [CrossRef]
- Stefanov, S.; Yankov, D.; Beschkov, V. Biotransformation of Phytosterols to Androstenedione in Two Phase Water-Oil Systems. Chem. Biochem. Eng. Q. 2006, 20, 421–427. [Google Scholar]
- Sripalakit, P.; Wichai, U.; Saraphanchotiwitthaya, A. Biotransformation of Various Natural Sterols to Androstenones by Mycobacterium Sp. and Some Steroid-Converting Microbial Strains. J. Mol. Catal. B Enzym. 2006, 41, 49–54. [Google Scholar] [CrossRef]
- Ahire, J.J.; Bhat, A.A.; Thakare, J.M.; Pawar, P.B.; Zope, D.G.; Jain, R.M.; Chaudhari, B.L. Cholesterol Assimilation and Biotransformation by Lactobacillus helveticus. Biotechnol. Lett. 2012, 34, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, P.N.; Chaudhari, B.L.; Chincholkar, S.B. Cholesterol Biotransformation to Androsta-1,4-Diene-3,17-Dione by Growing Cells of Chryseobacterium gleum. Biotechnol. Lett. 2010, 32, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhao, F.; Chen, D.; Li, D. Cloud Point System as a Tool to Improve the Efficiency of Biotransformation. Enzym. Microb. Technol. 2005, 36, 589–594. [Google Scholar] [CrossRef]
- Su, L.; Shen, Y.; Zhang, W.; Gao, T.; Shang, Z.; Wang, M. Cofactor Engineering to Regulate NAD+/NADH Ratio with Its Application to Phytosterols Biotransformation. Microb. Cell Fact. 2017, 16, 182. [Google Scholar] [CrossRef] [PubMed]
- Bragin, E.Y.; Shtratnikova, V.Y.; Dovbnya, D.V.; Schelkunov, M.I.; Pekov, Y.A.; Malakho, S.G.; Egorova, O.V.; Ivashina, T.V.; Sokolov, S.L.; Ashapkin, V.V.; et al. Comparative Analysis of Genes Encoding Key Steroid Core Oxidation Enzymes in Fast-Growing Mycobacterium spp. Strains. J. Steroid Biochem. Mol. Biol. 2013, 138, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yang, H.; Kuang, M.; Tu, Z.; Wang, X. Comparative Genomic Analysis of Mycobacterium neoaurum MN2 and MN4 Substrate and Product Tolerance. 3 Biotech 2017, 7, 181. [Google Scholar] [CrossRef]
- Rodríguez-García, A.; Fernández-Alegre, E.; Morales, A.; Sola-Landa, A.; Lorraine, J.; Macdonald, S.; Dovbnya, D.; Smith, M.C.M.; Donova, M.; Barreiro, C. Complete Genome Sequence of ‘Mycobacterium neoaurum’ NRRL B-3805, an Androstenedione (AD) Producer for Industrial Biotransformation of Sterols. J. Biotechnol. 2016, 224, 64–65. [Google Scholar] [CrossRef]
- Shtratnikova, V.Y.; Schelkunov, M.I.; Dovbnya, D.V.; Pekov, Y.A.; Bragin, E.Y.; Ashapkin, V.V.; Donova, M.V. Complete Genome Sequence of Mycobacterium sp. Strain VKM Ac-1817D, Capable of Producing 9α-Hydroxy-Androst-4-Ene-3,17-Dione from Phytosterol. Genome Announc. 2015, 3, e01447-14. [Google Scholar] [CrossRef] [Green Version]
- Andryushina, V.A.; Rodina, N.V.; Stytsenko, T.S.; Huy, L.D.; Druzhinina, A.V.; Yaderetz, V.V.; Voishvillo, N.E. Conversion of Soybean Sterols into 3,17-Diketosteroids Using Actinobacteria Mycobacterium neoaurum, Pimelobacter simplex, and Rhodococcus erythropolis. Appl. Biochem. Microbiol. 2011, 47, 270–273. [Google Scholar] [CrossRef]
- Luo, B.H. Conversion of Sterols into Androst-4-Ene-3,17-Dione (AD) by Fermentation of Mycobacterium sp. Immobilized on Diatomite in Organic-Aqueous System. Food Sci. 2009. Available online: https://www.semanticscholar.org/paper/Conversion-of-Sterols-into-Androst-4-ene-3%2C17-dione-Bing-hua/3defd6168244b12fb0faa3a820e3abcac7ccab0e (accessed on 6 January 2022).
