Synthesis, Crystal Structure and Magnetic Properties of a Trinuclear Copper(II) Complex Based on P-Cresol-Substituted Bis(α-Nitronyl Nitroxide) Biradical
Abstract
:1. Introduction
2. Results
2.1. Synthesis of the Inorganic Complex
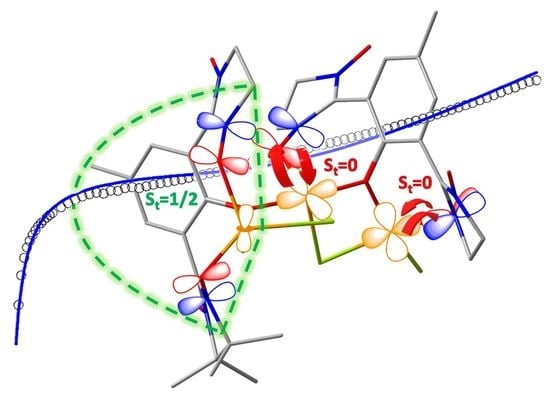
2.2. Crystal Structure
2.3. Magnetic Behaviour
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Complex Cu3(NIT2Ph)2Cl4
3.3. Single-Crystal X-ray Diffraction
3.4. Magnetic Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Caneschi, A.; Gatteschi, D.; Sessoli, R.; Rey, P. Toward molecular magnets: The metal-radical approach. Acc. Chem. Res. 1989, 22, 392–398. [Google Scholar] [CrossRef]
- Ullman, E.F.; Osiecki, J.H.; Boocock, D.G.B.; Darcy, R. Stable free radicals. X. Nitronyl nitroxide monoradicals and biradicals as possible small molecule spin labels. J. Am. Chem. Soc. 1972, 94, 7049–7059. [Google Scholar] [CrossRef]
- Tanimoto, R.; Suzuki, S.; Kozaki, M.; Okada, K. Nitronyl Nitroxide as a Coupling Partner: Pd-Mediated Cross-coupling of (Nitronyl nitroxide-2-ido)(triphenylphosphine)gold(I) with Aryl Halides. Chem. Lett. 2014, 43, 678–680. [Google Scholar] [CrossRef]
- Caneschi, A.; Gatteschi, D.; Hoffman, S.K.; Laugier, J.; Rey, P.; Sessoli, R. Crystal and molecular structure, magnetic properties and EPR spectra of a trinuclear copper(II) complex with bridging nitronyl nitroxides. Inorg. Chem. 1988, 27, 2390–2392. [Google Scholar] [CrossRef]
- Caneschi, A.; Ferraro, F.; Gatteschi, D.; Rey, P.; Sessoli, R. Structure and magnetic properties of a chain compound formed by copper(II) and a tridentate nitronyl nitroxide radical. Inorg. Chem. 1991, 30, 3162–3166. [Google Scholar] [CrossRef]
- Caneschi, A.; Gatteschi, D.; Sessoli, R.; Rey, P. Structure and magnetic properties of a ring of four spins formed by manganese(II) and a pyridine substituted nitronyl nitroxide. Inorg. Chim. Acta 1991, 184, 67–71. [Google Scholar] [CrossRef]
- Fegy, K.; Sanz, N.; Luneau, D.; Belorizky, E.; Rey, P. Proximate Nitroxide Ligands in the Coordination Spheres of Manganese(II) and Nickel(II) Ions. Precursors for High-Dimensional Molecular Magnetic Materials. Inorg. Chem. 1998, 37, 4518–4523. [Google Scholar] [CrossRef]
- Romanov, V.; Tretyakov, E.; Selivanova, G.; Li, J.; Bagryanskaya, I.; Makarov, A.; Luneau, D. Synthesis and Structure of Fluorinated (Benzo[d]imidazol-2-yl)methanols: Bench Compounds for Diverse Applications. Crystals 2020, 10, 786. [Google Scholar] [CrossRef]
- Romanov, V.; Bagryanskaya, I.; Gritsan, N.; Gorbunov, D.; Vlasenko, Y.; Yusubov, M.; Zaytseva, E.; Luneau, D.; Tretyakov, E. Assembly of Imidazolyl-Substituted Nitronyl Nitroxides into Ferromagnetically Coupled Chains. Crystals 2019, 9, 219. [Google Scholar] [CrossRef] [Green Version]
- Mikuriya, M.; Tanaka, K.; Handa, M.; Hiromitsu, I.; Yoshioka, D.; Luneau, D. Adduct complexes of ruthenium(II,III) propionate dimer with pyridyl nitroxides. Polyhedron 2005, 24, 2658–2664. [Google Scholar] [CrossRef]
