Investigation and Biological Assessment of Rumex vesicarius L. Extract: Characterization of the Chemical Components and Antioxidant, Antimicrobial, Cytotoxic, and Anti-Dengue Vector Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Characterization of R. vesicarius Extract
2.2. Biological Characteristics of the Plant Extracts
2.2.1. Antioxidant Activity—DPPH Assay
2.2.2. Antibacterial Activity
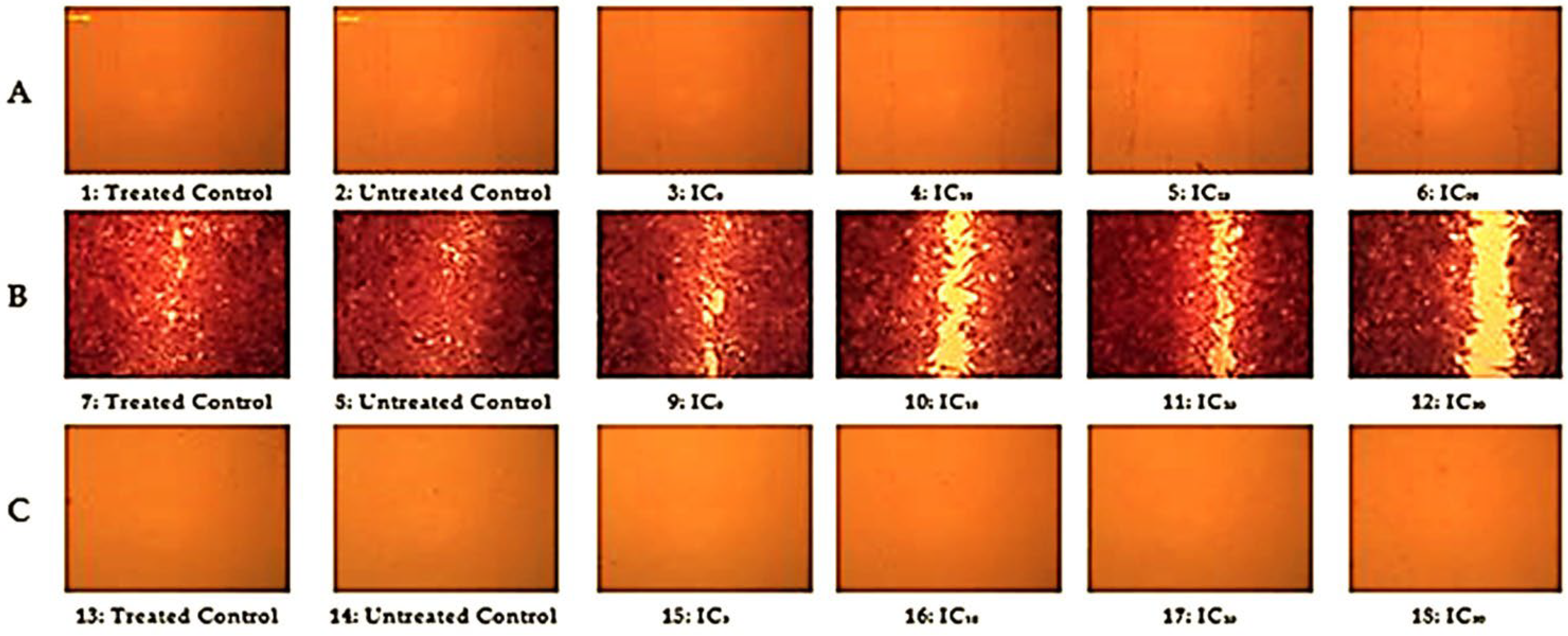
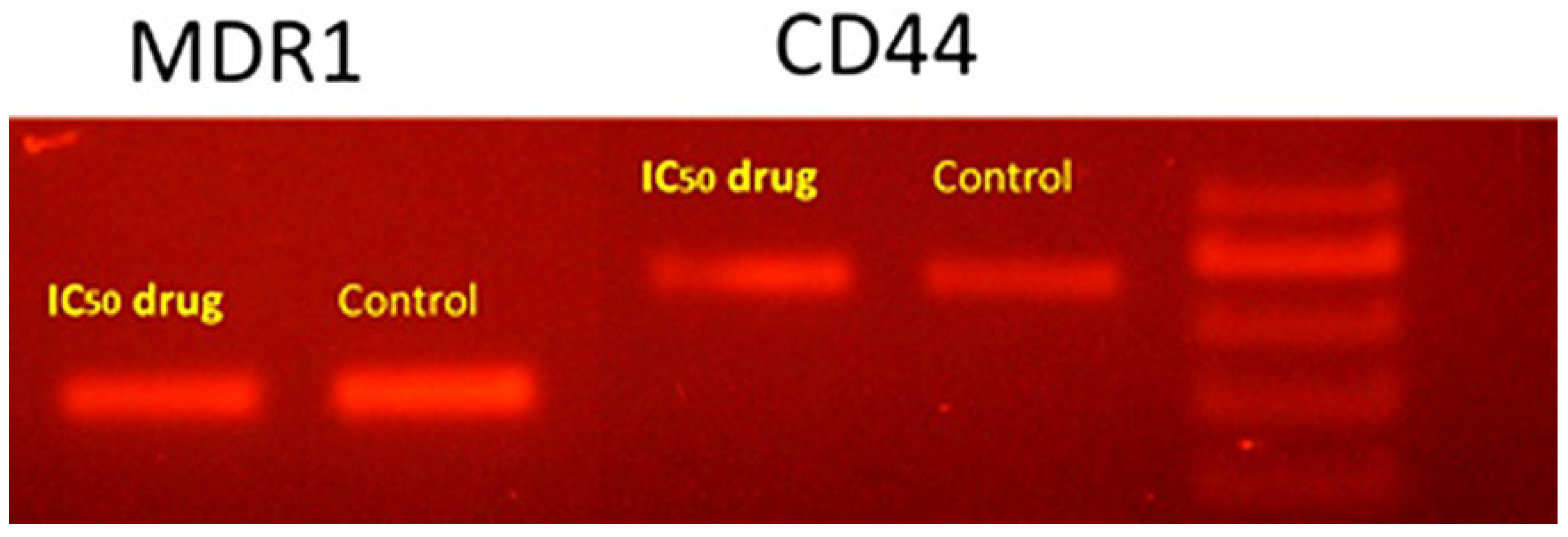
2.2.3. Cytotoxicity and Cell Migration Analysis
DNA Fragmentation
The EC50 Value of R. vesicarius Extract
2.2.4. Larvicidal Bioassay
3. Materials and Methods
3.1. Plant Material and Extraction Process
3.2. Gas Chromatography–Mass Spectrometry Analysis (GC-MS)
3.3. Antioxidant DPPH Assay
3.4. Assessment of the Antibacterial Activity
3.5. Cytotoxicity and Cell Proliferation
3.6. Cell Motility Assay
3.7. Conventional PCR
3.8. Mosquitocidal Assay
3.8.1. Aedes aegypti Colony
3.8.2. Larvicidal Activity of Tested Plant Extracts
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Hussain, K.; Shahazad, A.; Zia-ul-Hussnain, S. An ethnobotanical survey of important wild medicinal plants of Hattar district Haripur, Pakistan. Ethnobot. Leafl. 2008, 2008, 5. [Google Scholar]
- Sultan, M.S.; Elsayed, A.; El-Amir, Y.A. In vitro effect of plant parts extract of Senecio glaucus L. On pathogenic bacteria. Biointerface Res. Appl. Chem. 2022, 12, 3800–3810. [Google Scholar]
- Gusain, P.; Uniyal, D.; Joga, R. Conservation and Sustainable Use of Medicinal Plants. In Preparation of Phytopharmaceuticals for the Management of Disorders; Elsevier: Amsterdam, The Netherlands, 2021; pp. 409–427. [Google Scholar]
- David, B.; Wolfender, J.-L.; Dias, D.A. The pharmaceutical industry and natural products: Historical status and new trends. Phytochem. Rev. 2015, 14, 299–315. [Google Scholar]
- Tackholm, V. Students’ Flora of Egypt; Cairo University: Giza, Egypt, 1974. [Google Scholar]
- Boulos, L. Flora of Egypt. Checklist; Al Hadara Publishing: Cairo, Egypt, 1995. [Google Scholar]
- Boulos, L. Flora of Egypt; Al Hadara Publishing: Cairo, Egypt, 1999; Volume 1. [Google Scholar]
- Zohary, M. Flora Palaestina, Part 1; Israel Academy of Sciences and Humanities: Jerusalem, Israel, 1966; Volume 76. [Google Scholar]
- Boulos, L. Medicinal Plants of North Africa; Clair River: Algonac, MI, USA, 1983; Volume 4800, p. 109. [Google Scholar]
- Trease, G.; Evans, W. Pharmacognosy; Balliere Tindall Press: London, UK, 1983. [Google Scholar]
- Litvinenko, Y.A.; MuzychKina, R. Phytochemical investigation of biologically active substances in certain Kazakhstan Rumex species. 1. Chem. Nat. Compd. 2003, 39, 446–449. [Google Scholar] [CrossRef]
- El-Bakry, A.; Mostafa, H.; Alam, E. Antioxidant activity of Rumex vesicarius L. at the vegetative stage of growth. Asian J. Pharm. Clin. Res. 2012, 5, 111–117. [Google Scholar]
- Gomaa, S.B.; Saleh, N.M. Medicinal attributes of Rumex vesicarius (Polygonaceae) growing in Sakaka, AlJouf, Saudi Arabia. Med. J. Cairo Univ. 2014, 82, 917–926. [Google Scholar]
- Tukappa, N.K.A.; Londonkar, R.L.; Nayaka, H.B.; Kumar, C.B.S. Cytotoxicity and hepatoprotective attributes of methanolic extract of Rumex vesicarius L. Biol. Res. 2015, 48, 19. [Google Scholar] [CrossRef] [Green Version]
- Hafaz, M.F.; Soliman, H.M.; Abbas, M.A.; Gebreil, A.S.; El-Amier, Y.A. Potential Assessment of Rumex spp. as a source of bioactive compounds and biological activity. Biointerface Res. Appl. Chemi. 2022, 12, 1824–1834. [Google Scholar]