- Wang, H.; Yang, F.; Cheng, X.; Huang, Y.; Su, Z. Crystal Structure and Characterization of 3-Ketosteroid-Δ1-Dehydrogenase from Mycobacterium Strain HGMS2GL. Biophys. J. 2018, 114, 583a. [Google Scholar] [CrossRef]
- Li, Y.; Zengxia, L.; Qian, C.; Tao, X.; Jiping, S. Degradation of beta-Sitosterol to Androst-1, 4-Diene-3,17-Dione by Mycobacterium sp. Acta Acad. Med. Shanghai 2002, 29, 280–283. [Google Scholar]
- Yang, Y.; Yang, S.; Wu, Z. Development of 9α-Hydroxy-Androst-4-Ene-3,17-Dione (9α-OH-AD) through Cleaving Sterol Sidechain by Fermentation of Mycobacterium fortuitum. Chin. J. Appl. Environ. Biol. 2015, 21, 256–262. [Google Scholar] [CrossRef]
- Xiao, X.; He, J.-K.; Guan, Y.-X.; Yao, S.-J. Effect of Cholinium Amino Acids Ionic Liquids As Cosolvents on the Bioconversion of Phytosterols by Mycobacterium sp. Resting Cells. ACS Sustain. Chem. Eng. 2020, 8, 17124–17132. [Google Scholar] [CrossRef]
- Shtratnikova, V.Y.; Schelkunov, M.I.; Dovbnya, D.V.; Bragin, E.Y.; Donova, M.V. Effect of Methyl-β-Cyclodextrin on Gene Expression in Microbial Conversion of Phytosterol. Appl. Microbiol. Biotechnol. 2017, 101, 4659–4667. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Li, H.; Xu, Y.; Liu, W.; Zhang, X.; Gong, J.; Xu, Z.; Shi, J. Effects of a Nonionic Surfactant TX-40 on 9α-Hydroxyandrost-4-Ene-3,17-Dione Biosynthesis and Physiological Properties of Mycobacterium sp. LY-1. Process Biochem. 2019, 87, 89–94. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, G.; Ge, F.; Li, W.; Zeng, L.; Cao, W. Efficient Biotransformation of Cholesterol to Androsta-1,4-Diene-3,17-Dione by a Newly Isolated Actinomycete Gordonia neofelifaecis. World J. Microbiol. Biotechnol. 2011, 27, 759–765. [Google Scholar] [CrossRef]
- Zhou, P.; Fang, Y.; Yao, H.; Li, H.; Wang, G.; Liu, Y. Efficient Biotransformation of Phytosterols to Dehydroepiandrosterone by Mycobacterium sp. Appl. Biochem. Biotechnol. 2018, 186, 496–506. [Google Scholar] [CrossRef]
- Li, X.; Chen, T.; Peng, F.; Song, S.; Yu, J.; Sidoine, D.N.; Cheng, X.; Huang, Y.; He, Y.; Su, Z. Efficient Conversion of Phytosterols into 4-Androstene-3,17-Dione and Its C1,2-Dehydrogenized and 9α-Hydroxylated Derivatives by Engineered Mycobacteria. Microb. Cell Fact. 2021, 20, 158. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Y.; Shen, Y.; Zhang, X.; Xu, S.; Shang, Z.; Xia, M.; Wang, M. Efficient Production of Androstenedione by Repeated Batch Fermentation in Waste Cooking Oil Media through Regulating NAD+/NADH Ratio and Strengthening Cell Vitality of Mycobacterium neoaurum. Bioresour. Technol. 2019, 279, 209–217. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Y.; Shen, Y.; Zhang, X.; Zan, Z.; Xia, M.; Luo, J.; Wang, M. Efficient Repeated Batch Production of Androstenedione Using Untreated Cane Molasses by Mycobacterium neoaurum Driven by ATP Futile Cycle. Bioresour. Technol. 2020, 309, 123307. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-H.; Xu, L.-Q.; Yao, K.; Xiong, L.-B.; Tao, X.-Y.; Liu, M.; Wang, F.-Q.; Wei, D.-Z. Engineered 3-Ketosteroid 9α-Hydroxylases in Mycobacterium neoaurum: An Efficient Platform for Production of Steroid Drugs. Appl. Environ. Microbiol. 2018, 84, e02777-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Cabezón, L.; Galán, B.; García, J.L. Engineering Mycobacterium smegmatis for Testosterone Production. Microb. Biotechnol. 2017, 10, 151–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pendharkar, G.B.; Anjum, S.D.; Patil, S. Enhanced Biotransformation of Phytosterols, a Byproduct of Soybean Refineries, to a Key Intermediate Used for Synthesis of Steroidal Drugs. Asian J. Pharm. Clin. Res. 2014, 7, 178–180. [Google Scholar]
- Malaviya, A.; Gomes, J. Enhanced Biotransformation of Sitosterol to Androstenedione by Mycobacterium sp. Using Cell Wall Permeabilizing Antibiotics. J. Ind. Microbiol. Biotechnol. 2008, 35, 1235–1239. [Google Scholar] [CrossRef]
- Shao, M.; Zhang, X.; Rao, Z.; Xu, M.; Yang, T.; Li, H.; Xu, Z. Enhanced Production of Androst-1,4-Diene-3,17-Dione by Mycobacterium neoaurum JC-12 Using Three-Stage Fermentation Strategy. PLoS ONE 2015, 10, e0137658. [Google Scholar] [CrossRef]
- Sun, H.; Yang, J.; He, K.; Wang, Y.-P.; Song, H. Enhancing Production of 9α-Hydroxy-Androst-4-Ene-3,17-Dione (9-OHAD) from Phytosterols by Metabolic Pathway Engineering of Mycobacteria. Chem. Eng. Sci. 2021, 230, 116195. [Google Scholar] [CrossRef]
