Smart Biopolymer-Based Nanocomposite Materials Containing pH-Sensing Colorimetric Indicators for Food Freshness Monitoring
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Barberry and Saffron Anthocyanin Extraction
2.3. Fabrication of Smart Indicators
2.4. Instrumental Analysis
2.5. pH-Sensitivity of Colorimetric Films
2.6. UV-Vis Spectroscopy Analysis of Smart Colorimetric Indicators
2.7. Sensitivity of Colorimetric Indicators to Ammonia Vapor
2.8. Physical and Mechanical Properties of Films
2.8.1. Color Properties
2.8.2. Films Thickness
2.8.3. Mechanical Resistance
2.8.4. Moisture Content and Water Solubility of Films
2.8.5. Water Vapor Permeability
2.9. Antibacterial Activity
2.10. Antioxidant Capacity
2.11. Monitoring of Fish Freshness
2.12. Statistical Analysis
3. Results and Discussion
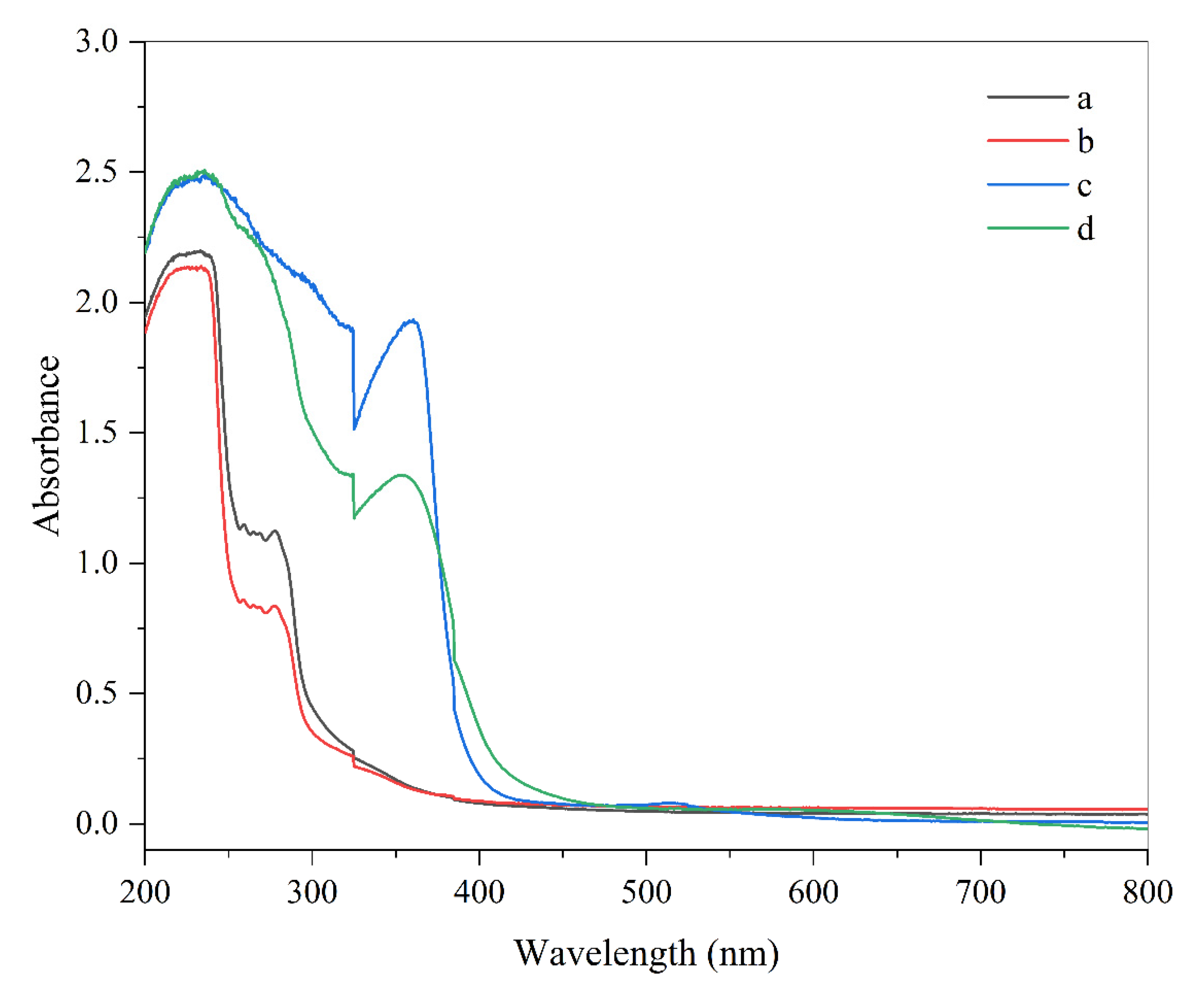
3.1. Absorbance Spectrum of Smart Indicators
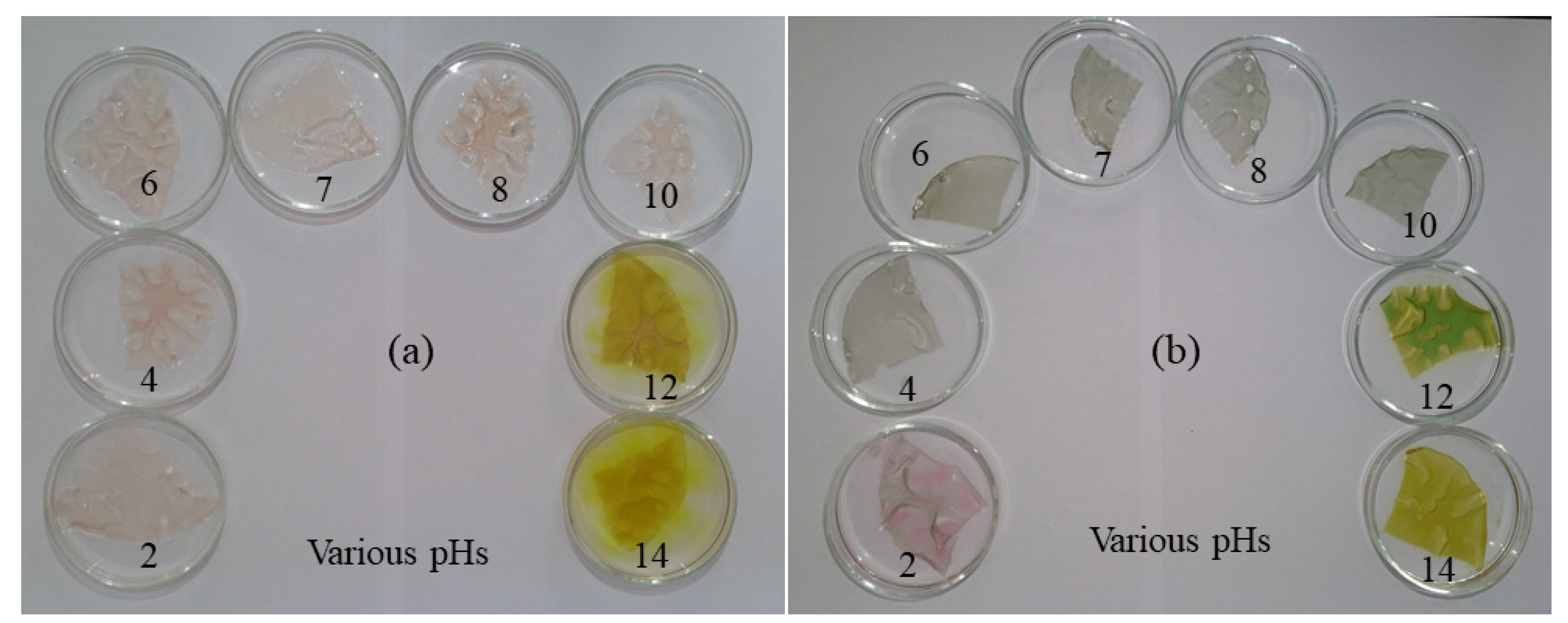
3.2. pH Dependence of Colorimetric Indicator
3.3. Ammonia-Sensitivity Test
3.4. Characterization of Colorimetric Indicators
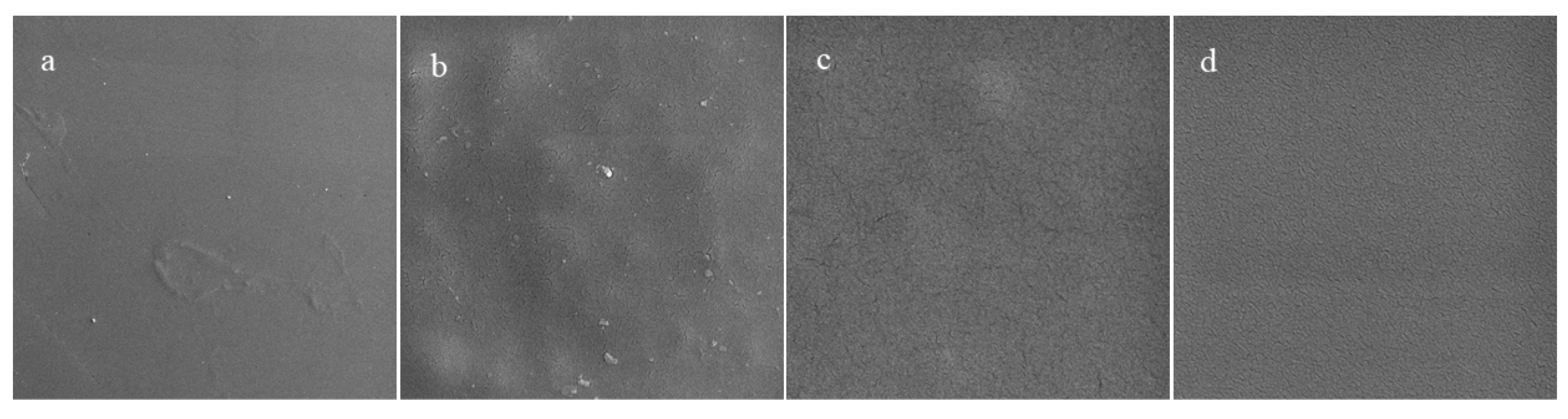
3.4.1. Surface Morphology
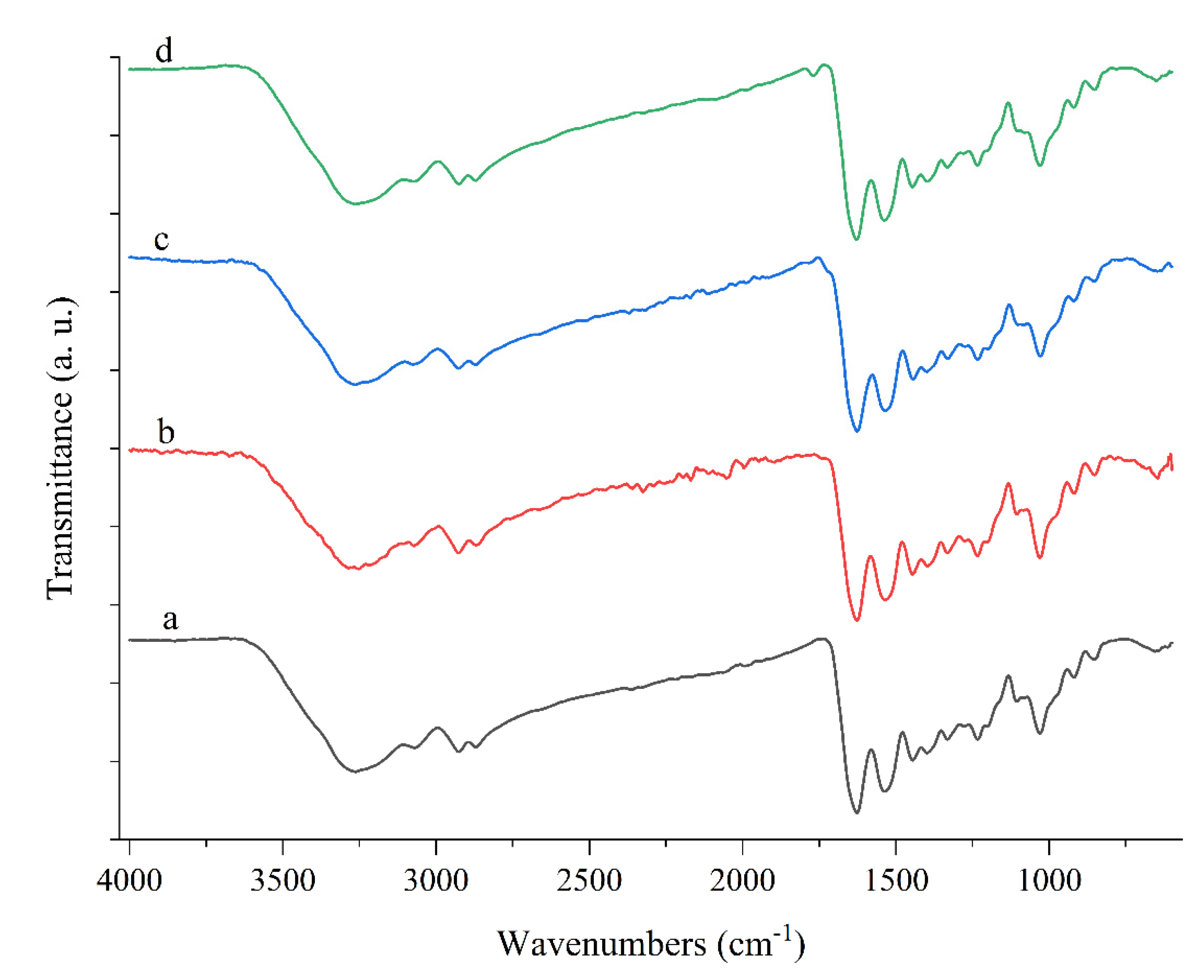
3.4.2. FTIR Analysis
3.5. Physical, Mechanical, and Optical Properties of Films
3.5.1. Color Characteristics
3.5.2. Transparency
3.5.3. Mechanical Properties
3.5.4. Water Vapor Permeability
3.5.5. Water Solubility of Films
3.5.6. Thickness
3.6. Antimicrobial Activity
3.7. Antioxidant Activity
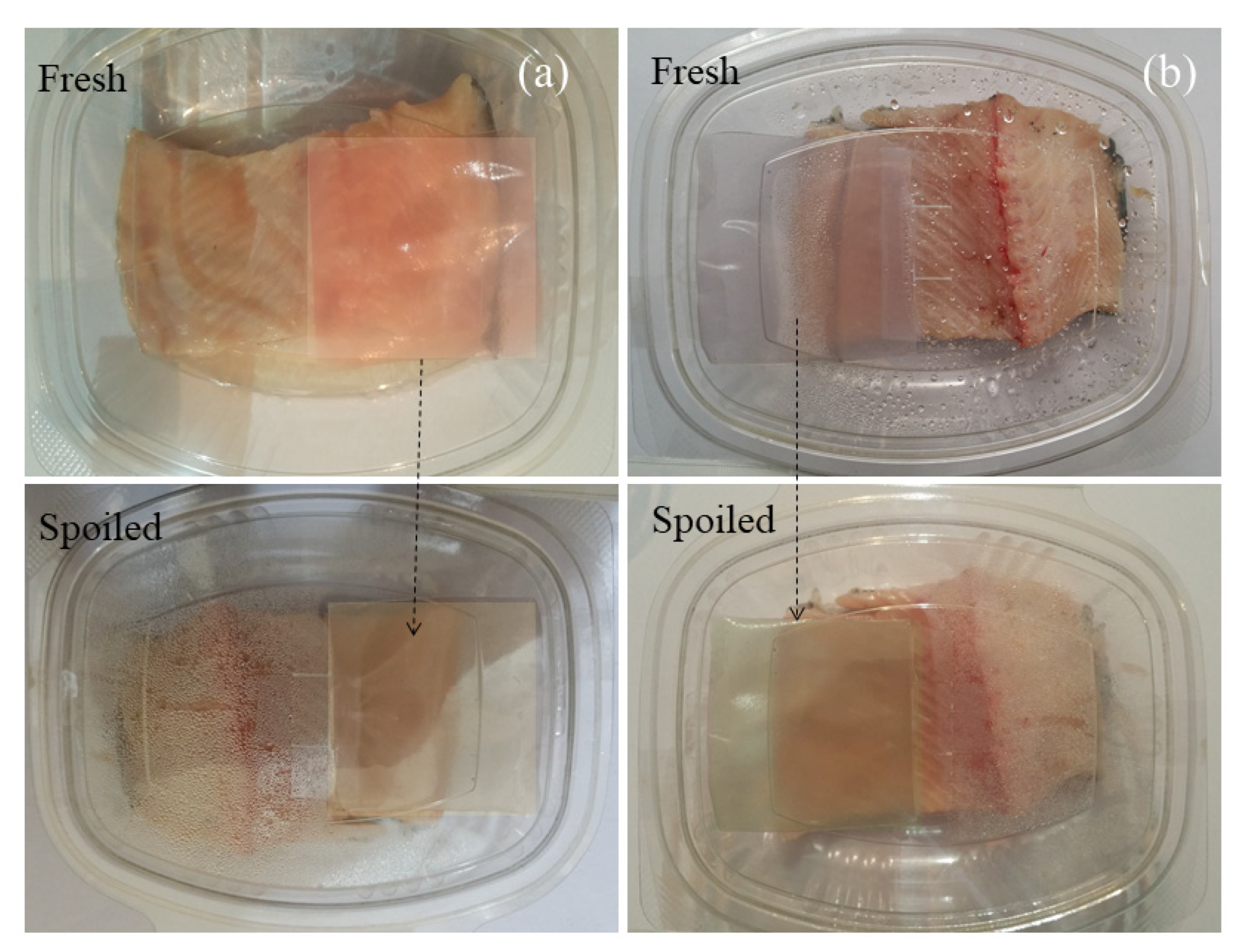
