A Panchromatic Cyclometalated Iridium Dye Based on 2-Thienyl-Perimidine
Abstract
:1. Introduction
2. Results and Discussion
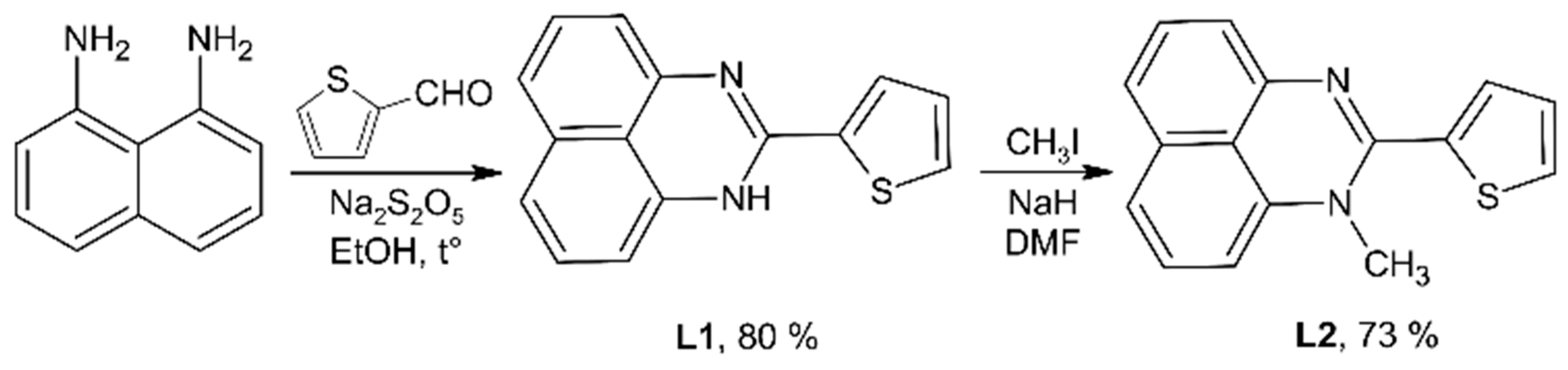
2.1. Synthesis and Crystal Structures of Ligands
2.2. Optical Properties of Ligands