- Yuan, J.-J.; Guan, Y.-X.; Yao, S.-J. Evaluation of Biocompatible Ionic Liquids for Their Application in Phytosterols Bioconversion by Mycobacterium sp. Resting Cells. ACS Sustain. Chem. Eng. 2017, 5, 10702–10709. [Google Scholar] [CrossRef]
- Li, Y.; Lu, F.; Sun, T.; Du, L. Expression of KsdD Gene Encoding 3-Ketosteroid-Δ 1 -Dehydrogenase from Arthrobacter Simplex in Bacillus subtilis. Lett. Appl. Microbiol. 2007, 44, 563–568. [Google Scholar] [CrossRef]
- Feng, L.H.; Pan, L.J.; Jiang, S.T.; Yang, Y.; Luo, B.H. Fermentation of Immobilized Mycobacterium sp.BD 696-6 for Production of Androst-4-Ene-3,17-Dione from Rapeseed Sterols. Food Sci. 2009, 30, 172–175. [Google Scholar]
- Xie, R.; Shen, Y.; Qin, N.; Wang, Y.; Su, L.; Wang, M. Genetic Differences in KsdD Influence on the ADD/AD Ratio of Mycobacterium neoaurum. J. Ind. Microbiol. Biotechnol. 2015, 42, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Wang, F.-Q.; Zhang, H.-C.; Wei, D.-Z. Identification and Engineering of Cholesterol Oxidases Involved in the Initial Step of Sterols Catabolism in Mycobacterium neoaurum. Metab. Eng. 2013, 15, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Zhang, X.; Rao, Z.; Xu, M.; Yang, T.; Xu, Z.; Yang, S. Identification of Steroid C27 Monooxygenase Isoenzymes Involved in Sterol Catabolism and Stepwise Pathway Engineering of Mycobacterium neoaurum for Improved Androst-1,4-Diene-3,17-Dione Production. J. Ind. Microbiol. Biotechnol. 2019, 46, 635–647. [Google Scholar] [CrossRef] [PubMed]
- Claudino, M.J.C.; Soares, D.; van Keulen, F.; Marques, M.P.C.; Cabral, J.M.S.; Fernandes, P. Immobilization of Mycobacterial Cells onto Silicone—Assessing the Feasibility of the Immobilized Biocatalyst in the Production of Androstenedione from Sitosterol. Bioresour. Technol. 2008, 99, 2304–2311. [Google Scholar] [CrossRef]
- Su, L.; Shen, Y.; Gao, T.; Luo, J.; Wang, M. Improvement of AD Biosynthesis Response to Enhanced Oxygen Transfer by Oxygen Vectors in Mycobacterium neoaurum TCCC 11979. Appl. Biochem. Biotechnol. 2017, 182, 1564–1574. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, X.; Wang, X.; Wang, L.; Xia, M.; Luo, J.; Shen, Y.; Wang, M. Improving Phytosterol Biotransformation at Low Nitrogen Levels by Enhancing the Methylcitrate Cycle with Transcriptional Regulators PrpR and GlnR of Mycobacterium neoaurum. Microb. Cell Fact. 2020, 19, 13. [Google Scholar] [CrossRef]
- Xiong, L.-B.; Liu, H.-H.; Song, X.-W.; Meng, X.-G.; Liu, X.-Z.; Ji, Y.-Q.; Wang, F.-Q.; Wei, D.-Z. Improving the Biotransformation of Phytosterols to 9α-Hydroxy-4-Androstene-3,17-Dione by Deleting EmbC Associated with the Assembly of Cell Envelope in Mycobacterium neoaurum. J. Biotechnol. 2020, 323, 341–346. [Google Scholar] [CrossRef]
- Wei, W.; Wang, F.; Fan, S.; Wei, D. Inactivation and Augmentation of the Primary 3-Ketosteroid-Δ 1—Dehydrogenase in Mycobacterium neoaurum NwIB-01: Biotransformation of Soybean Phytosterols to 4-Androstene- 3,17-Dione or 1,4-Androstadiene-3,17-Dione. Appl. Environ. Microbiol. 2010, 76, 4578–4582. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.-B.; Wang, M.; Li, H.-N.; Wang, Y.-B.; Luo, J.-M. Influence of Hydroxypropyl-β-Cyclodextrin on Phytosterol Biotransformation by Different Strains of Mycobacterium neoaurum. J. Ind. Microbiol. Biotechnol. 2012, 39, 1253–1259. [Google Scholar] [CrossRef]
- Xu, X.W.; Gao, X.Q.; Feng, J.X.; Wang, X.D.; Wei, D.Z. Influence of Temperature on Nucleus Degradation of 4-Androstene-3, 17-Dione in Phytosterol Biotransformation by Mycobacterium sp. Lett. Appl. Microbiol. 2015, 61, 63–68. [Google Scholar] [CrossRef]
- Shao, M.; Zhao, Y.; Liu, Y.; Yang, T.; Xu, M.; Zhang, X.; Rao, Z. Intracellular Environment Improvement of Mycobacterium neoaurum for Enhancing Androst-1,4-Diene-3,17-Dione Production by Manipulating NADH and Reactive Oxygen Species Levels. Molecules 2019, 24, 3841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, S.T.; Hu, J.; Yang, Y. Medium Optimization of Androst-4-Ene-3, 17-Dione Fermentation from Sterols by Mycobacterium sp. in Two-Phase Systems. Food Sci. 2007, 28, 386–389. [Google Scholar]
- Ying, Y.; Shaotong, J.; Junping, Z.; Jinyan, H.; Lili, C. Method for Detecting the Transformation of Phytosterol to Androst-4-Ene-3, 17-Dione with Microorganism. Food Ferment. Ind. 2008, 11, 152–155. [Google Scholar]
- Xu, Y.-G.; Guan, Y.-X.; Wang, H.-Q.; Yao, S.-J. Microbial Side-Chain Cleavage of Phytosterols by Mycobacteria in Vegetable Oil/Aqueous Two-Phase System. Appl. Biochem. Biotechnol. 2014, 174, 522–533. [Google Scholar] [CrossRef]