3.8. Monitoring Fish Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Chen, M.; Yan, T.; Huang, J.; Zhou, Y.; Hu, Y. Fabrication of halochromic smart films by immobilizing red cabbage anthocyanins into chitosan/oxidized-chitin nanocrystals composites for real-time hairtail and shrimp freshness monitoring. Int. J. Biol. Macromol. 2021, 179, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Sani, M.A.; Azizi-Lalabadi, M.; Tavassoli, M.; Mohammadi, K.; McClements, D. Recent advances in the development of smart and active biodegradable packaging materials. Nanomaterials 2021, 11, 1331. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh-Sani, M.; Mohammadian, E.; Rhim, J.-W.; Jafari, S.M. pH-sensitive (halochromic) smart packaging films based on natural food colorants for the monitoring of food quality. Trends Food Sci. Technol. 2020, 105, 93–144. [Google Scholar] [CrossRef]
- Ahmadi, A.; Ahmadi, P.; Sani, M.A.; Ehsani, A.; Ghanbarzadeh, B. Functional biocompatible nanocomposite films consisting of selenium and zinc oxide nanoparticles embedded in gelatin/cellulose nanofiber matrices. Int. J. Biol. Macromol. 2021, 175, 87–97. [Google Scholar] [CrossRef]
- Tavassoli, M.; Sani, M.A.; Khezerlou, A.; Ehsani, A.; McClements, D.J. Multifunctional nanocomposite active packaging materials: Immobilization of quercetin, lactoferrin, and chitosan nanofiber particles in gelatin films. Food Hydrocoll. 2021, 118, 106747. [Google Scholar] [CrossRef]