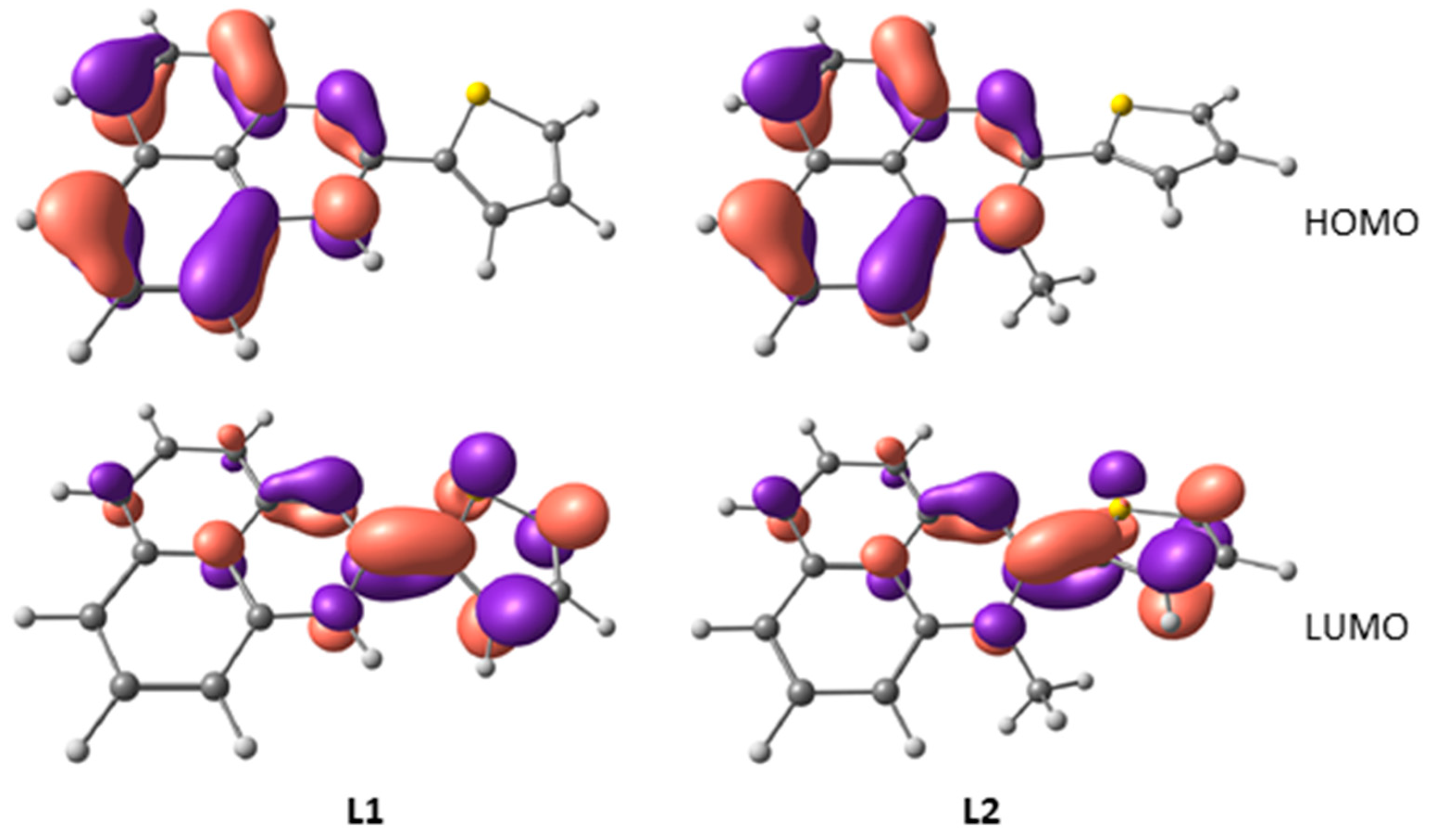
2.3. DFT Calculations of Perimidine Ligands
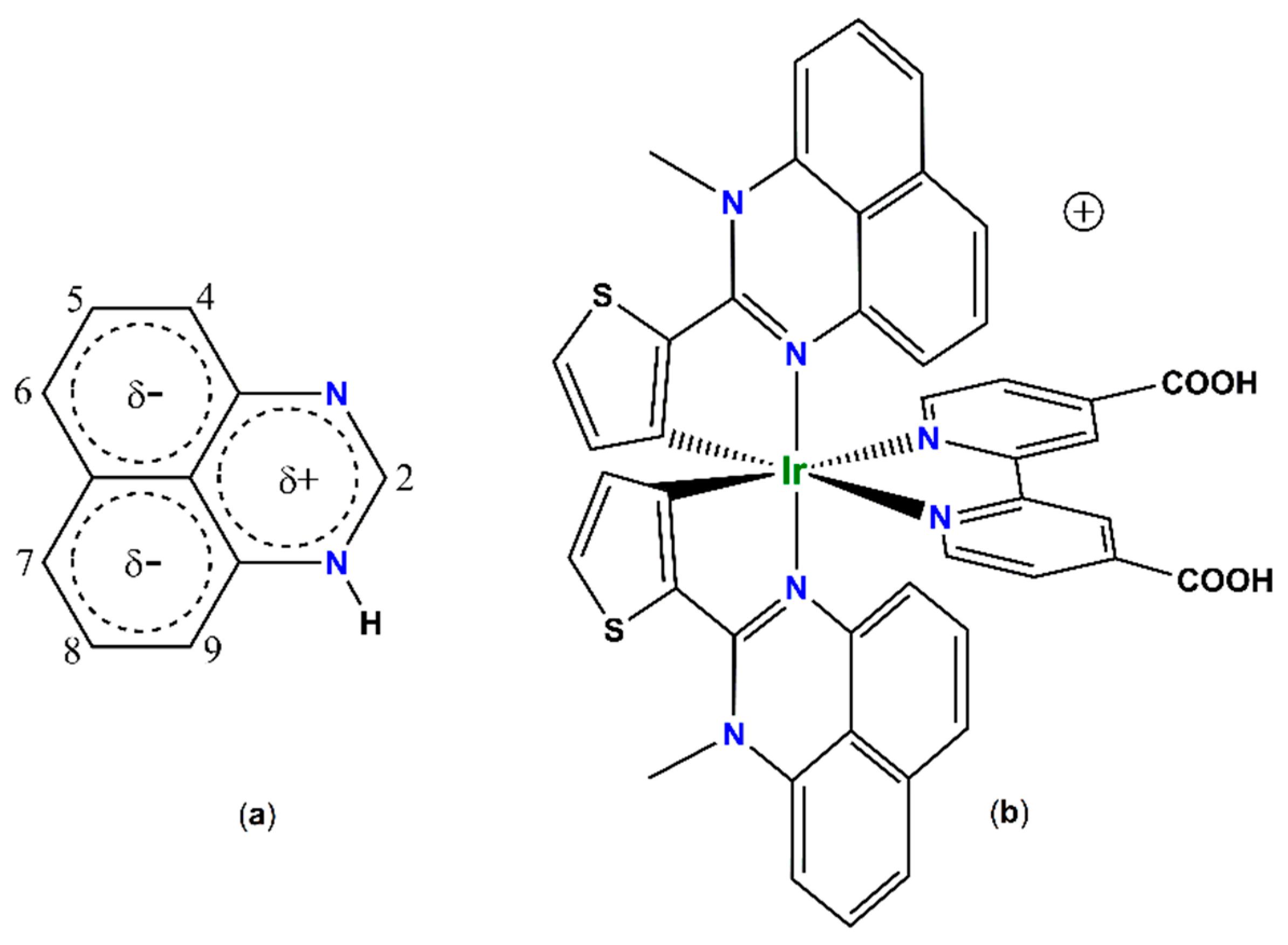
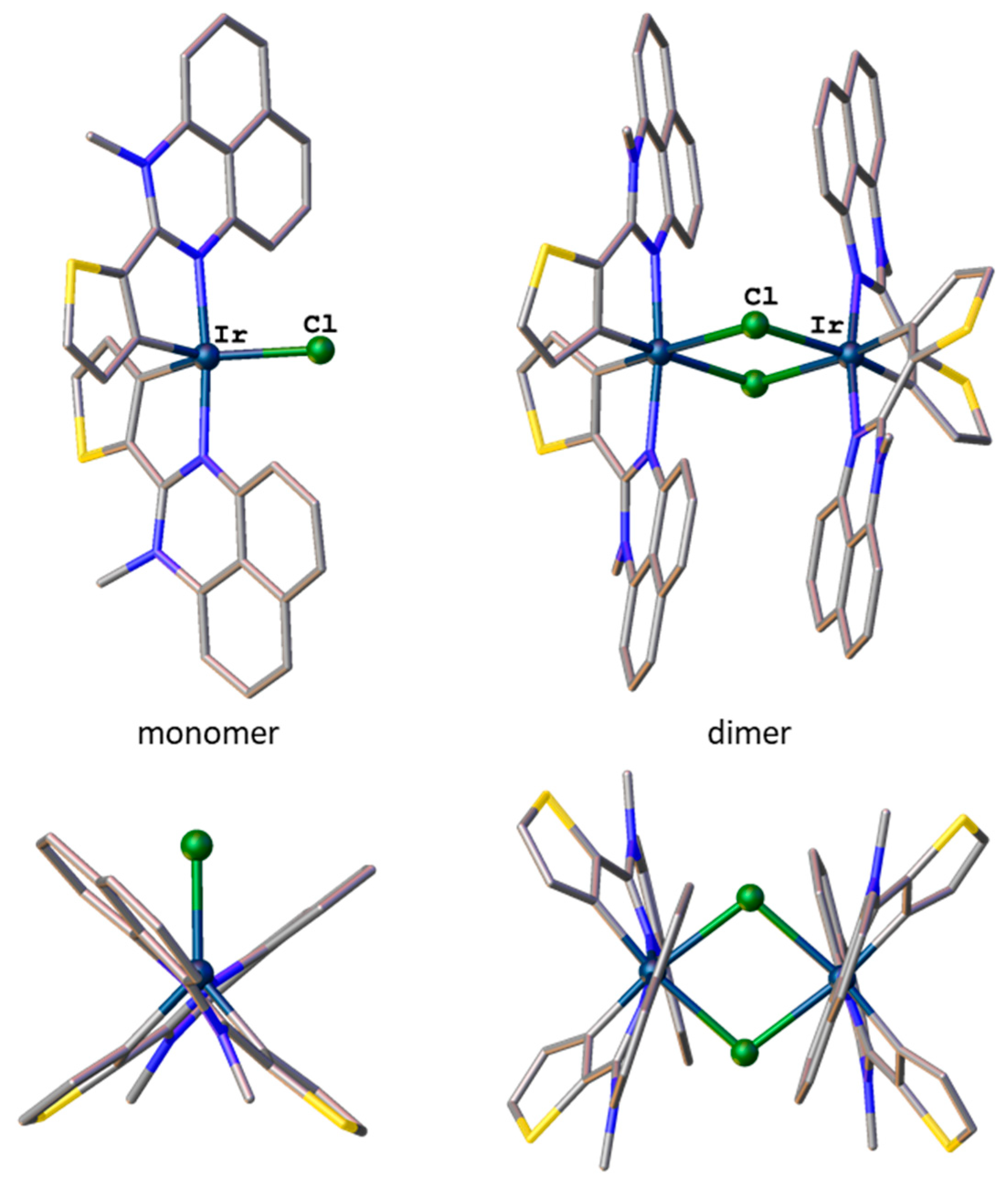
2.4. Geometry and Electronic Structure of Iridium(III) Cyclometalated Chlorides Based on Perimidines: A DFT Study