- Naghibi, F.; Yazdi, M.T.; Sahebgharani, M.; Daloii, M.R.N. Microbial Transformation of Cholesterol by Mycobacterium smgmatis. J. Sci. Islam. Repub. Iran 2002, 13, 103–106. [Google Scholar]
- Lin, Y.; Song, X.; Fu, J.; Lin, J.; Qu, Y. Microbial Transformation of Phytosterol in Corn Flour and Soybean Flour to 4-Androstene-3,17-Dione by Fusarium moniliforme Sheld. Bioresour. Technol. 2009, 100, 1864–1867. [Google Scholar] [CrossRef]
- Sallam, L.A.R.; Osman, M.E.; Hamdy, A.A.; Zaghlol, G.M. Microbial Transformation of Phytosterols Mixture from Rice Bran Oil Unsaponifiable Matter by Selected Bacteria. World J. Microbiol. Biotechnol. 2008, 24, 1643–1656. [Google Scholar] [CrossRef]
- Che, C.B.; Liu, J.C.; Wu, B.H. Microbial Transformation of Soybean Sterol. J. Harbin Univ. Sci. Technol. 2002, 7, 93–95. [Google Scholar]
- Wang, Z.; Zhao, F.; Hao, X.; Chen, D.; Li, D. Model of Bioconversion of Cholesterol in Cloud Point System. Biochem. Eng. J. 2004, 19, 9–13. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, X.; Wang, M.; Li, H.; Shi, J.; Xu, Z. Mutation Breeding of High 9α-Hydroxy-Androst-4-Ene-3,17- Dione Transforming Strains from Phytosterols and Their Conversion Process Optimization. Chin. J. Biotechnol. 2017, 33, 1198–1206. [Google Scholar] [CrossRef]
- Donova, M.V.; Gulevskaya, S.A.; Dovbnya, D.V.; Puntus, I.F. Mycobacterium sp. Mutant Strain Producing 9α-Hydroxyandrostenedione from Sitosterol. Appl. Microbiol. Biotechnol. 2005, 67, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, X.; Wang, Y.; Yao, P.; Zhang, R.; Feng, J.; Wu, Q.; Zhu, D.; Ma, Y. New Product Identification in the Sterol Metabolism by an Industrial Strain Mycobacterium neoaurum NRRL B-3805. Steroids 2018, 132, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, R.; Wu, Y.; Wang, D.; Wei, D. Nitrate Metabolism Decreases the Steroidal Alcohol Byproduct Compared with Ammonium in Biotransformation of Phytosterol to Androstenedione by Mycobacterium neoaurum. Appl. Biochem. Biotechnol. 2020, 190, 1553–1560. [Google Scholar] [CrossRef] [PubMed]
- Malaviya, A.; Gomes, J. Nutrient Broth/PEG200/TritonX114/Tween80/Chloroform Microemulsion as a Reservoir of Solubilized Sitosterol for Biotransformation to Androstenedione. J. Ind. Microbiol. Biotechnol. 2008, 35, 1435–1440. [Google Scholar] [CrossRef] [PubMed]
- Dovbnya, D.; Khomutov, S.; Kollerov, V.; Donova, M.V. Obtaining of 11α-Hydroxyandrost-4-Ene-3,17-Dione from Natural Sterols. In Methods in Molecular Biology; Humana Press: New York, NY, USA, 2017; Volume 1645, pp. 259–269. ISBN 9781493971831. [Google Scholar]
- Zhang, X.; Peng, Y.; Su, Z.; Chen, Q.; Ruan, H.; He, G. Optimization of Biotransformation from Phytosterol to Androstenedione by a Mutant Mycobacterium neoaurum ZJUVN-08. J. Zhejiang Univ. Sci. B 2013, 14, 132–143. [Google Scholar] [CrossRef] [Green Version]
- Su, L.; Shen, Y.; Xia, M.; Shang, Z.; Xu, S.; An, X.; Wang, M. Overexpression of Cytochrome P450 125 in Mycobacterium: A Rational Strategy in the Promotion of Phytosterol Biotransformation. J. Ind. Microbiol. Biotechnol. 2018, 45, 857–867. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Jiang, S.; Cao, L.; Li, Y. Preparing Androstenones by Microbial Degradation of Phytosterol in Biphasic System. Trans. Chin. Soc. Agric. Mach. 2008, 9, 79–82, 86. [Google Scholar]
- Mancilla, R.A.; Pavez-Díaz, R.; Amoroso, A. Production and Biotransformation of Phytosterol Microdispersions to Produce 4-Androstene-3,17-Dione. In Methods Mol Biol.; Humana Press: New York, NY, USA, 2017; pp. 159–165. [Google Scholar]
- Venu Gopal, S.K.; Naik, S.; Somal, P.; Sharma, P.; Arjuna, A.; Ul Hassan, R.; Khajuria, R.K.; Qazi, G.N. Production of 17-Keto Androstene Steroids by the Side Chain Cleavage of Progesterone with Bacillus sphaericus. Biocatal. Biotransform. 2008, 26, 272–279. [Google Scholar] [CrossRef]
- Egorova, O.V.; Gulevskaya, S.A.; Puntus, I.F.; Filonov, A.E.; Donova, M.V. Production of Androstenedione Using Mutants Of Mycobacterium sp. J. Chem. Technol. Biotechnol. 2002, 77, 141–147. [Google Scholar] [CrossRef]
- Lo, C.-K.; Pan, C.-P.; Liu, W.-H. Production of Testosterone from Phytosterol Using a Single-Step Microbial Transformation by a Mutant of Mycobacterium sp. J. Ind. Microbiol. Biotechnol. 2002, 28, 280–283. [Google Scholar] [CrossRef]