- Al-Rumaih, M.M.; Al-Saad, F.; Warsy, A. Seasonal variation in mineral content of different organs during development of Rumex vesicarius L. Saudi J. Biol. Sci. 2002, 9, 69–79. [Google Scholar]
- Sankar, N.R.; Devamma, M.N.; Giridhar, D. First report of Alternaria alternata causing leaf spot on Rumex vesicarius in India. Australas. Plant Dis. Notes 2012, 7, 17–18. [Google Scholar] [CrossRef] [Green Version]
- Belanger, J.; Balakrishna, M.; Latha, P.; Katumalla, S.; Johns, T. Contribution of selected wild and cultivated leafy vegetables from South India to lutein and beta-carotene intake. Asia Pac. J. Clin. Nutr. 2010, 19, 417–424. [Google Scholar] [PubMed]
- Saleh, N.A.; El-Hadidi, M.N.; Arafa, R.F. Flavonoids and anthraquinones of some Egyptian Rumex species (Polygonaceae). Biochem. Syst. Ecol. 1993, 21, 301–303. [Google Scholar] [CrossRef]
- Alfawaz, M.A. Chemical composition of hummayd (Rumex vesicarius) grown in Saudi Arabia. J. Food Compos. Anal. 2006, 19, 552–555. [Google Scholar] [CrossRef]
- Gopal, R.; Vijayakumaran, M.; Venkatesan, R.; Kathiroli, S. Marine organisms in Indian medicine and their future prospects. Indian J. Nat. Prod. Resourc. 2008, 7, 139–145. [Google Scholar]
- Mostafa, H.; Elbakry, A.; Eman, A.A. Evaluation of antibacterial and antioxidant activities of different plant parts of Rumex vesicarius L. (Polygonaceae). Int. J. Pharm. Pharm. Sci 2011, 3, 109–118. [Google Scholar]
- Weeratunga, P.; Rodrigo, C.; Fernando, S.D.; Rajapakse, S. Control methods for Aedes albopictus and Aedes aegypti. Cochrane Database Syst. Rev. 2017, 2017, CD012759. [Google Scholar]
- Carvajal-Lago, L.; Ruiz-López, M.J.; Figuerola, J.; Martínez-de la Puente, J. Implications of diet on mosquito life history traits and pathogen transmission. Environ. Res. 2021, 195, 110893. [Google Scholar] [PubMed]
- Khare, C.P. Indian Medicinal Plants: An Illustrated Dictionary; Springer Science & Business Media, LLC: Berlin/Heidelberg, Germany, 2007; p. 564. [Google Scholar]
- El-Hawary, S.A.; Sokkar, N.M.; Ali, Z.Y.; Yehia, M.M. A profile of bioactive compounds of Rumex vesicarius L. J. Food Sci. 2011, 76, C1195–C1202. [Google Scholar] [CrossRef]
- Alazzam, S.; Sharqi, M.M.; AlMehemdi, A.F. Phytochemical analysis, antioxidant and cytotoxic potential of Rumex vesicarius extracts. Med.-Leg. Update 2020, 20, 449–454. [Google Scholar]
- Nishina, A.; Kubota, K.; Kameoka, H.; Osawa, T. Antioxidizing component, musizin, inrumex japonicus houtt. J. Am. Oil Chem. Soc. 1991, 68, 735–739. [Google Scholar] [CrossRef]
- Demirezer, L.Ö.; Kuruüzüm-Uz, A.; Bergere, I.; Schiewe, H.-J.; Zeeck, A. The structures of antioxidant and cytotoxic agents from natural source: Anthraquinones and tannins from roots of Rumex patientia. Phytochemistry 2001, 58, 1213–1217. [Google Scholar] [CrossRef]
- Al-Ismail, K.; Hamdan, M.; Al-Delaimy, K. Antioxidant and anti Bacillus cereus activities of selected plant extracts. Jordan J. Agric. Sci. 2006, 2, 64–74. [Google Scholar]
- Özen, T. Antioxidant activity of wild edible plants in the Black Sea Region of Turkey. Grasas Y Aceites 2010, 61, 86–94. [Google Scholar] [CrossRef]
- Li, C.; Liu, S. Screening of Chinese plant extracts for antioxidant activity. Mod. Pharm. Res. 2009, 2, 31–35. [Google Scholar]
- Monzote, L.; Stamberg, W.; Staniek, K.; Gille, L. Toxic effects of carvacrol, caryophyllene oxide, and ascaridole from essential oil of Chenopodium ambrosioides on mitochondria. Toxicol. Appl. Pharmacol. 2009, 240, 337–347. [Google Scholar] [CrossRef]