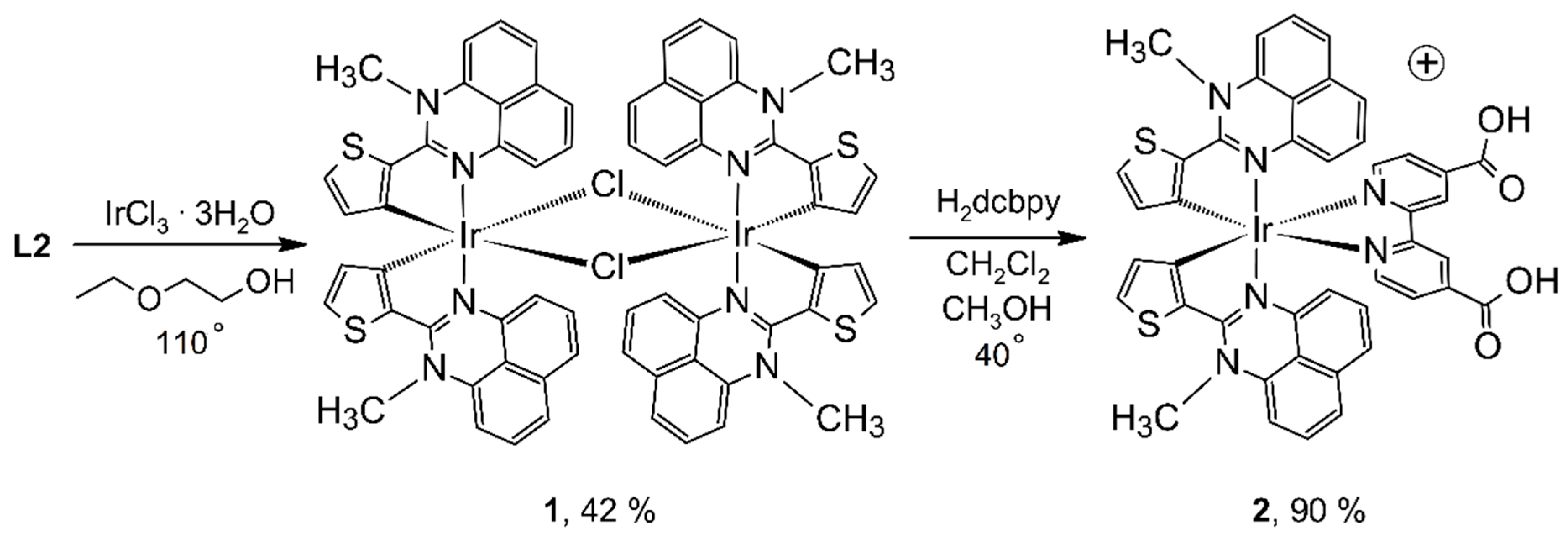
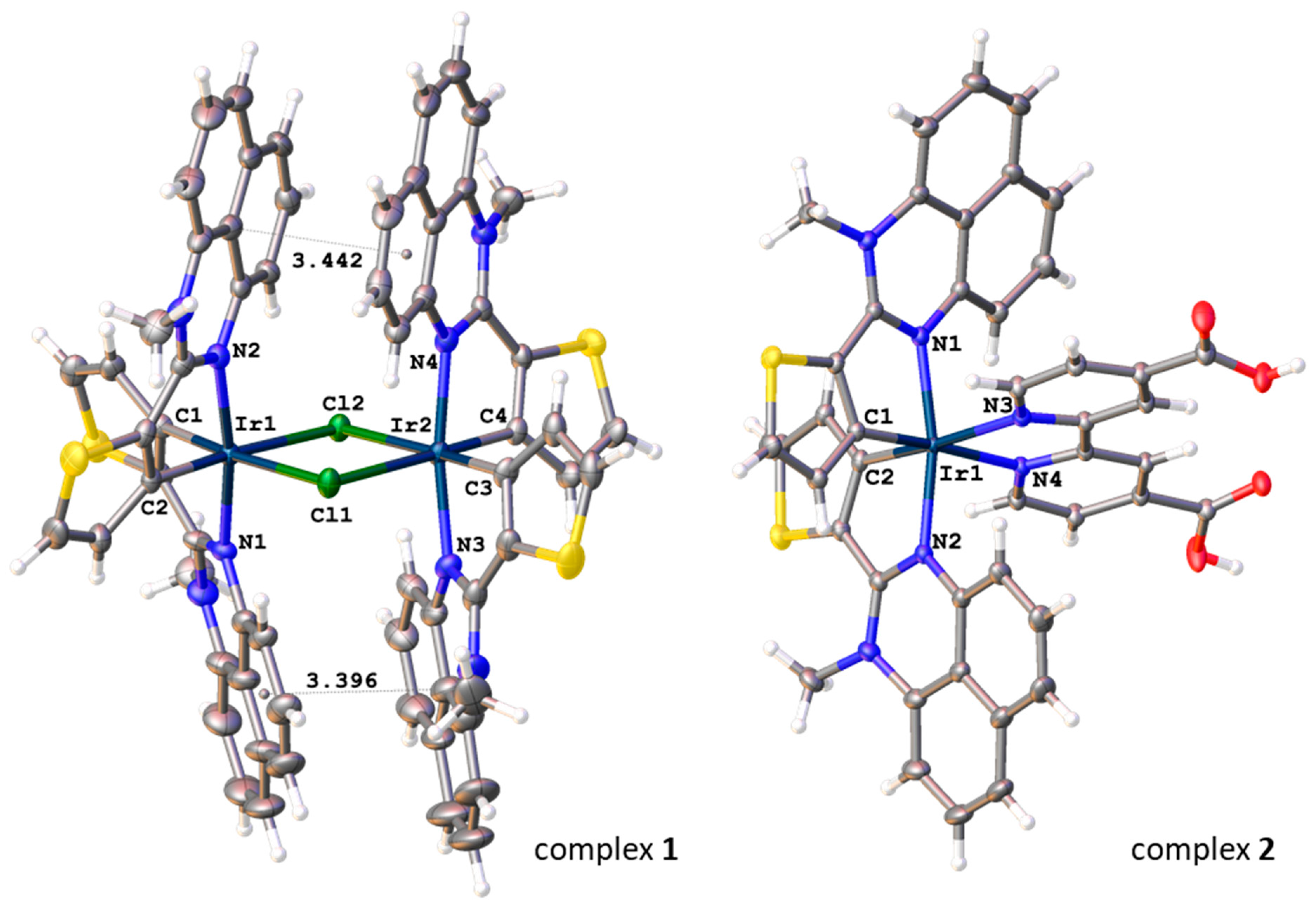
2.5. Synthesis and Structure of Cyclometalated Iridium(III) Complexes with Perimidine Ligands
2.6. Optical and Redox Properties of Complex 2
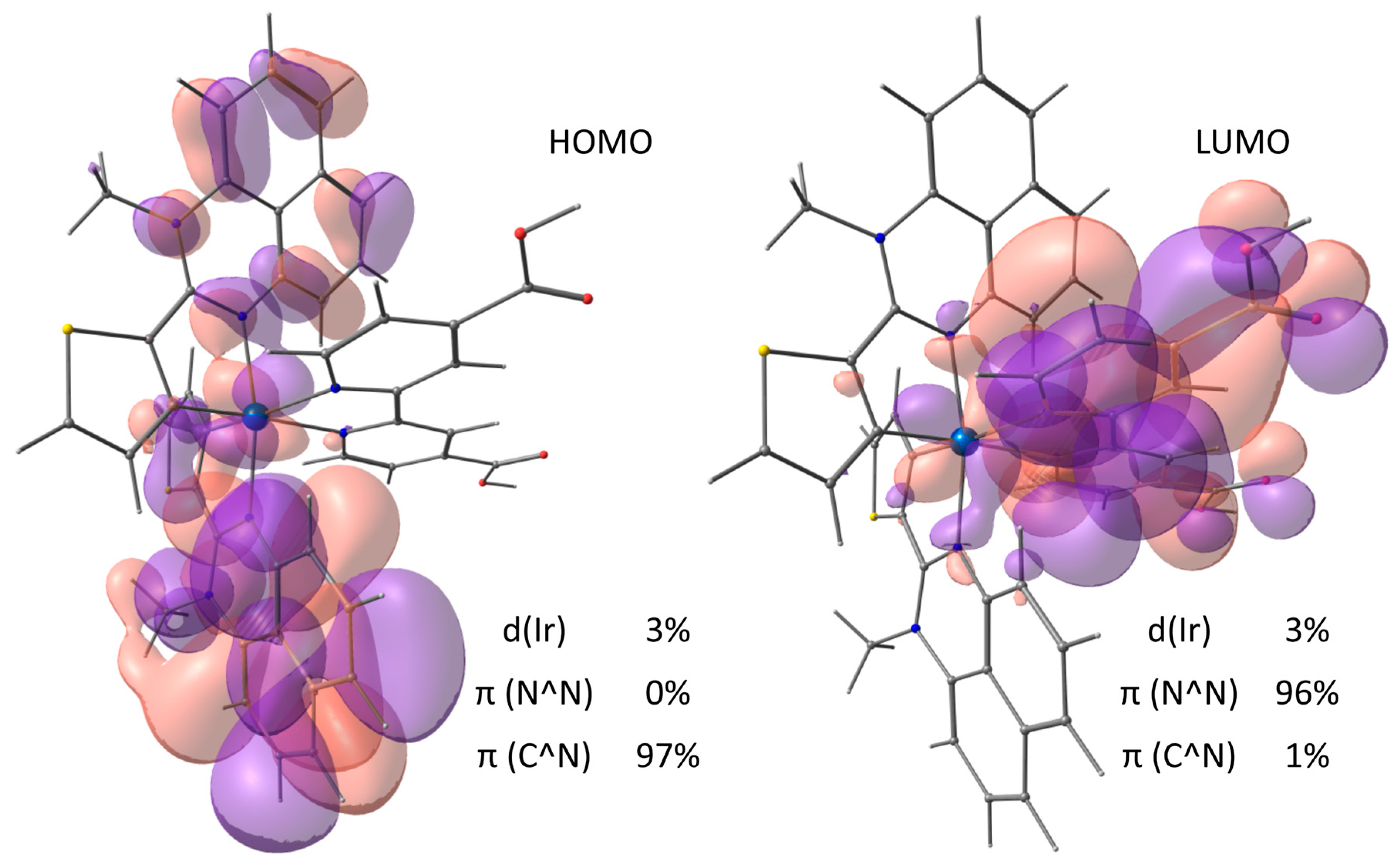
2.7. DFT/TDDFT Calculations of Complex 2
3. Materials and Methods
3.1. General Comment
3.2. Synthesis
3.3. X-ray Crystallography
3.3.1. Crystal Growth Conditions
- L1:
- Slow evaporation of the solution of perimidine L1 in dichloromethane.