- Alizadeh-Sani, M.; Tavassoli, M.; Mohammadian, E.; Ehsani, A.; Khaniki, G.J.; Priyadarshi, R.; Rhim, J.-W. pH-responsive color indicator films based on methylcellulose/chitosan nanofiber and barberry anthocyanins for real-time monitoring of meat freshness. Int. J. Biol. Macromol. 2021, 166, 741–750. [Google Scholar] [CrossRef]
- Alizadeh Sani, M.; Tavassoli, M.; Salim, S.A.; Azizi-Lalabadi, M.; McClements, D.J. Development of green halochromic smart and active packaging materials: TiO2 nanoparticle- and anthocyanin-loaded gelatin/κ-carrageenan films. Food Hydrocoll. 2022, 124, 107324. [Google Scholar] [CrossRef]
- Sani, M.A.; Tavassoli, M.; Hamishehkar, H.; McClements, D.J. Carbohydrate-based films containing pH-sensitive red barberry anthocyanins: Application as biodegradable smart food packaging materials. Carbohydr. Polym. 2021, 255, 117488. [Google Scholar] [CrossRef]
- Sani, M.A.; Maleki, M.; Eghbaljoo-Gharehgheshlaghi, H.; Khezerlou, A.; Mohammadian, E.; Liu, Q.; Jafari, S.M. Titanium dioxide nanoparticles as multifunctional surface-active materials for smart/active nanocomposite packaging films. Adv. Colloid Interface Sci. 2021, 300, 102593. [Google Scholar] [CrossRef]
- Alizadeh-Sani, M.; Tavassoli, M.; McClements, D.J.; Hamishehkar, H. Multifunctional halochromic packaging materials: Saffron petal anthocyanin loaded-chitosan nanofiber/methyl cellulose matrices. Food Hydrocoll. 2021, 111, 106237. [Google Scholar] [CrossRef]