- Sosa, A.A.; Bagi, S.H.; Hameed, I.H. Analysis of bioactive chemical compounds of Euphorbia lathyrus using gas chromatography-mass spectrometry and fourier-transform infrared spectroscopy. J. Pharmacogn. Phytother. 2016, 8, 109–126. [Google Scholar]
- Okwu, D.E. Citrus fruits: A rich source of phytochemicals and their roles in human health. Int. J. Chem. Sci. 2008, 6, 451–471. [Google Scholar]
- Al-Snafi, A.E. The medical benefit of Gnaphalium luteoalbum-A review. IOSR J. Pharm. 2019, 9, 40–44. [Google Scholar]
- Lu-Martínez, A.A.; Báez-González, J.G.; Castillo-Hernández, S.; Amaya-Guerra, C.; Rodríguez-Rodríguez, J.; García-Márquez, E. Studied of Prunus serotine oil extracted by cold pressing and antioxidant effect of P. longiflora essential oil. J. Food Sci. Technol. 2021, 58, 1420–1429. [Google Scholar] [CrossRef]
- Koleva, I.I.; Niederländer, H.A.; van Beek, T.A. An on-line HPLC method for detection of radical scavenging compounds in complex mixtures. Anal. Chem. 2000, 72, 2323–2328. [Google Scholar] [CrossRef]
- Koleva, I.I.; Van Beek, T.A.; Linssen, J.P.; Groot, A.D.; Evstatieva, L.N. Screening of plant extracts for antioxidant activity: A comparative study on three testing methods. Phytochem. Anal. Int. J. Plant Chem. Biochem. Tech. 2002, 13, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Alam, E.A. Antibacterial and antioxidant activities of seedlings of Rumex vesicarius L.(Polygonaceae). J. Med. Plants Res. 2013, 7, 2158–2164. [Google Scholar]
- Pan, X.; Kadla, J.F.; Ehara, K.; Gilkes, N.; Saddler, J.N. Organosolv ethanol lignin from hybrid poplar as a radical scavenger: Relationship between lignin structure, extraction conditions, and antioxidant activity. J. Agric. Food Chem. 2006, 54, 5806–5813. [Google Scholar] [CrossRef]
- Gu, F.; Kim, J.M.; Hayat, K.; Xia, S.; Feng, B.; Zhang, X. Characteristics and antioxidant activity of ultrafiltrated Maillard reaction products from a casein–glucose model system. Food Chem. 2009, 117, 48–54. [Google Scholar] [CrossRef]
- El-Amier, Y.A.; Soufan, W.; Almutairi, K.F.; Zaghloul, N.S.; Abd-ElGawad, A.M. Proximate composition, bioactive compounds, and antioxidant potential of wild halophytes grown in coastal salt marsh habitats. Molecules 2021, 27, 28. [Google Scholar] [CrossRef]
- Panduraju, T.; Rao, P.; Kumar, V. A study on antimicrobial activity of Rumex vesicarius Linn. Int. J. Pharm. Technol. 2009, 1, 21–25. [Google Scholar]
- Tukappa, A.; Londonkar, R.L. Evaluation of antibacterial and antioxidant activities of different methanol extract of Rumex vesicarius L. Am. J. Drug Discov. Dev. 2013, 3, 72–83. [Google Scholar] [CrossRef]
- Yıldırım, A.; Mavi, A.; Kara, A.A. Determination of antioxidant and antimicrobial activities of Rumex crispus L. extracts. J. Agric. Food Chem. 2001, 49, 4083–4089. [Google Scholar] [CrossRef]
- Alzoreky, N.; Nakahara, K. Antibacterial activity of extracts from some edible plants commonly consumed in Asia. Int. J. Food Microbiol. 2003, 80, 223–230. [Google Scholar] [CrossRef]
- Harshaw, D.; Nahar, L.; Vadla, B.; Sarker, S.D. Bioactivity of Rumex obtusifolius (Polygonaceae). Arch. Biol. Sci. 2010, 62, 387–392. [Google Scholar] [CrossRef]
- Elmogy, S.; Ismail, M.A.; Hassan, R.Y.; Noureldeen, A.; Darwish, H.; Fayad, E.; Elsaid, F.; Elsayed, A. Biological Insights of Fluoroaryl-2, 2′-Bichalcophene Compounds on Multi-Drug Resistant Staphylococcus aureus. Molecules 2020, 26, 139. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, A.; Mohamedin, A.; Ata, T.; Ghazala, N. Molecular characterization of multidrug resistant clinical Escherichia coli isolates. Am. J. Biochem. Mol. Biol. 2016, 6, 72–83. [Google Scholar] [CrossRef]