- L2:
- Slow evaporation of the solution of perimidine L2 in dichloromethane.
- 1:
- Recrystallization of complex 1 from hot toluene.
- 2:
- Addition of the saturated methanolic solution of NH4PF6 to the solution of complex 2 in dichloromethane/methanol mixture.
3.3.2. Crystallography Details
3.3.3. Photovoltaic Measurements
3.3.4. Density Functional Theory Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Mao, H.-T.; Li, G.-F.; Shan, G.-G.; Wang, X.-L.; Su, Z.-M. Recent Progress in Phosphorescent Ir(III) Complexes for Nondoped Organic Light-Emitting Diodes. Coord. Chem. Rev. 2020, 413, 213283. [Google Scholar] [CrossRef]
- Wen, L.-L.; Zang, C.-X.; Gao, Y.; Li, G.-F.; Shan, G.-G.; Wang, X.-L.; Shao, K.-Z.; Xie, W.-F.; Su, Z.-M. Rational Design of Ir(III) Phosphors to Strategically Manage Charge Recombination for High-Performance White Organic Light-Emitting Diodes. Inorg. Chem. 2022, 61, 3736–3745. [Google Scholar] [CrossRef]
- Bevernaegie, R.; Wehlin, S.A.M.; Elias, B.; Troian-Gautier, L. A Roadmap Towards Visible Light Mediated Electron Transfer Chemistry with Iridium(III) Complexes. ChemPhotoChem 2021, 5, 217–234. [Google Scholar] [CrossRef]
- Yiu, S.; Ho, P.; Kwok, Y.; He, X.; Wang, Y.; Yu, W.; Ho, C.; Huang, S. Development of Strong Visible-Light-Absorbing Cyclometalated Iridium(III) Complexes for Robust and Efficient Light-Driven Hydrogen Production. Chem. Eur. J. 2022, 28, e202104575. [Google Scholar] [CrossRef]
- Baranoff, E.; Yum, J.H.; Graetzel, M.; Nazeeruddin, M.K. Cyclometallated Iridium Complexes for Conversion of Light into Electricity and Electricity into Light. J. Organomet. Chem. 2009, 694, 2661–2670. [Google Scholar] [CrossRef]
- Dragonetti, C.; Valore, A.; Colombo, A.; Righetto, S.; Trifiletti, V. Simple Novel Cyclometallated Iridium Complexes for Potential Application in Dye-Sensitized Solar Cells. Inorg. Chim. Acta 2012, 388, 163–167. [Google Scholar] [CrossRef]
- Baranoff, E.; Yum, J.H.; Jung, I.; Vulcano, R.; Gratzel, M.; Nazeeruddin, M.K. Cyclometallated Iridium Complexes as Sensitizers for Dye-Sensitized Solar Cells. Chem. Asian J. 2010, 5, 496–499. [Google Scholar] [CrossRef]
- Bezzubov, S.I.; Kiselev, Y.M.; Churakov, A.V.; Kozyukhin, S.A.; Sadovnikov, A.A.; Grinberg, V.A.; Emets, V.V.; Doljenko, V.D. Iridium(III) 2-Phenylbenzimidazole Complexes: Synthesis, Structure, Optical Properties, and Applications in Dye-Sensitized Solar Cells. Eur. J. Inorg. Chem. 2016, 2016, 347–354. [Google Scholar] [CrossRef]
- Bezzubov, S.I.; Zharinova, I.S.; Khusyainova, A.A.; Kiselev, Y.M.; Taydakov, I.V.; Varaksina, E.A.; Metlin, M.T.; Tobohova, A.S.; Korshunov, V.M.; Kozyukhin, S.A.; et al. Aromatic Β-Diketone as a Novel Anchoring Ligand in Iridium(III) Complexes for Dye-Sensitized Solar Cells. Eur. J. Inorg. Chem. 2020, 2020, 3277–3286. [Google Scholar] [CrossRef]
- Légalité, F.; Escudero, D.; Pellegrin, Y.; Blart, E.; Jacquemin, D.; Fabrice, O. “Iridium Effect” in Cyclometalated Iridium Complexes for p-Type Dye Sensitized Solar Cells. Dye. Pigment. 2019, 171, 107693. [Google Scholar] [CrossRef]