- Mohammadian, E.; Alizadeh-Sani, M.; Jafari, S.M. Smart monitoring of gas/temperature changes within food packaging based on natural colorants. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2885–2931. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Priyadarshi, R.; Ezati, P.; Rhim, J.-W. Curcumin and its uses in active and smart food packaging applications-a comprehensive review. Food Chem. 2022, 375, 131885. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Rhim, J.-W. Fabrication of Carboxymethyl Cellulose/Agar-Based Functional Films Hybridized with Alizarin and Grapefruit Seed Extract. ACS Appl. Bio Mater. 2021, 4, 4470–4478. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, H.; Su, H.-M.; Imani, S.M.; Alkhaldi, K.; Filipe, C.D.M.; Didar, T.F. Intelligent food packaging: A review of smart sensing technologies for monitoring food quality. ACS Sens. 2019, 4, 808–821. [Google Scholar] [CrossRef] [PubMed]
- Kurek, M.; Garofulić, I.E.; Bakić, M.T.; Scetar, M.; Uzelac, V.D.; Galić, K. Development and evaluation of a novel antioxidant and pH indicator film based on chitosan and food waste sources of antioxidants. Food Hydrocoll. 2018, 84, 238–246. [Google Scholar] [CrossRef]
- Wu, C.; Sun, J.; Zheng, P.; Kang, X.; Chen, M.; Li, Y.; Ge, Y.; Hu, Y.; Pang, J. Preparation of an intelligent film based on chitosan/oxidized chitin nanocrystals incorporating black rice bran anthocyanins for seafood spoilage monitoring. Carbohydr. Polym. 2019, 222, 115006. [Google Scholar] [CrossRef] [PubMed]
- Sohany, M.; Tawakkal, I.S.M.A.; Ariffin, S.H.; Shah, N.N.A.K.; Yusof, Y.A. Characterization of Anthocyanin Associated Purple Sweet Potato Starch and Peel-Based pH Indicator Films. Foods 2021, 10, 2005. [Google Scholar] [CrossRef]