- Maccelli, A.; Vitanza, L.; Imbriano, A.; Fraschetti, C.; Filippi, A.; Goldoni, P.; Maurizi, L.; Ammendolia, M.G.; Crestoni, M.E.; Fornarini, S. Satureja montana L. Essential oils: Chemical profiles/phytochemical screening, antimicrobial activity and o/w nanoemulsion formulations. Pharmaceutics 2019, 12, 7. [Google Scholar] [CrossRef] [Green Version]
- Salehi, B.; Krochmal-Marczak, B.; Skiba, D.; Patra, J.K.; Das, S.K.; Das, G.; Popović-Djordjević, J.B.; Kostić, A.Ž.; Anil Kumar, N.V.; Tripathi, A. Convolvulus plant-A comprehensive review from phytochemical composition to pharmacy. Phytother. Res. 2020, 34, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Sökmen, A.; Sökmen, M.; Daferera, D.; Polissiou, M.; Candan, F.; Ünlü, M.; Akpulat, H.A. The in vitro antioxidant and antimicrobial activities of the essential oil and methanol extracts of Achillea biebersteini Afan. (Asteraceae). Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2004, 18, 451–456. [Google Scholar]
- Shareef, H.K.; Muhammed, H.J.; Hussein, H.M.; Hameed, I.H. Antibacterial effect of ginger (Zingiber officinale) roscoe and bioactive chemical analysis using gas chromatography mass spectrum. Orient. J. Chem. 2016, 32, 20–40. [Google Scholar] [CrossRef] [Green Version]
- Fayed, E.M.; Abd-EIGawad, A.M.; Elshamy, A.I.; El-Halawany, E.S.F.; EI-Amier, Y.A. Essential oil of Deverra tortuosa aerial parts: Detailed chemical profile, allelopathic, antimicrobial, and antioxidant activities. Chem. Biodivers. 2021, 18, e2000914. [Google Scholar] [CrossRef]
- Zaki, A.A.; Ali, Z.; Wang, Y.-H.; El-Amier, Y.A.; Khan, S.I.; Khan, I.A. Cytotoxic steroidal saponins from Panicum turgidum Forssk. Steroids 2017, 125, 14–19. [Google Scholar] [CrossRef]
- Dikmen, M.; Ozturk, N.; Ozturk, Y. The antioxidant potency of Punica granatum L. Fruit peel reduces cell proliferation and induces apoptosis on breast cancer. J. Med. Food 2011, 14, 1638–1646. [Google Scholar] [CrossRef] [Green Version]
- Zaki, F. Field application of plant extracts against the aphid, B. brassicae and the whitefly, B. abaci and their side effects on their predators and parasites. Arch. Phytopathol. Plant Prot. 2008, 41, 462–466. [Google Scholar] [CrossRef]
- Khacha-Ananda, S.; Tragoolpua, K.; Chantawannakul, P.; Tragoolpua, Y. Antioxidant and anti-cancer cell proliferation activity of propolis extracts from two extraction methods. Asian Pac. J. Cancer Prev. 2013, 14, 6991–6995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bazou, D.; Coakley, W.T.; Hayes, A.J.; Jackson, S.K. Long-term viability and proliferation of alginate-encapsulated 3-D HepG2 aggregates formed in an ultrasound trap. Toxicol. Vitr. 2008, 22, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xiang, Q.; Du, L.; Song, G.; Wang, Y.; Liu, X. The interaction of sesamol with DNA and cytotoxicity, apoptosis, and localization in HepG2 cells. Food Chem. 2013, 141, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, P.; Arunkumar, K.; Sivakumar, L.; Murugan, M.; Murugan, K. Anticancer effect of fucoidan on cell proliferation, cell cycle progression, genetic damage and apoptotic cell death in HepG2 cancer cells. Toxicol. Rep. 2019, 6, 556–563. [Google Scholar]
- Ahmad, Z.; Sarmidi, M.; Hasham, R. Evaluation of wound closure activity of cocos nucifera oil on scratched monolayer of human dermal fibroblasts. Chem. Eng. Trans. 2017, 56, 1657–1662. [Google Scholar]
- Grada, A.; Otero-Vinas, M.; Prieto-Castrillo, F.; Obagi, Z.; Falanga, V. Research techniques made simple: Analysis of collective cell migration using the wound healing assay. J. Investig. Dermatol. 2017, 137, e11–e16. [Google Scholar] [CrossRef] [Green Version]