- Bobo, M.V.; Paul, A.; Robb, A.J.; Arcidiacono, A.M.; Smith, M.D.; Hanson, K.; Vannucci, A.K. Bis-Cyclometalated Iridium Complexes Containing 4,4′-Bis(Phosphonomethyl)-2,2′-Bipyridine Ligands: Photophysics, Electrochemistry, and High-Voltage Dye-Sensitized Solar Cells. Inorg. Chem. 2020, 59, 6351–6358. [Google Scholar] [CrossRef]
- Sinopoli, A.; Wood, C.J.; Gibson, E.A.; Elliott, P.I.P. Hybrid Cyclometalated Iridium Coumarin Complex as a Sensitiser of Both N- and p-Type DSSCs. Eur. J. Inorg. Chem. 2016, 2016, 2887–2890. [Google Scholar] [CrossRef]
- Sinopoli, A.; Wood, C.J.; Gibson, E.A.; Elliott, P.I.P. New Cyclometalated Iridium(III) Dye Chromophore Complexes for p-Type Dye-Sensitised Solar Cells. Dye. Pigment. 2017, 140, 269–277. [Google Scholar] [CrossRef] [Green Version]
- Hierlinger, C.; Flint, H.V.; Cordes, D.B.; Slawin, A.M.Z.; Gibson, E.A.; Jacquemin, D.; Guerchais, V.; Zysman-Colman, E. A Panchromatic, near Infrared Ir(III) Emitter Bearing a Tripodal C^N^C Ligand as a Dye for Dye-Sensitized Solar Cells. Polyhedron 2018, 140, 109–115. [Google Scholar] [CrossRef] [Green Version]
- Sachs, F. Ueber Ringschlüsse in Peristellung Der Naphtalinreihe. Justus Liebigs Ann. Chem. 1909, 365, 53–134. [Google Scholar] [CrossRef]
- Pozharskii, A.F.; Gulevskaya, A.V.; Claramunt, R.M.; Alkorta, I.; Elguero, J. Perimidines: A Unique π-Amphoteric Heteroaromatic System. Russ. Chem. Rev. 2020, 89, 1204–1260. [Google Scholar] [CrossRef]
- Sahiba, N.; Agarwal, S. Recent Advances in the Synthesis of Perimidines and Their Applications. Top. Curr. Chem. 2020, 378, 44. [Google Scholar] [CrossRef]
- Harry, N.A.; Ujwaldev, S.M.; Aneeja, T.; Anilkumar, G. A Comprehensive Overview of Perimidines: Synthesis, Chemical Transformations, and Applications. Curr. Org. Chem. 2021, 25, 248–271. [Google Scholar] [CrossRef]
- Booysen, I.N.; Ebinumoliseh, I.; Sithebe, S.; Akerman, M.P.; Xulu, B. Coordination Behaviours of Perimidine Ligands Incorporating Fused N-Donor Heterocyclics towards Rhenium(I) and -(V). Polyhedron 2016, 117, 755–760. [Google Scholar] [CrossRef]
- Koo, J.Y.; Yakiyama, Y.; Lee, G.R.; Lee, J.; Choi, H.C.; Morita, Y.; Kawano, M. Selective Formation of Conductive Network by Radical-Induced Oxidation. J. Am. Chem. Soc. 2016, 138, 1776–1779. [Google Scholar] [CrossRef]
- Kim, J.; Koo, J.Y.; Lee, Y.H.; Kojima, T.; Yakiyama, Y.; Ohtsu, H.; Oh, J.H.; Kawano, M. Structural Investigation of Chemiresistive Sensing Mechanism in Redox-Active Porous Coordination Network. Inorg. Chem. 2017, 56, 8735–8738. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.Y.; Koo, J.Y.; Ohtsu, H.; Yakiyama, Y.; Kim, K.; Hashizume, D.; Kawano, M. An Organic Mixed-Valence Ligand for Multistate Redox-Active Coordination Networks. Angew. Chem. Int. Ed. 2018, 57, 4717–4721. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.F.; Ma, C.; McQueen, C.M.A.; Ward, J.S. Iridium Complexes of Perimidine-Based N-Heterocyclic Carbene Pincer Ligands via Aminal C–H Activation. Dalt. Trans. 2018, 47, 1577–1587. [Google Scholar] [CrossRef] [PubMed]