- Yong, H.; Wang, X.; Zhang, X.; Liu, Y.; Qin, Y.; Liu, J. Effects of anthocyanin-rich purple and black eggplant extracts on the physical, antioxidant and pH-sensitive properties of chitosan film. Food Hydrocoll. 2019, 94, 93–104. [Google Scholar] [CrossRef]
- Liu, D.; Cui, Z.; Shang, M.; Zhong, Y. A colorimetric film based on polyvinyl alcohol/sodium carboxymethyl cellulose incorporated with red cabbage anthocyanin for monitoring pork freshness. Food Packag. Shelf Life 2021, 28, 100641. [Google Scholar] [CrossRef]
- Zhang, J.; Zou, X.; Zhai, X.D.; Huang, X.W.; Jiang, C.P.; Holmes, M. Preparation of an intelligent pH film based on biodegradable polymers and roselle anthocyanins for monitoring pork freshness. Food Chem. 2019, 272, 306–312. [Google Scholar] [CrossRef]
- Alpaslan, D.; Dudu, T.E.; Şahiner, N.; Aktas, N. Synthesis and preparation of responsive poly (Dimethyl acrylamide/gelatin and pomegranate extract) as a novel food packaging material. Mater. Sci. Eng. C 2020, 108, 110339. [Google Scholar] [CrossRef] [PubMed]
- Yong, H.; Wang, X.; Bai, R.; Miao, Z. Development of antioxidant and intelligent pH-sensing packaging films by incorporating purple-fleshed sweet potato extract into chitosan matrix. Food Hydrocoll. 2019, 90, 216–224. [Google Scholar] [CrossRef]
- Wu, C.; Li, Y.; Sun, J.; Lu, Y.; Tong, C.; Wang, L.; Yan, Z.; Pang, J. Novel konjac glucomannan films with oxidized chitin nanocrystals immobilized red cabbage anthocyanins for intelligent food packaging. Food Hydrocoll. 2020, 98, 105245. [Google Scholar] [CrossRef]