- Luneau, D.; Rey, P. Magnetism of metal-nitroxide compounds involving bis-chelating imidazole and benzimidazole substituted nitronyl nitroxide free radicals. Coord. Chem. Rev. 2005, 249, 2591–2611. [Google Scholar] [CrossRef]
- Sutter, J.-P.; Kahn, M.L.; Golhen, S.; Ouahab, L.; Kahn, E.O. Synthesis and Magnetic Behavior of Rare-Earth Complexes with N,O-Chelating Nitronyl Nitroxide Triazole Ligands: Example of a [GdIII{Organic Radical}2] Compound with anS=9/2 Ground State. Chem. Eur. J. 1998, 4, 571–576. [Google Scholar] [CrossRef]
- Luneau, D.; Stroh, C.; Cano, J.; Ziessel, E.R. Synthesis, Structure, and Magnetism of a 1D Compound Engineered from a Biradical [5,5′-Bis(3″-oxide-1″-oxyl-4″,4″,5″,5″-tetramethylimidazolin-2″-yl)-2,2′-bipyridine] and Mn II (hfac) 2. Inorg. Chem. 2005, 44, 633–637. [Google Scholar] [CrossRef]
- Hase, S.; Shiomi, D.; Sato, K.; Takui, T. Phenol-substituted nitronyl nitroxide biradicals with a triplet (S = 1) ground state. J. Mater. Chem. 2001, 11, 756–760. [Google Scholar] [CrossRef]
- Petrov, P.A.; Tret"yakov, E.V.; Romanenko, G.; Ovcharenko, V.I.; Sagdeev, R.Z. Seven-membered metallocycle in the CuIIcomplex with deprotonated 2-(2-hydroxy-3-nitrophenyl)-4,4,5,5-tetramethyl-4,5-dihydro-1H-imidazole-1-oxyl 3-oxide. Bull. Acad. Sci. USSR Div. Chem. Sci. 2004, 53, 109–113. [Google Scholar] [CrossRef]
- Liu, R.; Liu, L.; Fang, D.; Xu, J.; Zhao, S.; Xu, W. Synthesis, Structure, and Magnetic Properties of Two Novel Dinuclear Complexes involving Lanthanide-phenoxo Anion Radical. J. Inorg. Gen. Chem. 2015, 641, 728–731. [Google Scholar] [CrossRef]
- Spinu, C.A.; Pichon, C.; Ionita, G.; Mocanu, T.; Calancea, S.; Raduca, M.; Sutter, J.-P.; Hillebrand, M.; Andruh, M. Synthesis, crystal structure, magnetic, spectroscopic, and theoretical investigations of two new nitronyl-nitroxide complexes. J. Coord. Chem. 2021, 74, 279–293. [Google Scholar] [CrossRef]
- Cassaro, R.; Friedman, J.; Lahti, P.M. Copper(II) coordination compounds with sterically constraining pyrenyl nitronyl nitroxide and imino nitroxide. Polyhedron 2016, 117, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Haraguchi, M.; Tretyakov, E.; Gritsan, N.; Romanenko, G.; Gorbunov, D.; Bogomyakov, A.; Maryunina, K.; Suzuki, S.; Kozaki, M.; Shiomi, D.; et al. (Azulene-1,3-diyl)-bis(nitronyl nitroxide) and (Azulene-1,3-diyl)-bis(iminonitroxide) and Their Copper Complexes. Chem. Asian J. 2017, 12, 2929–2941. [Google Scholar] [CrossRef]
- Fidan, I.; Luneau, D.; Ahsen, V.; Hirel, C. Revisiting the Ullman’s Radical Chemistry for Phthalocyanine Derivatives. Chem. A Eur. J. 2018, 24, 5359–5365. [Google Scholar] [CrossRef] [Green Version]
- Önal, E.; Fidan, I.; Luneau, D.; Hirel, C. Through the challenging synthesis of tetraphenylporphyrin derivatives bearing nitroxide moieties. J. Porphyr. Phthalocyanines 2019, 23, 584–588. [Google Scholar] [CrossRef]
- Fidan, I.; Önal, E.; Yerli, Y.; Luneau, D.; Ahsen, V.; Hirel, C. Synthetic Access to a Pure Polyradical Architecture: Nucleophilic Insertion of Nitronyl Nitroxide on a Cyclotriphosphazene Scaffold. ChemPlusChem 2017, 82, 1384–1389. [Google Scholar] [CrossRef] [PubMed]