- Sandhya, J.; Veeralakshmi, S.; Kalaiselvam, S. Tripolyphosphate crosslinked Triticum aestivum (wheatgrass) functionalized antimicrobial chitosan: Ameliorating effect on physicochemical, mechanical, in vitro cytocompatibility and cell migration properties. J. Biomol. Struct. Dyn. 2021, 39, 1635–1644. [Google Scholar] [CrossRef]
- Mujoriya, R.; Bodla, R.B. A study on wheat grass and its nutritional value. Food Sci. Qual. Manag. 2011, 2, 1–8. [Google Scholar]
- Durairaj, V.; Hoda, M.; Shakya, G.; Babu, S.P.P.; Rajagopalan, R. Phytochemical screening and analysis of antioxidant properties of aqueous extract of wheatgrass. Asian Pac. J. Trop. Med. 2014, 7, S398–S404. [Google Scholar] [CrossRef] [Green Version]
- Luqman, S.; Rizvi, S.I. Protection of lipid peroxidation and carbonyl formation in proteins by capsaicin in human erythrocytes subjected to oxidative stress. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2006, 20, 303–306. [Google Scholar] [CrossRef]
- Macdougall, J. Analysis of Dose-Response Studies-E Max Model. In Dose Finding in Drug Development; Springer: Berlin/Heidelberg, Germany, 2006; pp. 127–145. [Google Scholar]
- Sundaram, V.; Mohammed, S.; Srinivasan, M.; Johnson, J.; Suepaul, R.; Pargass, I.; John, C.; Ramdhanie, D.; Lallack, S.; Daniel, E. Acute and subacute toxicity evaluation of hydroalcoholic extract from the stem bark of Bois Bande (Parinari campestris Aubl. 1772) in rats. BMC Pharmacol. Toxicol. 2021, 22, 51. [Google Scholar] [CrossRef] [PubMed]
- Nasir, S.; Nasir, I.; Asrar, M.; Debboun, M. Larvicidal and pupicidal action of medicinal plant extracts against dengue mosquito Aedes albopictus (Skuse)(Diptera: Culicidae). Indian J. Anim. Res. 2017, 51, 155–158. [Google Scholar] [CrossRef] [Green Version]
- Ullah, Z.; Ijaz, A.; Mughal, T.K.; Zia, K. Larvicidal activity of medicinal plant extracts against Culex quinquefasciatus Say. (Culicidae, Diptera). Int. J. Mosq. Res. 2018, 5, 47–51. [Google Scholar]
- Hassanain, A.N.; Zeinhom, A.S.; Mokhtar, M.M.; Shaapan, M.R.; Hassanain, A.M.; Zaky, S. Comparison between insecticidal activity of lantana camara extract and its synthesized nanoparticles against Anopheline mosquitoes. Pak. J. Biol. Sci. 2019, 22, 327–334. [Google Scholar]
- Shehata, A.Z. Biological activity of Prunus domestica (Rosaceae) and Rhamnus cathartica (Rhamnaceae) leaves extracts against the mosquito vector, Culex pipiens L. (Diptera: Culicidae). Egypt. Acad. J. Biol. Sci. F Toxicol. Pest Control. 2019, 11, 65–73. [Google Scholar] [CrossRef]
- Dey, P.; Goyary, D.; Chattopadhyay, P.; Kishor, S.; Karmakar, S.; Verma, A. Evaluation of larvicidal activity of Piper longum leaf against the dengue vector, Aedes aegypti, malarial vector, Anopheles stephensi and filariasis vector, Culex quinquefasciatus. S. Afr. J. Bot. 2020, 132, 482–490. [Google Scholar] [CrossRef]
- Farag, S.; Adel Hussein, M.; Hafez, S.E.; Khaled, A.S.; Kamel, O.M.H.M.; Zyaan, O.H. Ultra-structural studies on the midgut of Culex pipiens larvae treated with pomegranate peel extract, Punica granatum. J. Egypt. Soc. Parasitol. 2018, 48, 77–84. [Google Scholar] [CrossRef]
- Souza, M.M.; Silva, B.D.; Costa, C.S.; Badiale-Furlong, E. Free phenolic compounds extraction from Brazilian halophytes, soybean and rice bran by ultrasound-assisted and orbital shaker methods. An. Da Acad. Bras. Ciências 2018, 90, 3363–3372. [Google Scholar] [CrossRef]