- Liu, J.; Yong, H.; Liu, Y.; Qin, Y.; Kan, J.; Liu, J. Preparation and characterization of active and intelligent films based on fish gelatin and haskap berries (Lonicera caerulea L.) extract. Food Packag. Shelf Life 2019, 22, 100417. [Google Scholar] [CrossRef]
- Ebrahimi Tirtashi, F.; Moradi, M.; Tajik, H.; Forough, M.; Ezati, P.; Kuswandi, B. Cellulose/chitosan pH-responsive indicator incorporated with carrot anthocyanins for intelligent food packaging. Int. J. Biol. Macromol. 2019, 136, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Gasti, T.; Dixit, S.; D’souza, O.J.; Hiremani, V.D.; Vootla, S.K.; Masti, S.P.; Chougale, R.B.; Malabadi, R.B. Smart biodegradable films based on chitosan/methylcellulose containing Phyllanthus reticulatus anthocyanin for monitoring the freshness of fish fillet. Int. J. Biol. Macromol. 2021, 187, 451–461. [Google Scholar] [CrossRef]
- Jancikova, S.; Jamróz, E.; Kulawik, P.; Tkaczewska, J.; Dordevic, D. Furcellaran/gelatin hydrolysate/rosemary extract composite films as active and intelligent packaging materials. Int. J. Biol. Macromol. 2019, 131, 19–28. [Google Scholar] [CrossRef]
- Kanatt, S.R. Development of active/intelligent food packaging film containing Amaranthus leaf extract for shelf life extension of chicken/fish during chilled storage. Food Packag. Shelf Life 2020, 24, 100506. [Google Scholar] [CrossRef]
- Alpaslan, D.; Dudu, T.E.; Aktas, N. Synthesis of Smart Food Packaging from Poly (Gelatin-co-Dimethyl Acrylamide)/Citric Acid-Red Apple peel Extract. Soft Mater. 2021, 19, 64–77. [Google Scholar] [CrossRef]