- Zhang, R.; Xu, X.; Cao, H.; Yuan, C.; Yuminaga, Y.; Zhao, S.; Shi, J.; Zhang, B. Purification, Characterization, and Application of a High Activity 3-Ketosteroid-Δ1-Dehydrogenase from Mycobacterium neoaurum DSM 1381. Appl. Microbiol. Biotechnol. 2019, 103, 6605–6616. [Google Scholar] [CrossRef] [PubMed]
- Gulla, V.; Banerjee, T.; Patil, S. Quantitative TLC Analysis of Steroid Drug Intermediates Formed During Bioconversion of Soysterols. Chromatographia 2008, 68, 663–667. [Google Scholar] [CrossRef]
- Malaviya, A.; Gomes, J. Rapid Screening and Isolation of a Fungus for Sitosterol to Androstenedione Biotransformation. Appl. Biochem. Biotechnol. 2009, 158, 374–386. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Shen, Y.; Gao, T.; Cui, L.; Luo, J.; Wang, M. Regulation of NAD (H) Pool by Overexpression of Nicotinic Acid Phosphoribosyltransferase for AD (D) Production in Mycobacterium neoaurum. In Advances in Applied Biotechnology, Proceedings of the 3rd International Conference on Applied Biotechnology (ICAB2016), Tianjin, China, 25–27 November 2016; Springer: Singapore, 2018; Lecture Notes in Electrical Engineering; pp. 357–364. [Google Scholar]
- Mondaca, M.-A.; Vidal, M.; Chamorro, S.; Vidal, G. Selection of Biodegrading Phytosterol Strains. In Microbial Steroids; Humana Press: New York, NY, USA, 2017; pp. 143–150. [Google Scholar]
- Eisa, M.; El-Refai, H.; Amin, M. Single Step Biotransformation of Corn Oil Phytosterols to Boldenone by a Newly Isolated Pseudomonas aeruginosa. Biotechnol. Rep. 2016, 11, 36–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, N.; Shen, Y.; Yang, X.; Su, L.; Tang, R.; Li, W.; Wang, M. Site-Directed Mutagenesis under the Direction of in Silico Protein Docking Modeling Reveals the Active Site Residues of 3-Ketosteroid-Δ1-Dehydrogenase from Mycobacterium neoaurum. World J. Microbiol. Biotechnol. 2017, 33, 146. [Google Scholar] [CrossRef]
- Carvalho, F.; Marques, M.P.C.; de Carvalho, C.C.C.R.; Cabral, J.M.S.; Fernandes, P. Sitosterol Bioconversion with Resting Cells in Liquid Polymer Based Systems. Bioresour. Technol. 2009, 100, 4050–4053. [Google Scholar] [CrossRef]
- Ríos, L.O.L.; Luengo, J.M.; Fernández-Cañón, J.M. Steroid 11-Alpha-Hydroxylation by the Fungi Aspergillus nidulans and Aspergillus ochraceus. In Microbial Steroids; Humana Press: New York, NY, USA, 2017; pp. 271–287. [Google Scholar]
- Dogra, N.; Qazi, G.N. Steroid Biotransformation by Different Strains Of Micrococcus sp. Folia Microbiol. 2001, 46, 17–20. [Google Scholar] [CrossRef]
- Sukhodolskaya, G.V.; Nikolayeva, V.M.; Khomutov, S.M.; Donova, M.V. Steroid-1-Dehydrogenase of Mycobacterium sp. VKM Ac-1817D Strain Producing 9α-Hydroxy-Androst-4-Ene-3,17-Dione from Sitosterol. Appl. Microbiol. Biotechnol. 2007, 74, 867–873. [Google Scholar] [CrossRef]
- Cruz, A.; Angelova, B.; Fernandes, P.; Cabral, J.M.S.; Pinheiro, H.M. Study of Key Operational Parameters for the Side-Chain Cleavage of Sitosterol by Free Mycobacterial Cells in Bis-(2-Ethylhexyl) Phthalate. Biocatal. Biotransform. 2004, 22, 189–194. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, S.; Pan, L.; Yi, S. Study on Biotransformation of AD from Phytosterol in Two-Phase Systems. Food Ferment. Ind. 2008, 34, 61–64. [Google Scholar]
- Xu, Y.; Fan, Y.; Luo, L.; Wang, H.; Guan, Y.; Yao, S. Study on Substrate and Solubilizer in Side-Chain Cleavage of Sterols by Mycobacterium sp. MB 3863. J. Chem. React. Eng. Technol. 2015, 31, 423–429. [Google Scholar]
- Grishko, V.V.; Nogovitsyna, E.M.; Ivshina, I.B. The Biocatalytic Obtainment of Physiologically Active Compounds Based on Vegetative β-Sitosterol. Catal. Ind. 2009, 1, 157–164. [Google Scholar] [CrossRef]
- Su, L.; Xu, S.; Shen, Y.; Xia, M.; Ren, X.; Wang, L.; Shang, Z.; Wang, M. The Sterol Carrier Hydroxypropyl-β-Cyclodextrin Enhances the Metabolism of Phytosterols by Mycobacterium neoaurum. Appl. Environ. Microbiol. 2020, 86, e00441-20. [Google Scholar] [CrossRef] [PubMed]
- Awadhiya, P.; Banerjee, T. Tween 80 Alters the Production Ratio of Pharmaceutically Important Steroid Intermediates, 4-AD and ADD during Biotransformation of Soy Sterol by Mycobacterium sp. NRRL B-3805. Int. J. Pharm. Sci. Res. 2018, 9, 1935–1941. [Google Scholar] [CrossRef]
- Wang, X.; Hua, C.; Xu, X.; Wei, D. Two-Step Bioprocess for Reducing Nucleus Degradation in Phytosterol Bioconversion by Mycobacterium neoaurum NwIB-R10hsd4A. Appl. Biochem. Biotechnol. 2019, 188, 138–146. [Google Scholar] [CrossRef]