- De Dobbeleer, I.; Gummersbach, J.; Huebschmann, H.-J.; Mayer, A.; Silcock, P. Analyzing PBDEs in house dust samples with the Thermo Scientific TSQ Quantum XLS Ultra GC-MS/MS in EI-SRM mode. Dreieich Ger. Fish. Sci. 2012, 5, 1–6. [Google Scholar]
- Miguel, M.G. Antioxidant activity of medicinal and aromatic plants. A review. Flavour Fragr. J. 2010, 25, 291–312. [Google Scholar] [CrossRef]
- Abd-ElGawad, A.M.; Elshamy, A.I.; El-Amier, Y.A.; El Gendy, A.E.-N.G.; Al-Barati, S.A.; Dar, B.A.; Al-Rowaily, S.L.; Assaeed, A.M. Chemical composition variations, allelopathic, and antioxidant activities of Symphyotrichum squamatum (Spreng.) Nesom essential oils growing in heterogeneous habitats. Arab. J. Chem. 2020, 13, 4237–4245. [Google Scholar] [CrossRef]
- Parejo, I.; Codina, C.; Petrakis, C.; Kefalas, P. Evaluation of scavenging activity assessed by Co (II)/EDTA-induced luminol chemiluminescence and DPPH·(2, 2-diphenyl-1-picrylhydrazyl) free radical assay. J. Pharmacol. Toxicol. Methods 2000, 44, 507–512. [Google Scholar] [CrossRef]
- Kim, E.; Kim, H.-J.; Yang, S.-M.; Kim, C.-G.; Choo, D.-W.; Kim, H.-Y. Rapid identification of Staphylococcus species isolated from food samples by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. J. Microbiol. Biotechnol. 2019, 29, 548–557. [Google Scholar] [CrossRef]
- Sardari, S.; Amin, G.; Micetich, R.G.; Daneshtalab, M. Phytopharmaceuticals. Part 1. Antifungal activity of selected Iranian and Canadian plants. Pharm. Biol. 1998, 36, 180–188. [Google Scholar] [CrossRef]
- Terblanche, U.; Semakalu, C.; Mtunzi, F.; Pillay, M. Screening of variables influencing extraction yield of Cotyledon orbiculata: 23 full factorial design. Int. J. Pharmacogn. Phytochem. Res. 2017, 9, 303–312. [Google Scholar]
- Zhu, Q.; Jiang, L.; Wang, X. The expression of duffy antigen receptor for chemokines by epithelial ovarian cancer decreases growth potential. Oncol. Lett. 2017, 13, 4302–4306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aktas, H.G.; Ayan, H. Oleuropein: A potential inhibitor for prostate cancer cell motility by blocking voltage-gated sodium channels. Nutr. Cancer 2021, 73, 1758–1767. [Google Scholar] [CrossRef]
- El-Sheikh, T.M.; Hassan, M.I.; Moselhy, W.A.; Amer, M.S.; Shehata, A.Z. Evaluation of the biological activity of some Cupressus semprevirens (Cupressaceae) extracts against the mosquito vector Culex pipiens L. (Diptera: Culicidae). Egypt. Acad. J. Biol. Sci. A Entomol. 2011, 4, 33–48. [Google Scholar] [CrossRef] [Green Version]
- Briggs, J.D. Reduction of adult house-fly emergence by the effects of Bacillus spp. on the development of immature forms. J. Insect Pathol. 1960, 2, 418–432. [Google Scholar]
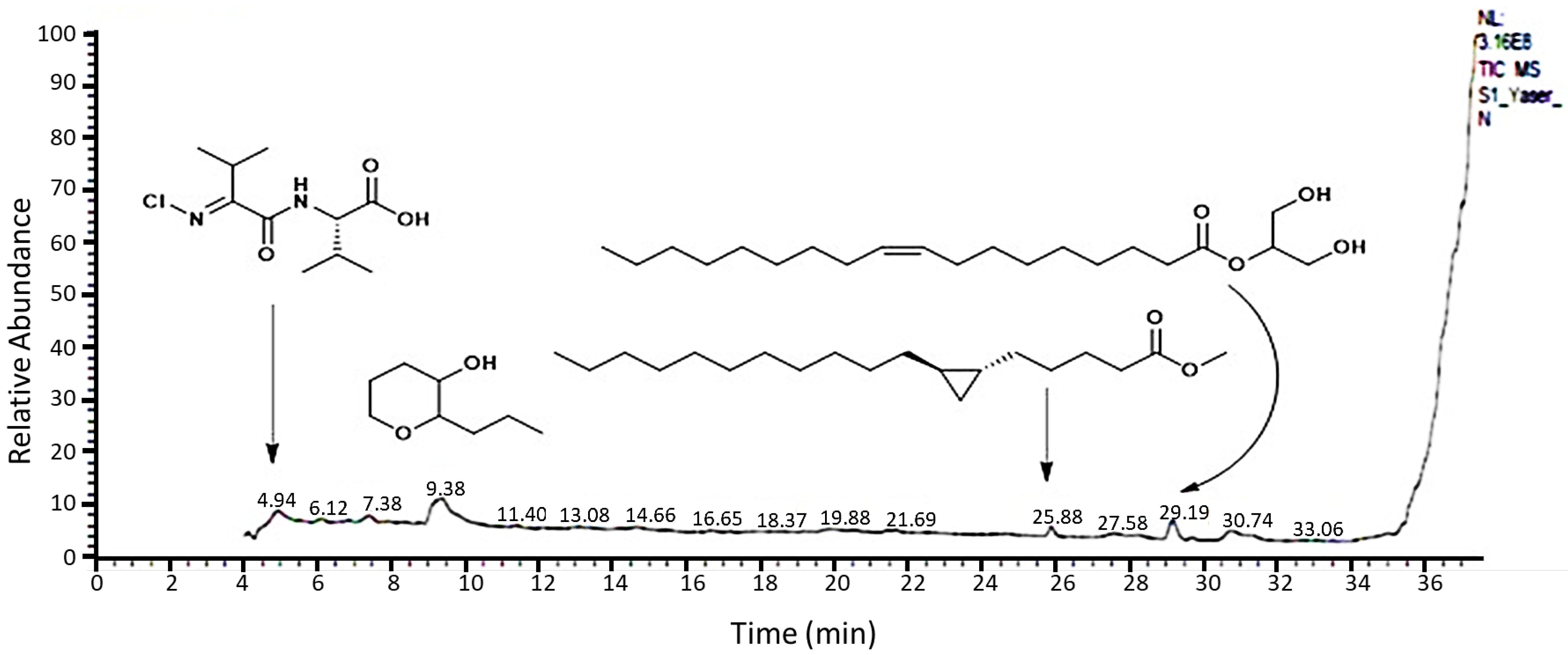


| Entry | Chemical Name | Classification | Rt | MF | Composition % |
|---|---|---|---|---|---|
| Oxygenated Hydrocarbon | |||||
| 1 | (Z)-2-Ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine | Aryl substituted cyclic amine | 4.21 | C20H23N | 0.92 |
| 2 | 3-(2-Oxocyclohexyl)propanenitrile | Oxygenated hydrocarbon | 5.54 | C9H13NO | 0.41 |
| 3 | Ethyl 2-hydroxycyclohexane-1-carboxylate | Oxygenated hydrocarbon | 9.22 | C9H16O3 | 18.96 |
| 4 | 2-Propyltetrahydro-2H-pyran-3-ol | Oxygenated hydrocarbon | 9.38 | C8H16O2 | 11.18 |
| 5 | Ascaridole epoxide | Oxygenated hydrocarbon | 12.99 | C10H16O3 | 0.67 |
| 6 | 3,5-Heptadienal, 2-ethylidene-6-methyl-“(2Z,3E)-2-ethylidene-6-methylhepta-3,5-dienal” | Oxygenated hydrocarbon | 13.07 | C10H14O | 0.28 |
| 7 | 1,25-Dihydroxyvitamin D3, TMS derivative | Oxygenated hydrocarbon | 34.16 | C30H52O3Si | 0.20 |
| 8 | Deoxyspergualin | Polyamine spermidine | 8.74 | C17H37N7O3 | 0.36 |