- Morozov, V.; Tretyakov, E. Spin polarization in graphene nanoribbons functionalized with nitroxide. J. Mol. Model. 2019, 25, 58. [Google Scholar] [CrossRef] [PubMed]
- Lecourt, C.; Izumi, Y.; Maryunina, K.; Inoue, K.; Bélanger-Desmarais, N.; Reber, C.; Desroches, C.; Luneau, D. Hypersensitive pressure-dependence of the conversion temperature of hysteretic valence tautomeric manganese–nitronyl nitroxide radical 2D-frameworks. Chem. Commun. 2021, 57, 2376–2379. [Google Scholar] [CrossRef]
- Lecourt, C.; Izumi, Y.; Khrouz, L.; Toche, F.; Chiriac, R.; Bélanger-Desmarais, N.; Reber, C.; Fabelo, O.; Inoue, K.; Desroches, C.; et al. Thermally-induced hysteretic valence tautomeric conversions in the solid state via two-step labile electron transfers in manganese-nitronyl nitroxide 2D-frameworks. Dalton Trans. 2020, 49, 15646–15662. [Google Scholar] [CrossRef]
- Lannes, A.; Suffren, Y.; Tommasino, J.B.; Chiriac, R.; Toche, F.; Khrouz, L.; Molton, F.; Duboc, C.; Kieffer, I.; Hazemann, J.-L.; et al. Room Temperature Magnetic Switchability Assisted by Hysteretic Valence Tautomerism in a Layered Two-Dimensional Manganese-Radical Coordination Framework. J. Am. Chem. Soc. 2016, 138, 16493–16501. [Google Scholar] [CrossRef]
- Zheludev, A.; Barone, V.; Bonnet, M.; Delley, B.; Grand, A.; Ressouche, E.; Rey, P.; Subra, R.; Schweizer, J. Spin density in a nitronyl nitroxide free radical. Polarized neutron diffraction investigation and ab initio calculations. J. Am. Chem. Soc. 1994, 116, 2019–2027. [Google Scholar] [CrossRef]
- Vaz, M.G.; Andruh, M. Molecule-based magnetic materials constructed from paramagnetic organic ligands and two different metal ions. Coord. Chem. Rev. 2020, 427, 213611. [Google Scholar] [CrossRef]
- Luneau, D.; Borta, A.; Chumakov, Y.; Jacquot, J.-F.; Jeanneau, E.; Lescop, C.; Rey, P. Molecular magnets based on two-dimensional Mn(II)–nitronyl nitroxide frameworks in layered structures. Inorg. Chim. Acta 2008, 361, 3669–3676. [Google Scholar] [CrossRef]
- Minguet, M.; Luneau, D.; Lhotel, E.; Villar, V.; Paulsen, C.; Amabilino, D.B.; Veciana, J. An Enantiopure Molecular Ferromagnet. Angew. Chem. Int. Ed. 2002, 41, 586–589. [Google Scholar] [CrossRef]
- Stumpf, H.O.; Pei, Y.; Kahn, O.; Ouahab, L.; Grandjean, D. A Molecular-Based Magnet with a Fully Interlocked Three-Dimensional Structure. Science 1993, 261, 447–449. [Google Scholar] [CrossRef] [PubMed]
- Bernot, K.; Pointillart, F.; Rosa, P.; Etienne, M.; Sessoli, R.; Gatteschi, D. Single molecule magnet behaviour in robust dysprosium–biradical complexes. Chem. Commun. 2010, 46, 6458–6460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coronado, E.; Giménez-Saiz, C.; Recuenco, A.; Tarazón, A.; Romero, F.M.; Camón, A.; Luis, F. Single-Molecule Magnetic Behavior in a Neutral Terbium(III) Complex of a Picolinate-Based Nitronyl Nitroxide Free Radical. Inorg. Chem. 2011, 50, 7370–7372. [Google Scholar] [CrossRef]