- Sripalakit, P.; Saraphanchotiwitthaya, A. Utilization of Phytosterol-Containing Vegetable Oils as a Substrate for Production of Androst-4-Ene-3,17-Dione and Androsta-1,4-Diene-3,17-Dione by Using Mycobacterium sp. Biocatal. Agric. Biotechnol. 2016, 8, 18–23. [Google Scholar] [CrossRef]
- Rumijowska-Galewicz, A.; Ziółkowski, A.; Korycka-Machała, M.; Sedlaczek, L. Alterations in Lipid Composition of Mycobacterium vaccae Cell Wall Outer Layer Enhance β-Sitosterol Degradation. World J. Microbiol. Biotechnol. 2000, 16, 237–244. [Google Scholar] [CrossRef]
- Korycka-Machała, M.; Rumijowska-Galewicz, A.; Dziadek, J. The Effect of Ethambutol on Mycobacterial Cell Wall Permeability to Hydrophobic Compounds. Pol. J. Microbiol. 2005, 54, 5–11. [Google Scholar]
- Thygs, F.B.; Merz, J. Downstream Process Synthesis for Microbial Steroids. Methods Mol. Biol. 2017, 1645, 321–345. [Google Scholar] [CrossRef]
- Ahmed, E.M. Utilization of Concrete as a Carrier for Bacterial Cells during Bioconversion of Some Sterols. Int. J. Chem. Sci. 2014, 12, 413–428. [Google Scholar]
- Liu, X.; Hao, X.; Zhang, R.; Feng, W.; Zhu, C.; Zhang, B.; Shi, J. Construction of Engineering Bacteria for Degrading Phytosterol to Androst-1, 4-Diene-3, 17-Dione and the Optimization of Transformation Medium. Sci. Technol. Food Ind. 2018, 39, 110–116. [Google Scholar]
- Xiao, G.; Xue, H.; Cheng, G.; Bao, X. Effect of Hydroxypropyl-β-Cyclodextrin on the Side-Chain Bioconversion of Phytosterols by Mycobacterium sp.NRRL B-3683. J. Chem. Eng. Chin. Univ. 2009, 23, 440–444. [Google Scholar]
- Liao, W.H.; Rao, Z.M.; Shen, W.; Fang, H.Y.; Zhuge, J. Optimization of the Fermentation Parameters for Transformation Phytosterol into Androstenone by Strain Bacillus sp ST06-95. Sci. Technol. Food Ind. 2008, 8. [Google Scholar]
- Chen, X.M.; Li, J.X.; Wang, H.Z.; Meng, Q.X. Research on Fermentation Process of Microbial Transformation of Phytosterol to Testosterone. Chem. Bioeng. 2007. [Google Scholar]
- Yuan, J.-J.; Guan, Y.-X.; Wang, Y.-T.; Wang, H.-Q.; Yao, S.-J. Side-Chain Cleavage of Phytosterols by Mycobacterium sp. MB 3683 in a Biphasic Ionic Liquid/Aqueous System. J. Chem. Technol. Biotechnol. 2016, 91, 2631–2637. [Google Scholar] [CrossRef]
- Wan, M.; Xu, L.; Zhang, J.; Shi, W.; Wang, X. The Optimization of Biotransformation from Phytosterol to Androstenedione by a Substrate-Tolerantmutant Strain MN4. J. Jiangxi Norm. Univ. 2016. [Google Scholar]
- USPTO United States Patent and Trademark Office. Available online: https://www.uspto.gov/ (accessed on 19 December 2021).
- Transparency Market Research. Androstenedione Market Transparency Market Research. Androstenedione Market—Global Industry Analysis, Size, Share, Growth, Trends, and Forecast 2019–2027. Available online: https://www.transparencymarketresearch.com/androst (accessed on 6 January 2022).
- Gupta, R.S.; Lo, B.; Son, J. Phylogenomics and Comparative Genomic Studies Robustly Support Division of the Genus Mycobacterium into an Emended Genus Mycobacterium and Four Novel Genera. Front. Microbiol. 2018, 9, 67. [Google Scholar] [CrossRef] [Green Version]
- Oren, A.; Garrity, G. List of New Names and New Combinations Previously Effectively, but Not Validly, Published. Int. J. Syst. Evol. Microbiol. 2018, 68, 1411–1417. [Google Scholar] [CrossRef]
- Wovcha, M.G.; Biggs, C.B.; Pyke, T.R. Biologically Pure Culture of Mutant Mycobacterium. U.S. Patent 4,339,539, 13 July 1982. [Google Scholar]
- Noh, S.-K.; Kim, M.-K.; Yoon, W.-T.; Park, K.-M.; Park, S.-O. Method for Preparation of Androst-4-Ene-3,17-Dione and Androsta-1,4-Diene-3,17-Dione. U.S. Patent 7,241,589, 10 July 2007. [Google Scholar]
- Weber, A.; Kennecke, M. Process for the Preparation of 4-Androstene-3,17-Dione and of 1,4-Androstadiene-3,17-Dione. U.S. Patent 5,418,145, 2 June 1986. [Google Scholar]
- Barry, C.E. Interpreting Cell Wall “virulence Factors” of Mycobacterium Tuberculosis. Trends Microbiol. 2001, 9, 237–241. [Google Scholar] [CrossRef]
- Knight, J.C.; Wovcha, M.G. Composition of Matter and Process. U.S. Patent 4,039,381, 2 August 1977. [Google Scholar]
- Knight, J.C.; Wovcha, M.G. Composition of Matter and Process. U.S. Patent 4,042,459, 16 August 1977. [Google Scholar]
- Pyke, T.R.; Salmond, M.P. Microbial Transformation of Steroids. U.S. Patent 4,097,335, 27 June 1978. [Google Scholar]
- Knight, J.C.; Wovcha, M.G. Composition of Matter and Process. U.S. Patent 4,098,647, 4 July 1978. [Google Scholar]
- Knight, J.C.; Wovcha, M.G. Steroid Intermediates. U.S. Patent 4,176,123, 27 November 1979. [Google Scholar]