| 9 | Methyl 2,2,3,3,4,4,4-heptafluorobutanoate | Ester | 12.87 | C5H3F7O2 | 0.20 |
| 10 | Tetraacetyl-d-xylonic nitrile | Polyester | 14.63 | C14H17NO9 | 0.41 |
| Fatty Acids and Lipids | |||||
| 11 | High oleic safflower oil | Vegetable oil “80% oleic acid” | 4.45 | C21H22O11 | 2.93 |
| 12 | Methyl octadeca-8,11-diynoate | Fatty acid | 6.13 | C19H30O2 | 3.82 |
| 13 | (2-Phenyl-1,3-dioxolan-4-yl)methyl oleate | Fatty-acid derivative | 7.40 | C28H44O4 | 5.75 |
| 14 | bis(2-Ethylhexyl) adipate | Ester of fatty acid | 7.94 | C22H42O4 | 0.80 |
| 15 | d-Lyxo-d-manno-nononic-1,4-lactone | Lactone of tetronic acid | 11.31 | C9H16O9 | 0.56 |
| 16 | tert-Butyl palmitate | Ester of fatty acid | 14.52 | C20H40O2 | 0.26 |
| 17 | Oleic acid | Fatty acid | 14.67 | C18H34O2 | 0.57 |
| 18 | 9-Hexadecenoic acid | Fatty acid | 15.20 | C16H30O2 | 0.17 |
| 19 | trans-2-Dodecenoic acid | Fatty acid | 15.52 | C12H22O2 | 0.24 |
| 20 | 2-Hydroxypropane-1,3-diyl (9E,9’E)-bis(octadec-9-enoate) | Diester derivative of fatty acid | 16.64 | C39H72O5 | 0.94 |
| 21 | (E)-Octadec-13-enoic acid | Fatty acid | 17.13 | C18H34O2 | 2.74 |
| 22 | 2-Bromotetradecanoic acid | Fatty-acid derivative | 19.89 | C14H27BrO2 | 1.45 |
| 23 | [1,1’-Bicyclopropyl]-2-octanoic acid, 2’-hexyl-, methyl ester | Fatty-acid derivative | 19.96 | C21H38O2 | 0.90 |
| 24 | 8-((2R,3S)-3-Octyloxiran-2-yl)octanoic acid | Fatty acid | 21.41 | C18H34O3 | 1.09 |
| 25 | 2,3-Dihydroxypropyl stearate | Fatty acid | 21.69 | C21H42O4 | 1.23 |
| 26 | Methyl 5-((1R,2R)-2-undecylcyclopropyl)-pentanoate | Fatty-acid derivative | 25.88 | C20H38O2 | 4.50 |
| 27 | 2-Hydroxypropane-1,3-diyl dipalmitate | Ester of fatty acid | 28.31 | C35H68O5 | 2.87 |
| 28 | 1,3-Dihydroxypropan-2-yl oleate | Ester of fatty acid | 29.18 | C21H40O4 | 17.56 |
| 29 | Methyl 11-((2R,3R)-3-pentyloxiran-2-yl)undecanoate | Ester of fatty acid | 29.70 | C19H36O3 | 1.75 |
| 30 | 9-Octadecenoic acid,1,2,3-propanetriyl ester, (E,E,E)- | Ester of fatty acid | 31.35 | C57H104O6 | 1.23 |
| Carbohydrates | |||||
| 31 | d-Gala-l-ido-octonic amide “2,3,4,5,6,7,8-heptahydroxyoctanamide” | Carbohydrate amide | 6.50 | C8H17NO8 | 0.26 |
| 32 | Desulfosinigrin “1-S-[(1E)-N-hydroxy-3-butenimidoyl]-1-thiohexopyranose” | Glycoside | 6.64 | C10H17NO6S | 0.27 |
| 33 | l-Gala-l-ido-octose | Carbohydrate | 6.85 | C8H16O8 | 1.09 |
| 34 | Melezitose | Trisaccharide sugar | 8.79 | C18H32O16 | 0.34 |
| 35 | d-Ribo-hexos-3-ulose “(2S,4R,5R)-2,4,5,6-tetrahydroxy-3-oxohexanal” | Dicarbonyl sugar | 9.84 | C6H10O6 | 0.30 |
| 36 | 2,3-Dihydroxypropyl palmitate | 1-Monoacylglycerols | 4.89 | C19H38O4 | 3.80 |
| Amines | |||||
| 37 | N2,N4-Diisopropyl-6-(methylsulfonyl)-1,3,5-triazine-2,4-diamine | Hetryl amine | 4.14 | C10H19N5O2S | 2.44 |
| 38 | (E)-(2-(Chloroimino)-3-methylbutanoyl)-l-valine | Amino acid | 4.94 | C10H17ClN2O3 | 3.84 |
| 39 | S-(2-Aminoethyl) O-hydrogen sulfurothioate | Amino-thioester | 11.39 | C2H7NO3S2 | 0.59 |
| 40 | Glutamic acid | Amino acid | 11.46 | C5H9NO4 | 0.34 |
| 41 | Methyl N-acetyl-d-glucosamide | N-Acetyl-d-glucosamine | 10.35 | C9H17NO6 | 0.14 |
| Steroids | |||||
| 42 | Estra-1,3,5(10)-trien-17β-ol | Steroid | 20.54 | C18H24O | 0.34 |
| 43 | Ethyl iso-allocholate | Steroidal ester | 34.94 | C26H44O5 | 0.87 |
| Alkaloids | |||||
| 44 | 19,20-Didehydroyohimbinone | Indole alkaloid | 7.88 | C21H22N2O3 | 0.42 |
| Total | 99.99 | ||||
| Treatment | Conc. (mg/L) | Radical Scavenging Activity (%) | IC50 (mg/L) |
|---|---|---|---|
| Rumex vesicarius L | 5 | 10.64 ± 0.51 F | 28.89 |
| 10 | 33.05 ± 1.41 E | ||
| 20 | 46.04 ± 2.26 D | ||