- Li, H.; Jing, P.; Lu, J.; Xie, J.; Zhai, L.; Xi, L. Dipyridyl-Decorated Nitronyl Nitroxide–DyIII Single-Molecule Magnet with a Record Energy Barrier of 146 K. Inorg. Chem. 2021, 60, 7622–7626. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wu, Q.; Lu, J.; Jing, P.; Du, Y.; Li, L. Slow relaxation of magnetization in lanthanide–biradical complexes based on a functionalized nitronyl nitroxide biradical. Dalton Trans. 2020, 49, 17414–17420. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-L.; Li, L.-C.; Liao, D.-Z. Slow Magnetic Relaxation in Lanthanide Complexes with Chelating Nitronyl Nitroxide Radical. Inorg. Chem. 2010, 49, 4735–4737. [Google Scholar] [CrossRef]
- Bernot, K.; Bogani, L.; Caneschi, A.; Gatteschi, D.; Sessoli, R. A Family of Rare-Earth-Based Single Chain Magnets: Playing with Anisotropy. J. Am. Chem. Soc. 2006, 128, 7947–7956. [Google Scholar] [CrossRef]
- Bogani, L.; Sangregorio, C.; Sessoli, R.; Gatteschi, D. Molecular Engineering for Single-Chain-Magnet Behavior in a One-Dimensional Dysprosium-Nitronyl Nitroxide Compound. Angew. Chem. Int. Ed. 2005, 44, 5817–5821. [Google Scholar] [CrossRef]
- Houard, F.; Gendron, F.; Suffren, Y.; Guizouarn, T.; Dorcet, V.; Calvez, G.; Daiguebonne, C.; Guillou, O.; Le Guennic, B.; Mannini, M.; et al. Single-chain magnet behavior in a finite linear hexanuclear molecule. Chem. Sci. 2021, 12, 10613–10621. [Google Scholar] [CrossRef]
- Liu, R.; Li, L.; Wang, X.; Yang, P.; Wang, C.; Liao, D.; Sutter, J.-P. Smooth transition between SMM and SCM-type slow relaxing dynamics for a 1-D assemblage of {Dy(nitronyl nitroxide)2} units. Chem. Commun. 2010, 46, 2566–2568. [Google Scholar] [CrossRef]
- Xie, J.; Li, H.-D.; Yang, M.; Sun, J.; Li, L.-C.; Sutter, J.-P. Improved single-chain-magnet behavior in a biradical-based nitronyl nitroxide-Cu–Dy chain. Chem. Commun. 2019, 55, 3398–3401. [Google Scholar] [CrossRef]
- de Panthou, F.L.; Belorizky, E.; Calemczuk, R.; Luneau, D.; Marcenat, C.; Ressouche, E.; Turek, P.; Rey, P. A New Type of Thermally Induced Spin Transition Associated with an Equatorial.dblarw. Axial Conversion in a Copper(II)-Nitroxide Cluster. J. Am. Chem. Soc. 1995, 117, 11247–11253. [Google Scholar] [CrossRef]
- de Panthou, F.L.; Luneau, D.; Musin, R.; Öhrström, L.; Grand, A.; Turek, P.; Rey, P. Spin-Transition and Ferromagnetic Interactions in Copper(II) Complexes of a 3-Pyridyl-Substituted Imino Nitroxide. Dependence of the Magnetic Properties upon Crystal Packing. Inorg. Chem. 1996, 35, 3484–3491. [Google Scholar] [CrossRef]
- Fedin, M.; Veber, S.; Bagryanskaya, E.; Ovcharenko, V.I. Electron paramagnetic resonance of switchable copper-nitroxide-based molecular magnets: An indispensable tool for intriguing systems. Coord. Chem. Rev. 2015, 289–290, 341–356. [Google Scholar] [CrossRef]
- Luneau, D. Coordination Chemistry of Nitronyl Nitroxide Radicals Has Memory. Eur. J. Inorg. Chem. 2020, 2020, 597–604. [Google Scholar] [CrossRef]
- Tolstikov, S.; Tretyakov, E.; Fokin, S.; Suturina, E.; Romanenko, G.; Bogomyakov, A.; Stass, D.; Maryasov, A.; Fedin, M.; Gritsan, N.; et al. C(sp2)-Coupled Nitronyl Nitroxide and Iminonitroxide Diradicals. Chem. A Eur. J. 2014, 20, 2793–2803. [Google Scholar] [CrossRef]