- Knight, J.C.; Wovcha, M.G. Mycobacterium Fortuitum Strain. U.S. Patent 4,329,432, 11 May 1982. [Google Scholar]
- Wovcha, M.G.; Biggs, C.B.; Pyke, T.R. Process for Microbial Transformation of Steroids. U.S. Patent 4,211,841, 8 July 1980. [Google Scholar]
- Wovcha, M.G.; Biggs, C.B.; Pyke, T.R. Process for Preparing Androsta-1,4-Diene-3,17-Dione and Androst-4-Ene-3,17-Dione. U.S. Patent 4,293,645, 6 October 1981. [Google Scholar]
- Wovcha, M.G.; Biggs, C.B. Process for Preparing Androst-4-Ene-3,17-Dione. U.S. Patent 4,293,644, 6 October 1981. [Google Scholar]
- Wovcha, M.G.; Brooks, K.E. Composition of Matter and Process. U.S. Patent 4,293,646, 6 October 1981. [Google Scholar]
- Knight, J.C.; Wovcha, M.G. Process for the Microbial Transformation of Steroids. U.S. Patent 4,304,860, 8 December 1981. [Google Scholar]
- Wovcha, M.G.; Biggs, C.B.; Pyke, T.R. Mycobacterium fortuitum Strain. U.S. Patent 4,328,315, 4 May 1982. [Google Scholar]
- Wovcha, M.G.; Biggs, C.B. Mycobacterium phlei Mutants Convert Sterols to Androsta-1,4-Diene-3,17-Dione and Androsta-4-Ene-3,17-Dione. U.S. Patent 4,345,029, 17 August 1982. [Google Scholar]
- Wovcha, M.G.; Biggs, C.B. Microorganism Mutant Conversion of Sterols to Androsta-4-Ene-3,17-Dione. U.S. Patent 4,345,030, 17 August 1982. [Google Scholar]
- Wovcha, M.G.; Biggs, C.B.; Pyke, T.R. Mycobacterium fortuitum Mutant. U.S. Patent 4,345,033, 17 August 1982. [Google Scholar]
- Wovcha, M.; Brooks, K. Mycobacterium fortuitum Mutant. U.S. Patent 4,345,034, 17 August 1982. [Google Scholar]
- Wovcha, M.G.; Brooks, K.E. Mycobacterium fortuitum Mutant. U.S. Patent 4,358,538, 9 November 1982. [Google Scholar]
- The MarketWatch News Androstenedione Market Size 2021 Top Countries Data and Strategies That Explain Level of Competition and Future Forecasts in 2024. Available online: https://www.marketwatch.com/press-release/androstenedione-market-size-2021-top-countries-data-and-strategies-that-explain-level-of-competition-and-future-forecasts-in-2024-2021-07-08 (accessed on 18 December 2021).







| Meso Perspective Group | Micro Perspective Group | References |
|---|---|---|
| Microorganism | Genetic modification or genetic identification | [4,6,10,13,14,15,18,21,22,29,30,31,32,39,43,45,46,47,51,53,55,56,57,60,61,62,65,74,75,81,85,86,89,90,92,93,95,110,113] |
| Ks enzyme * | [6,10,13,14,15,29,35,43,53,55,57,62,87,93,97,104] | |
| Resting cells, cell wall modifications or immobilization | [11,19,34,38,49,52,54,58,100,106,107,109] | |
| Microbial selection | [24,37,70,71,89,91,96,101,109] | |
| Process Improvement | Chemical addition | [11,17,18,20,22,23,27,28,33,38,39,40,44,48,49,52,59,63,69,72,73,78,80,82,83,84,94,98,99,100,102,103,106,107,111,112,114] |
| Culture medium | [11,22,23,25,34,36,44,54,60,66,68,70,71,77,80,82,92,105,110,112,113,115] | |
| Biphasic system | [19,23,27,42,44,66,68,73,78,82,83,94,99,114] | |
| Operational mode or strategy | [13,14,16,44,45,50,79,101,104] | |
| Process variables | [22,23,36,54,59,64,80,86,112,113] | |
| Metabolic Intermediates and Hormones | PS ** or Co *** conversion into intermediates | [6,15,19,20,21,22,25,26,32,33,37,39,40,41,50,55,61,65,69,71,72,73,74,75,79,84,95,96,97,101] |
| Hormone production from PS ** | [14,42,47,51,86,92,113] | |
| Analytical Methods and others | Analytical methods | [12,67,88] |
| Others | [76,108] |
| Microorganism 1 | Substrate | Genetic Modifications | Reactional Conditions | Results | Differential | Ref. |
|---|---|---|---|---|---|---|
| Scientific Articles | ||||||
| Mycolicibacteriumneoaurum TCCC 11978 (MNR M3) | Sterol mixture, weight percentage: 51.7% β-sitosterol, 27.2%; stigmasterol, 17.1% campesterol, and 4.0% brassicasterol—Soybean oil | Cofactor engineering: modification of enzymes related to NADH * and NAD+ * metabolism | pH: 7.2; 30 °C; 140 rpm; 144 h | conversion ratio 94% | nicotinic acid in the phytosterols fermentation system to increase intracellular NAD+/NADH | [28] |
| Mycolicibacterium neoaurum TCCC 11979 | Phytosterol (98.4% purity) | - | 29 °C; 140 rpm; 120 h | molar yield of AD 55.8% | Oxygen vectors (n-hexadecane, perfluorohexane, soybean oil, PDMS, and PMPS *) | [59] |
| Mycolicibacteriumneoaurum TCCC 11978 (MNR M3) | Sterol mixture, weight percentage: 51.7% β-sitosterol, 27.2% stigmasterol, 17.1% campesterol, and 4.0% brassicasterol | Overexpression of cytochrome p450 125 (cyp125-3) | pH: 7.2; 30 °C; 140 rpm; 120 h | Conversion: 96%; 1.98 g·L−1 in 96 h | phytosterols (3 g·L−1) and HP-β-CD ** (25 mM) | [81] |