- da Silva Filipini, G.; Romani, V.P.; Martins, V.G. Biodegradable and active-intelligent films based on methylcellulose and jambolão (Syzygium cumini) skins extract for food packaging. Food Hydrocoll. 2020, 109, 106139. [Google Scholar] [CrossRef]

| Film Type | ||||
|---|---|---|---|---|
| Physical Properties | Gelatin | G/CsNFs | G/CsNFs/Bas | G/CsNFs/SPAs |
| Thickness (µm) | 88.5 ± 6 a | 100 ± 8 b | 118 ± 12 c | 115 ± 10 d |
| Transparency (%) | 90.4 ± 1.0 a | 85.5 ± 1.2 b | 83.2 ± 1.5 c | 74.9.0 ± 0.8 d |
| WVP (×10−11 g × m/kPa × m2 × h) | 2.45 ± 0.04 a | 1.20 ± 0.05 b | 1.10 ± 0.02 b | 1.15 ± 0.03 b |
| Water solubility (%) | 50.3 ± 1.2 a | 44.5 ± 1.5 b | 44.0 ± 1.7 b | 45.1 ± 1.9 b |
| Mechanical properties | ||||
| Tensile strength (MPa) | 53.4 ± 0.9 a | 65.05 ± 1.4 b | 35.68 ± 1.8 c | 41.5 ± 2.2 d |
| Elongation at break (%) | 1.25 ± 0.15 a | 1.07 ± 0.01 b | 5.30 ± 0.2 c | 6.8 ± 0.4 d |
| Color properties | ||||
| L value | 85.5 ± 1.4 a | 66.5 ± 1.5 b | 53.5 ± 1.0 c | 50.8 ± 2.0 d |
| a value | 0.33 ± 0.02 a | −0.33 ± 0.13 b | 23.3 ± 0.88 c | 5.3 ± 0.75 d |
| b value | 2.66 ± 0.15 a | 5.83 ± 0.8 b | 10.1 ± 0.66 c | −9.66 ± 0.5 d |
| ∆E value | 8.01 ± 0.5 a | 27.26 ± 0.15 b | 47.02 ± 1.7 c | 44.43 ± 1.5 d |
| Chroma (C*ab) | 2.68 ± 0.2 a | 5.8 ± 0.5 b | 25.39 ± 1.44 b | 11.02 ± 0.66 c |
| Hue angle (hab) | 83.0 ± 1.33 a | 90.33 ± 1.11 b | 27.0 ± 1.0 c | 318.66 ± 0.55 d |
| Antimicrobial activity (inhibition zone (mm)) | ||||