- Gurskaya, L.; Rybalova, T.; Beregovaya, I.; Zaytseva, E.; Kazantsev, M.; Tretyakov, E. Aromatic nucleophilic substitution: A case study of the interaction of a lithiated nitronyl nitroxide with polyfluorinated quinoline-N-oxides. J. Fluor. Chem. 2020, 237, 109613. [Google Scholar] [CrossRef]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; van Rijn, J.; Verschoor, G.C. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 7, 1349–1356. [Google Scholar] [CrossRef]
- Hoffmann, S.K.; Hodgson, D.J.; Hatfield, W.E. Crystal structures and magnetic and EPR studies of intradimer and interdimer exchange coupling in [M(en)3]2[Cu2Cl8]Cl2.cntdot.2H2O (M = Co, Rh, Ir) crystals. Inorg. Chem. 1985, 24, 1194–1201. [Google Scholar] [CrossRef]
- Bream, R.A.; Estes, E.D.; Hodgson, D.J. Structural characterization of dichloro[2-(2-methylaminoethyl)pyridine]copper(II). Inorg. Chem. 1975, 14, 1672–1675. [Google Scholar] [CrossRef]
- Estes, E.D.; Estes, W.E.; Hatfield, W.E.; Hodgson, D.J. Molecular structure of bis[dichloro(N,N,N’,N’-tetramethylenediaminecopper(II)], [Cu(tmen)Cl2]2. Inorg. Chem. 1975, 14, 106–109. [Google Scholar] [CrossRef]
- Sinn, E.; Robinson, W.T. X-Ray structure analyses and magnetic correlation of four copper(II) complexes. J. Chem. Soc. Chem. Commun. 1972, 6, 359–361. [Google Scholar] [CrossRef]
- Colomban, C.; Philouze, C.; Molton, F.; Leconte, N.; Thomas, F. Copper(II) complexes of N3O ligands as models for galactose oxidase: Effect of variation of steric bulk of coordinated phenoxyl moiety on the radical stability and spectroscopy. Inorg. Chim. Acta 2018, 481, 129–142. [Google Scholar] [CrossRef]
- Okuniewski, A.; Rosiak, D.; Chojnacki, J.; Becker, B. Coordination polymers and molecular structures among complexes of mercury(II) halides with selected 1-benzoylthioureas. Polyhedron 2015, 90, 47–57. [Google Scholar] [CrossRef]
- Rosiak, D.; Okuniewski, A.; Chojnacki, J. Novel complexes possessing Hg–(Cl, Br, I)⋯O C halogen bonding and unusual Hg 2 S 2 (Br/I) 4 kernel. The usefulness of τ′4 structural parameter. Polyhedron 2018, 146, 35–41. [Google Scholar] [CrossRef]
- Yang, L.; Powell, D.R.; Houser, R.P. Structural variation in copper(i) complexes with pyridylmethylamide ligands: Structural analysis with a new four-coordinate geometry index, τ4. Dalton Trans. 2007, 9, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Brookhart, M.; Green, M.L. Carbon hydrogen-transition metal bonds. J. Organomet. Chem. 1983, 250, 395–408. [Google Scholar] [CrossRef]
- Crabtree, R.H. Transition Metal Complexation ofσ Bonds. Angew. Chem. Int. Ed. 1993, 32, 789–805. [Google Scholar] [CrossRef]
- Brookhart, M.; Green, M.L.H.; Parkin, G. Agostic interactions in transition metal compounds. Proc. Natl. Acad. Sci. USA 2007, 104, 6908–6914. [Google Scholar] [CrossRef] [Green Version]
- Braga, D.; Grepioni, A.F.; Tedesco, E.; Biradha, K.; Desiraju, G.R. Hydrogen Bonding in Organometallic Crystals. 6. X−H---M Hydrogen Bonds and M---(H−X) Pseudo-Agostic Bonds. Organometallics 1997, 16, 1846–1856. [Google Scholar] [CrossRef]