- Coluccini, C.; Manfredi, N.; Calderon, E.H.; Salamone, M.M.; Ruffo, R.; Roberto, D.; Lobello, M.G.; De Angelis, F.; Abbotto, A. Photophysical and Electrochemical Properties of Thiophene-Based 2-Arylpyridines. Eur. J. Org. Chem. 2011, 2011, 5587–5598. [Google Scholar] [CrossRef]
- Kalle, P.; Tatarin, S.V.; Zakharov, A.Y.; Kiseleva, M.A.; Bezzubov, S.I. Synthesis and Comparative Structural Study of 2-(Pyridin-2-yl)-1H-Perimidine and Its Mono- and Di-N-Methylated Analogues. Acta Crystallogr. Sect. E Crystallogr. Commun. 2021, 77, 96–100. [Google Scholar] [CrossRef]
- Kalle, P.; Tatarin, S.V.; Kiseleva, M.A.; Zakharov, A.Y.; Smirnov, D.E.; Bezzubov, S.I. Syntheses and Crystal Structures of 2-(p-Tolyl)-1H-Perimidine Hemihydrate and 1-Methyl-2-(p-Tolyl)-1H-Perimidine. Acta Crystallogr. Sect. E Crystallogr. Commun. 2022, 78, 169–172. [Google Scholar] [CrossRef]
- Zysman-Colman, E. (Ed.) Iridium(III) in Optoelectronic and Photonics Applications; John Wiley & Sons, Ltd.: Chichester, UK, 2017; ISBN 9781119007166. [Google Scholar]
- Tatarin, S.V.; Kalle, P.; Taydakov, I.V.; Varaksina, E.A.; Korshunov, V.M.; Bezzubov, S.I. Sterically Hindered Phenanthroimidazole Ligands Drive the Structural Flexibility and Facile Ligand Exchange in Cyclometalated Iridium(iii) Complexes. Dalt. Trans. 2021, 50, 6889–6900. [Google Scholar] [CrossRef]
- Bilyalova, A.A.; Tatarin, S.V.; Kalle, P.; Smirnov, D.E.; Zharinova, I.S.; Kiselev, Y.M.; Dolzhenko, V.D.; Bezzubov, S.I. Synthesis, Structure, Optical, and Electrochemical Properties of Iridium(III) Complexes with 2-Arylphenantroimidazoles and Dibenzoylmethane. Russ. J. Inorg. Chem. 2019, 64, 207–215. [Google Scholar] [CrossRef]
- Bezzubov, S.I.; Kalle, P.; Bilyalova, A.A.; Tatarin, S.V.; Dolzhenko, V.D. Overcoming the Inertness of Iridium(III) in a Facile Single-Crystal to Single-Crystal Reaction of Iodine Vapor with a Cyclometalated Chloride Monomer. Chem. Eur. J. 2018, 24, 12779–12783. [Google Scholar] [CrossRef]
- Wang, S.; Duan, Y.; Du, C.X.; Wu, Y.J. A New Iridium(III) Complex with Efficient Photo- and Electro-Luminescent Properties. Inorg. Chem. Commun. 2011, 14, 1393–1395. [Google Scholar] [CrossRef]
- Solomatina, A.I.; Kuznetsov, K.M.; Gurzhiy, V.V.; Pavlovskiy, V.V.; Porsev, V.V.; Evarestov, R.A.; Tunik, S.P. Luminescent Organic Dyes Containing a Phenanthro[9,10-D] Imidazole Core and [Ir(N^C)(N^N)]+ Complexes Based on the Cyclometalating and Diimine Ligands of This Type. Dalt. Trans. 2020, 49, 6751–6763. [Google Scholar] [CrossRef] [PubMed]
- Takimoto, K.; Watanabe, Y.; Yoshida, J.; Sato, H. Five-Coordinate Iridium(III) Complex with ΔΛ Chirality. Dalt. Trans. 2021, 50, 13256–13263. [Google Scholar] [CrossRef]
- Nonoyama, M. Benzo[h]Quinolin-10-yl-N Iridium(III) Complexes. Bull. Chem. Soc. Jpn. 1974, 47, 767–768. [Google Scholar] [CrossRef] [Green Version]