| E. coli | - | 12.0 ± 1.8 a | 16.5 ± 0.9 b | 16.8 ± 2.3 b |
| S. aureus | - | 12.6 ± 1.1 a | 17.8 ± 1.3 b | 18.0 ± 0.9 b |
| Antioxidant activity | ||||
| DPPH radical scavenging (%) | - | 14.5 ± 2.0 a | 82.2 ± 1.7 b | 83.0 ± 1.5 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tavassoli, M.; Alizadeh Sani, M.; Khezerlou, A.; Ehsani, A.; Jahed-Khaniki, G.; McClements, D.J. Smart Biopolymer-Based Nanocomposite Materials Containing pH-Sensing Colorimetric Indicators for Food Freshness Monitoring. Molecules 2022, 27, 3168. https://doi.org/10.3390/molecules27103168
Tavassoli M, Alizadeh Sani M, Khezerlou A, Ehsani A, Jahed-Khaniki G, McClements DJ. Smart Biopolymer-Based Nanocomposite Materials Containing pH-Sensing Colorimetric Indicators for Food Freshness Monitoring. Molecules. 2022; 27(10):3168. https://doi.org/10.3390/molecules27103168
Chicago/Turabian StyleTavassoli, Milad, Mahmood Alizadeh Sani, Arezou Khezerlou, Ali Ehsani, Gholamreza Jahed-Khaniki, and David Julian McClements. 2022. "Smart Biopolymer-Based Nanocomposite Materials Containing pH-Sensing Colorimetric Indicators for Food Freshness Monitoring" Molecules 27, no. 10: 3168. https://doi.org/10.3390/molecules27103168
APA StyleTavassoli, M., Alizadeh Sani, M., Khezerlou, A., Ehsani, A., Jahed-Khaniki, G., & McClements, D. J. (2022). Smart Biopolymer-Based Nanocomposite Materials Containing pH-Sensing Colorimetric Indicators for Food Freshness Monitoring. Molecules, 27(10), 3168. https://doi.org/10.3390/molecules27103168