- Mei, X.; Wang, X.; Wang, J.; Ma, Y.; Li, L.; Liao, D. Dinuclear lanthanide complexes bridged by nitronyl nitroxide radical ligands with 2-phenolate groups: Structure and magnetic properties. New J. Chem. 2013, 37, 3620–3626. [Google Scholar] [CrossRef]
- Tandon, S.S.; Bunge, S.D.; Patel, N.; Wang, E.C.; Thompson, L.K. Self-Assembly of Antiferromagnetically-Coupled Copper(II) Supramolecular Architectures with Diverse Structural Complexities. Molecules 2020, 25, 5549. [Google Scholar] [CrossRef] [PubMed]
- Crawford, V.H.; Richardson, H.W.; Wasson, J.R.; Hodgson, D.J.; Hatfield, W.E. Relation between the singlet-triplet splitting and the copper-oxygen-copper bridge angle in hydroxo-bridged copper dimers. Inorg. Chem. 1976, 15, 2107–2110. [Google Scholar] [CrossRef]
- Thompson, L.K.; Mandal, S.K.; Tandon, S.S.; Bridson, J.N.; Park, M.K. Magnetostructural Correlations in Bis(μ2-phenoxide)-Bridged Macrocyclic Dinuclear Copper(II) Complexes. Influence of Electron-Withdrawing Substituents on Exchange Coupling. Inorg. Chem. 1996, 35, 3117–3125. [Google Scholar] [CrossRef]
- Khan, O. Molecular Magnetism; Wiley-VCH: Weinhein, Germany, 1993. [Google Scholar]
- Rajendiran, T.M.; Kannappan, R.; Mahalakshmy, R.; Rajeswari, J.; Venkatesan, R.; Rao, P. New unsymmetrical μ-phenoxo bridged binuclear copper(II) complexes. Transit. Met. Chem. 2003, 28, 447–454. [Google Scholar] [CrossRef]
- Caneschi, A.; Gatteschi, D.; Rey, P. The Chemistry and Magnetic Properties of Metal Nitronyl Nitroxide Complexes. Prog. Inorg. Chem. 1991, 39, 331–429. [Google Scholar] [CrossRef]
- Cogne, A.; Laugier, J.; Luneau, D.; Rey, P. Novel Square Planar Copper(II) Complexes with Imino or Nitronyl Nitroxide Radicals Exhibiting Large Ferro- and Antiferromagnetic Interactions. Inorg. Chem. 2000, 39, 5510–5514. [Google Scholar] [CrossRef] [PubMed]
- Chilton, N.F.; Anderson, R.P.; Turner, L.D.; Soncini, A.; Murray, K.S. PHI: A powerful new program for the analysis of anisotropic monomeric and exchange-coupled polynucleard- andf-block complexes. J. Comput. Chem. 2013, 34, 1164–1175. [Google Scholar] [CrossRef]
- Agilent. CrysAlis PRO; Agilent Technologies Ltd.: Yarnton, UK, 2014. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Bourhis, L.J.; Dolomanov, O.V.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. The anatomy of a comprehensive constrained, restrained refinement program for the modern computing environment –Olex2dissected. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 59–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pascal, P. Magnetochemical Researches. Ann. Chim. Phys. 1910, 19, 5–70. [Google Scholar]
- Bain, G.A.; Berry, J.F. Diamagnetic Corrections and Pascal’s Constants. J. Chem. Educ. 2008, 85, 532. [Google Scholar] [CrossRef]








| Formula | C42H58Cl4Cu3N8O10 |
| M (g mol−1) | 1167.38 |
| T (K) | 293(2) |
| λ (Å) | 0.71073 |
| Crystal system | Monoclinic |
| Space group | P21/n (#14) |
| a (Å) | 19.8080(9) |
| b (Å) | 11.4226(7) |
| c (Å) | 22.0120(15) |
| α (deg) | 90 |
| β (deg) | 99.450(5) |
| γ (deg) | 90 |
| V (Å3) | 4912.8(5) |
| Z | 4 |
| Dc (g cm−3) | 1.578 |
| F(000) | 2404 |
| θ range (deg.) | 2.565 to 29.287 |