| 30 | 53.80 ± 2.60 C | ||
| 40 | 59.83 ± 3.01 B | ||
| 50 | 74.28 ± 3.51 A | ||
| LSD0.05 | 1.81 *** | ||
| Ascorbic acid | 1 | 2.52 ± 0.01 F | 12.48 |
| 2.5 | 10.52 ± 0.02 E | ||
| 5 | 36.77 ± 0.17 D | ||
| 10 | 49.62 ± 0.31 C | ||
| 15 | 59.33 ± 1.12 B | ||
| 20 | 69.11 ± 1.43 A | ||
| LSD0.05 | 1.61 *** |
| Microbes | R. vesicarius (10 mg/mL) | Standard Antibiotic (10 mg/L) | |||
|---|---|---|---|---|---|
| Cephradin | Tetracycline | Azithromycin | Ampicillin | ||
| Gram-negative bacteria | |||||
| Escherichia coli | 21.07 ± 0.80 B | 16.37 ± 0.62 D | 19.61 ± 0.74 BC | 19.01 ± 0.72 B | 19.77 ± 0.75 C |
| Pseudomonas aeruginosa | 10.12 ± 0.38 E | 0.00 F | 0.00 E | 13.06 ± 0.49 C | 0.00 F |
| Salmonella Typhimurium | 23.46 ± 1.69 A | 0.00 F | 11.05 ± 0.42 D | 0.00 D | 0.00 F |
| Klebsiella pneumoniae | 14.38 ± 0.54 D | 11.55 ± 0.44 E | 19.09 ± 0.72 C | 12.44 ± 0.47 C | 6.08 ± 0.23 E |
| Gram-positive bacteria | |||||
| Bacillus cereus | 22.91 ± 0.96 AB | 19.67 ± 0.74 BC | 11.04 ± 0.62 D | 18.99 ± 0.82 B | 8.11 ± 0.31 D |
| Staphylococcus aureus | 17.83 ± 0.67 C | 20.14 ± 0.76 B | 21.77 ± 0.82 AB | 19.35 ± 0.73 B | 29.61 ± 1.72 A |
| Staphylococcus haemolyticus | 13.66 ± 0.52 D | 24.80 ± 1.94 A | 22.68 ± 0.96 A | 22.08 ± 0.93 A | 21.04 ± 0.99 C |
| Staphylococcus xylosus | 10.54 ± 0.40 E | 18.53 ± 1.70 C | 20.45 ± 0.77 ABC | 21.41 ± 0.81 A | 24.11 ± 1.81 B |
| LSD0.05 | 0.0000 *** | 0.0000 *** | 0.0000 *** | 0.0000 *** | 0.0000 *** |
| Sample | Conc. (µg/mL) | R1 [a] | R2 [a] | IC50 (µg/mL) [b] |
|---|---|---|---|---|
| R. vesicarius | 1000 | 0.22 | 0.218 | 501.4 |
| 500 | 0.92 | 0.96 | ||
| 125 | 1.6 | 1.6 | ||
| 62.5 | 1.608 | 1.683 | ||
| 31.3 | 1.63 | 1.64 | ||
| 0 | 1.3 | 1.3 |
| Conc. (mg/L) | R. vesicarius | |
|---|---|---|
| 24 h Post Treatment | 48 h Post Treatment | |
| 1200 | 28.9 ± 1.10 A | 42.6 ± 2.00 A |
| 1000 | 25.7 ± 1.00 B | 28.9 ± 1.10 B |
| 500 | 15.5 ± 1.10 C | 22.4 ± 1.20 C |
| 250 | 7.7 ± 1.10 D | 14.4 ± 2.20 D |
| 125 | 0.00 F | 1.1 ± 1.10 E |
| Control | 1.1 ± 1.10 E | 1.1 ± 1.10 E |
| F-value | 153.883 * | 111.955 * |
| p-value | (<0.001 *) | (<0.001 *) |
| LC50 | 19.99 | 14.97 |
| LC90 | 36.12 | 27.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salama, S.A.; AL-Faifi, Z.E.; Masood, M.F.; El-Amier, Y.A. Investigation and Biological Assessment of Rumex vesicarius L. Extract: Characterization of the Chemical Components and Antioxidant, Antimicrobial, Cytotoxic, and Anti-Dengue Vector Activity. Molecules 2022, 27, 3177. https://doi.org/10.3390/molecules27103177
Salama SA, AL-Faifi ZE, Masood MF, El-Amier YA. Investigation and Biological Assessment of Rumex vesicarius L. Extract: Characterization of the Chemical Components and Antioxidant, Antimicrobial, Cytotoxic, and Anti-Dengue Vector Activity. Molecules. 2022; 27(10):3177. https://doi.org/10.3390/molecules27103177
Chicago/Turabian StyleSalama, Salama A., Zarraq E. AL-Faifi, Mostafa F. Masood, and Yasser A. El-Amier. 2022. "Investigation and Biological Assessment of Rumex vesicarius L. Extract: Characterization of the Chemical Components and Antioxidant, Antimicrobial, Cytotoxic, and Anti-Dengue Vector Activity" Molecules 27, no. 10: 3177. https://doi.org/10.3390/molecules27103177
APA StyleSalama, S. A., AL-Faifi, Z. E., Masood, M. F., & El-Amier, Y. A. (2022). Investigation and Biological Assessment of Rumex vesicarius L. Extract: Characterization of the Chemical Components and Antioxidant, Antimicrobial, Cytotoxic, and Anti-Dengue Vector Activity. Molecules, 27(10), 3177. https://doi.org/10.3390/molecules27103177