| Mycolicibacteriumneoaurum TCC 11028 (MNR M3) | Phytosterol (98.4% purity/3 g·L−1) | Overexpression of nicotinic acid phosphoribosyltransferase (NAPRTase) | pH 7.2; 29 °C; 200 rpm; 96 h | molar yield of AD (D) (94.9%) | HP-CD ** (0 or 25 mM) | [90] |
| Mycolicibacterium neoaurum NwIB-R10hsd4A | Phytosterol | - | T1 30 °C; T2 37 °C | 24.7 g·L−1 | two-step bioprocess, cell culture at 30 °C and bioconversion with resting cells at 37 °C | [104] |
| Mycolicibacterium sp. VKM Ac-1817D | Phytosterol | - | 30 °C; 200 rpm | 11 mmol/L; 0.3 mmol/h/g dry cell | MCD **** | [39] |
| Mycolicibacterium sp. | 4.5% β-sitosterol; 26.4% campesterol; 17.7% stigmasterol; 3.6% brassicasterol | Deletion kshA1 and kstD1 ***** | pH = 8; T = 37 °C; 200 rpm; 72 h | AD, 3.1 g·L−1 | HP-β-CD ***; increase in culture temperature to 37 °C to reduce nucleus degradation | [64] |
| Mycolicibacterium neoaurum NwIB-01 | Soybean phytosterols | Inactivation and augmentation of the primary 3-Ketosteroid-δ1-Dehydrogenase | 30 °C; 300 rpm; airflow 0.5 vvm; 96 h | ADD, 4.23 g·L−1; AD, 1.76 g·L−1; (57.8% mole conversion) | - | [62] |
| Mycolicibacterium sp. MB 3683 | Phytosterol | - | 30 °C; 200 rpm; 30 h | 1.3–1.4 g·L−1 | Cholinium; amino acids Ionic liquids; Best: 1% (v/v) [Ch][Asp] | [38] |
| Mycolicibacterium neoaurum TCCC 11978 C2 | 51.7% sitosterol; 27.2% stigmasterol; 17.1% campesterol; 4.0% brassicasterol | - | 30 °C; 140 rpm; 120 h | 84.8% mole conversion | HP-β-CD *** | [102] |
| Mycolicibacterium neoaurum, Pimelobacter simplex, and Rhodococcus erythropolis | soybean sterols (20–30 g/L) | - | 30 °C; 220 rpm; phytosterol load of 30 g/L over 144 h | AD: 14.5–15.2 g·L−1 | Mixture of soy steroids (20–30 g/L) in the form of small crystals in suspension (particle size 5–15 μm) | [33] |
| Mycolicibacterium sp. DSM-2967 | phytosterol-containing vegetable oils | - | pH 7.8; at room temp.; 200 rpm | Best: with canola oil; yield: 7.92 mg/100 mL | Phytosterol-containing vegetable oils directly converted to AD | [105] |
| Mycolicibacterium sp. MB 3683 | Phytosterols | - | Ionic liquid addition at 84 h, 20:1 (v/v, aqueous/IL), | AD production reached 2.23 g·L−1 after 5 days | Ionic liquid to increase low substrate solubility | [114] |
| Alkalibacterium olivoapovliticus | olive oil | - | 72 h; 30 °C | Conversion: 90% | Concrete was used as a tool to immobilize the microorganism | [109] |
| Moraxella ovis | Rice bran oil (RBO) | - | 36 h, pH 7; 30 °C | 0.22 mg AD/40 mg RBO | The unsaponifiable matter of rice bran oil was used as a raw material | [71] |
| Mycolicibacterium sp. B-3805S/Mycolicibacterium sp. NRRL B-3683 | Phytosterol | nitrosoguanidine (NTG) mutagenesis | 5-L surface-aeration microprocessor-controlled fermentor; 30 °C | Conversion: 70.6% | - | [4] |
| Patents | ||||||
| Mycolicibacterium phlei NRRL B-8154 | Sitosterol, cholesterol, stigmasterol and campesterol | Nitrosoguanidine mutagenesis | 30 °C; 14 days | - | - | [120] |
| Mycolicibacterium fortuitum EUG-119 (KCCM-10259) | Cyclodextrin-sterol complex | - | 30 °C; 5 days; 200 rpm | - | - | [121] |
| Mycolicibacterium sp. NRRL B-3805 | Alpha-sitosterol (AS) | - | 30 °C; 4 days; 220 rpm | 160 mg AD/1000 mg AS | - | [122] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunes, V.O.; Vanzellotti, N.d.C.; Fraga, J.L.; Pessoa, F.L.P.; Ferreira, T.F.; Amaral, P.F.F. Biotransformation of Phytosterols into Androstenedione—A Technological Prospecting Study. Molecules 2022, 27, 3164. https://doi.org/10.3390/molecules27103164
Nunes VO, Vanzellotti NdC, Fraga JL, Pessoa FLP, Ferreira TF, Amaral PFF. Biotransformation of Phytosterols into Androstenedione—A Technological Prospecting Study. Molecules. 2022; 27(10):3164. https://doi.org/10.3390/molecules27103164
Chicago/Turabian StyleNunes, Victor Oliveira, Nathália de Castro Vanzellotti, Jully Lacerda Fraga, Fernando Luiz Pellegrini Pessoa, Tatiana Felix Ferreira, and Priscilla Filomena Fonseca Amaral. 2022. "Biotransformation of Phytosterols into Androstenedione—A Technological Prospecting Study" Molecules 27, no. 10: 3164. https://doi.org/10.3390/molecules27103164
APA StyleNunes, V. O., Vanzellotti, N. d. C., Fraga, J. L., Pessoa, F. L. P., Ferreira, T. F., & Amaral, P. F. F. (2022). Biotransformation of Phytosterols into Androstenedione—A Technological Prospecting Study. Molecules, 27(10), 3164. https://doi.org/10.3390/molecules27103164