| Limiting indices | −27 ≤ h ≤ 26, −15 ≤ k ≤ 14, −28 ≤ l ≤ 30 |
| Refl. collected | 31578 |
| Rint (%) | 6.66 |
| Num. param. | 622 |
| GOF on F2 | 1.036 |
| R (a), ωR (b) (all data) | 0.0648, 0.1643 |
| Δρmax/Δρmin (e.Å3) | 0.6/−0.7 |
| Cu 1 | |||
|---|---|---|---|
| Cu1-O5A | 1.934(3) | O5B-Cu1-O5A | 175.99(14) |
| Cu1-O5B | 1.937(3) | O1A-Cu-Cl2 | 148.64(11) |
| Cu1-O1A | 1.947(3) | Cu1-O1A-N1A | 126.4(3) |
| Cu1-Cl2 | 2.2596(14) | Cu1-Cl3-Cu2 | 82.91(5) |
| Cu1-Cl3 | 2.5869(15) | Cu1-O5B-Cu3 | 117.11(17) |
| Cu1---Cu2 | 3.211(1) | Cu1-O5A-Cu2 | 111.13(16) |
| Cu1---Cu3 | 3.281(1) | ||
| Cu 2 | |||
| Cu2-O3A | 1.918(4) | O5A-Cu2-Cl4 | 161.35(13) |
| Cu2-O5A | 1.960(3) | O3A-Cu2-Cl3 | 158.53(13) |
| Cu2-Cl4 | 2.1520(18) | Cu2-O3A-N3A | 112.7(4) |
| Cu2-Cl3 | 2.2492(17) | ||
| Cu 3 | |||
| Cu3-O5B | 1.909(3) | O5B-Cu3-Cl1 | 177.92(12) |
| Cu3-O3B | 1.967(4) | O3B-Cu3-Cl2 | 127.3(11) |
| Cu3-O1B | 2.110(4) | Cu3-O5B-Cu1 | 117.11(17) |
| Cu3-Cl1 | 2.1585(16) | Cu3-Cl2-Cu1 | 83.60(5) |
| Cu3-Cl2 | 2.6440(17) | Cu3-O1B-N1B | 125.8(3) |
| Cu3-O3B-N3B | 120.1(3) | ||
| Nitronyl Nitroxide Moieties | |||
| O1A-N1A * | 1.295(5) | O1B-N1B * | 1.300(5) |
| O2A-NA2 | 1.263(6) | O2B-N2B | 1.270(5) |
| O3A-N3A * | 1.310(5) | O3B-N3B * | 1.297(5) |
| O4A-N4A | 1.263(5) | O4A-N4A | 1.254(5) |
| Selected Planes | Atomic Deviation (Å) from Least-Square Planes | ϕ (°) | δ (°) | ||||
|---|---|---|---|---|---|---|---|
| Diradical A | O1, O3 | N1, N3 | C1, C14 | N2, N4 | O2, O4 | ||
| O1-N1-C1-N2-O2 | 0.065(3) | −0.105(4) | −0.075(4) | 0.031(4) | 0.044(4) | 50.6(2) | 55.08 |
| O3-N3-C14-N4-O4 | −0.029(4) | 0.031(4) | 0.039(4) | −0.001(4) | −0.029(4) | 45.2(1) | 82.92 |
| Diradical B | O1, O3 | N1, N3 | C1, C14 | N2, N4 | O2, O4 | ||
| O1-N1-C1-N2-O2 | 0.030(4) | −0.052(4) | −0.025(5) | 0.028(4) | 0.004(4) | 42.6(2) | 57.30 |
| O3-N3-C14-N4-O4 | 0.022(4) | −0.022(4) | −0.031(4) | −0.004(4) | 0.025(4) | 46.4(1) | 72.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grenda, S.; Beau, M.; Luneau, D. Synthesis, Crystal Structure and Magnetic Properties of a Trinuclear Copper(II) Complex Based on P-Cresol-Substituted Bis(α-Nitronyl Nitroxide) Biradical. Molecules 2022, 27, 3218. https://doi.org/10.3390/molecules27103218
Grenda S, Beau M, Luneau D. Synthesis, Crystal Structure and Magnetic Properties of a Trinuclear Copper(II) Complex Based on P-Cresol-Substituted Bis(α-Nitronyl Nitroxide) Biradical. Molecules. 2022; 27(10):3218. https://doi.org/10.3390/molecules27103218
Chicago/Turabian StyleGrenda, Sabrina, Maxime Beau, and Dominique Luneau. 2022. "Synthesis, Crystal Structure and Magnetic Properties of a Trinuclear Copper(II) Complex Based on P-Cresol-Substituted Bis(α-Nitronyl Nitroxide) Biradical" Molecules 27, no. 10: 3218. https://doi.org/10.3390/molecules27103218
APA StyleGrenda, S., Beau, M., & Luneau, D. (2022). Synthesis, Crystal Structure and Magnetic Properties of a Trinuclear Copper(II) Complex Based on P-Cresol-Substituted Bis(α-Nitronyl Nitroxide) Biradical. Molecules, 27(10), 3218. https://doi.org/10.3390/molecules27103218