- Garces, F.O.; King, K.A.; Watts, R.J. Synthesis, Structure, Electrochemistry, and Photophysics of Methyl-Substituted Phenylpyridine Ortho-Metalated Iridium(III) Complexes. Inorg. Chem. 1988, 27, 3464–3471. [Google Scholar] [CrossRef]
- Ajmal, A.; Majeed, I.; Malik, R.N.; Idriss, H.; Nadeem, M.A. Principles and Mechanisms of Photocatalytic Dye Degradation on TiO2 Based Photocatalysts: A Comparative Overview. RSC Adv. 2014, 4, 37003–37026. [Google Scholar] [CrossRef]
- Bezzubov, S.I.; Doljenko, V.D.; Troyanov, S.I.; Kiselev, Y.M. Tuning the Photophysical and Electrochemical Properties of Iridium(III) 2-Aryl-1-Phenylbenzimidazole Complexes. Inorg. Chim. Acta 2014, 415, 22–30. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spek, A.L. Single-Crystal Structure Validation with the Program PLATON. J. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Kohn, W.; Becke, A.D.; Parr, R.G. Density Functional Theory of Electronic Structure. J. Phys. Chem. 1996, 100, 12974–12980. [Google Scholar] [CrossRef] [Green Version]
- Becke, A.D. Density-Functional Exchange-Energy Approximation with Correct Asymptotic Behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Neese, F.; Wennmohs, F.; Hansen, A.; Becker, U. Efficient, Approximate and Parallel Hartree-Fock and Hybrid DFT Calculations. A “chain-of-Spheres” Algorithm for the Hartree-Fock Exchange. Chem. Phys. 2009, 356, 98–109. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced Basis Sets of Split Valence, Triple Zeta Valence and Quadruple Zeta Valence Quality for H to Rn: Design and Assessment of Accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Neese, F. The ORCA Program System. WIREs Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]

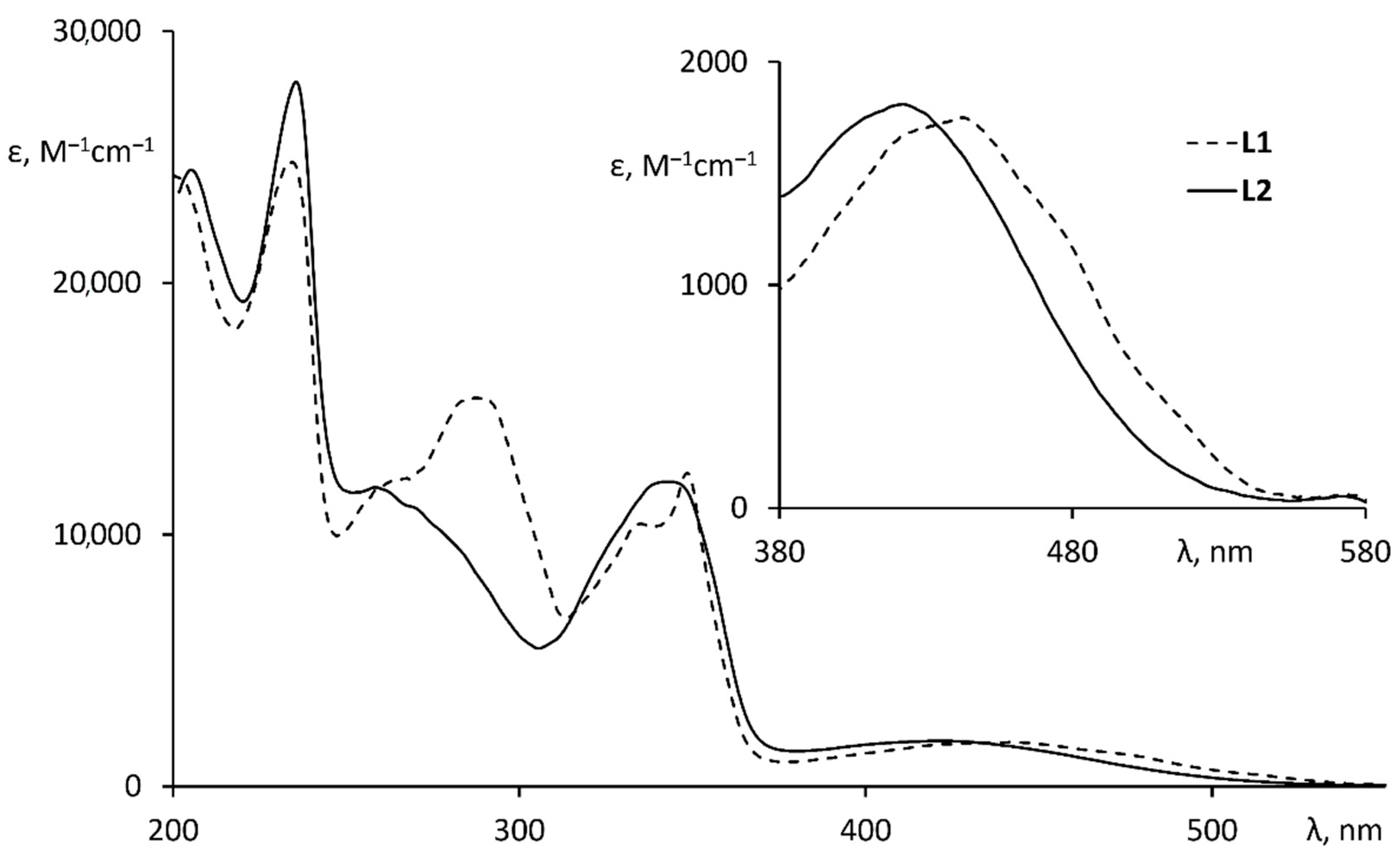
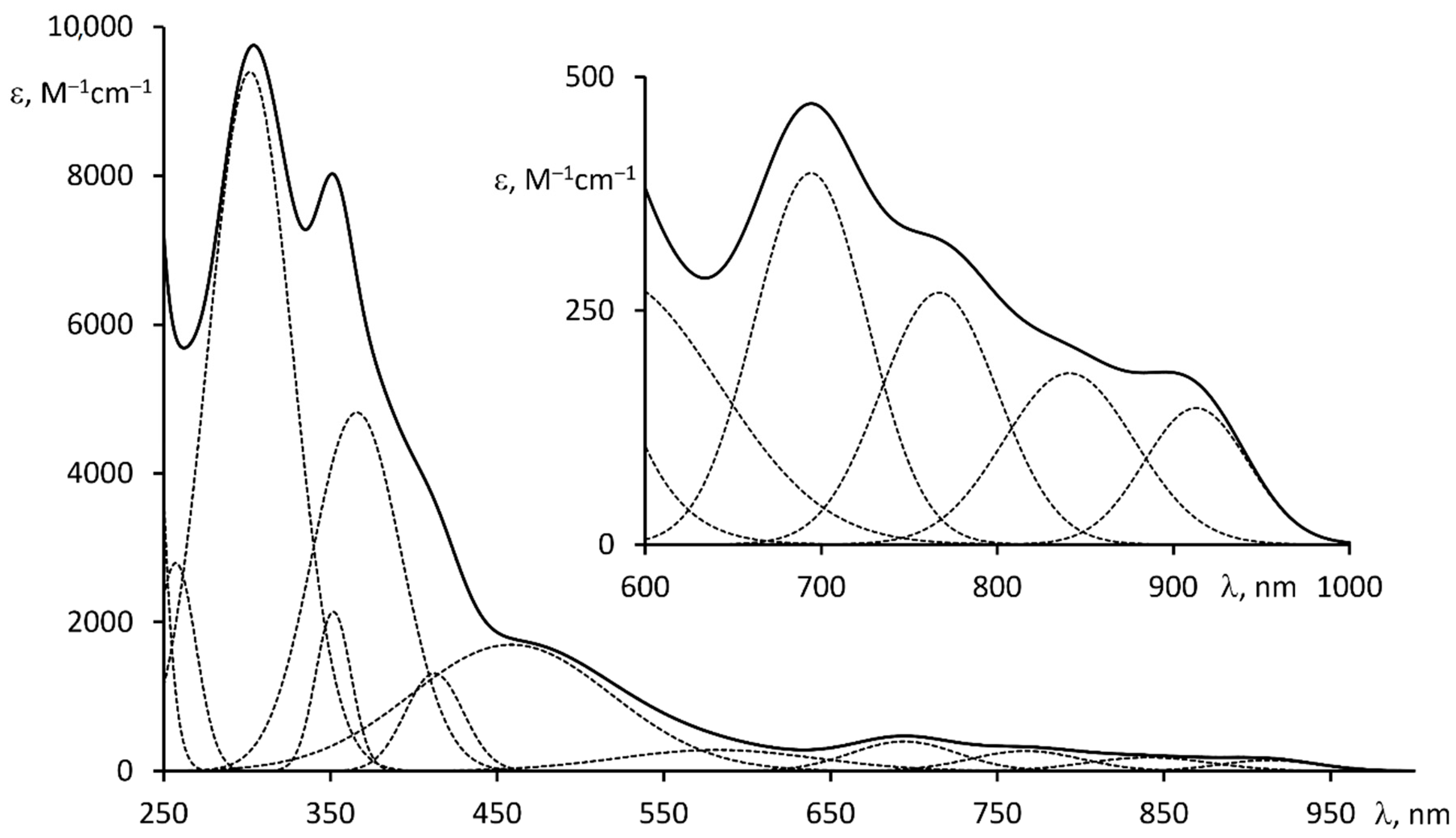
| λabs/nm (ε·10−3/M−1·cm−1) 1 | 302 (9.3), 353 (2), 368 (4.8), 416 (1.3), 460 (1.8), 582 (0.3), 694 (0.4), 770 (0.3), 840 (0.2), 912 (0.15) |
| Eox, V vs. E(Fc+/Fc) 2 | 0.54 3 |
| VOC, V | 0.13 |
| JSC, mA·cm−2 | 0.12 |
| FF | <0.1 |
| η, % | <0.002 |
| τrec, ms | 88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalle, P.; Kiseleva, M.A.; Tatarin, S.V.; Smirnov, D.E.; Zakharov, A.Y.; Emets, V.V.; Churakov, A.V.; Bezzubov, S.I. A Panchromatic Cyclometalated Iridium Dye Based on 2-Thienyl-Perimidine. Molecules 2022, 27, 3201. https://doi.org/10.3390/molecules27103201
Kalle P, Kiseleva MA, Tatarin SV, Smirnov DE, Zakharov AY, Emets VV, Churakov AV, Bezzubov SI. A Panchromatic Cyclometalated Iridium Dye Based on 2-Thienyl-Perimidine. Molecules. 2022; 27(10):3201. https://doi.org/10.3390/molecules27103201
Chicago/Turabian StyleKalle, Paulina, Marina A. Kiseleva, Sergei V. Tatarin, Daniil E. Smirnov, Alexander Y. Zakharov, Viktor V. Emets, Andrei V. Churakov, and Stanislav I. Bezzubov. 2022. "A Panchromatic Cyclometalated Iridium Dye Based on 2-Thienyl-Perimidine" Molecules 27, no. 10: 3201. https://doi.org/10.3390/molecules27103201







