Effect of Ionic and Non-Ionic Surfactant on Bovine Serum Albumin Encapsulation and Biological Properties of Emulsion-Electrospun Fibers
Abstract
:1. Introduction
2. Results
2.1. Characterization of the Electrospinning Solution
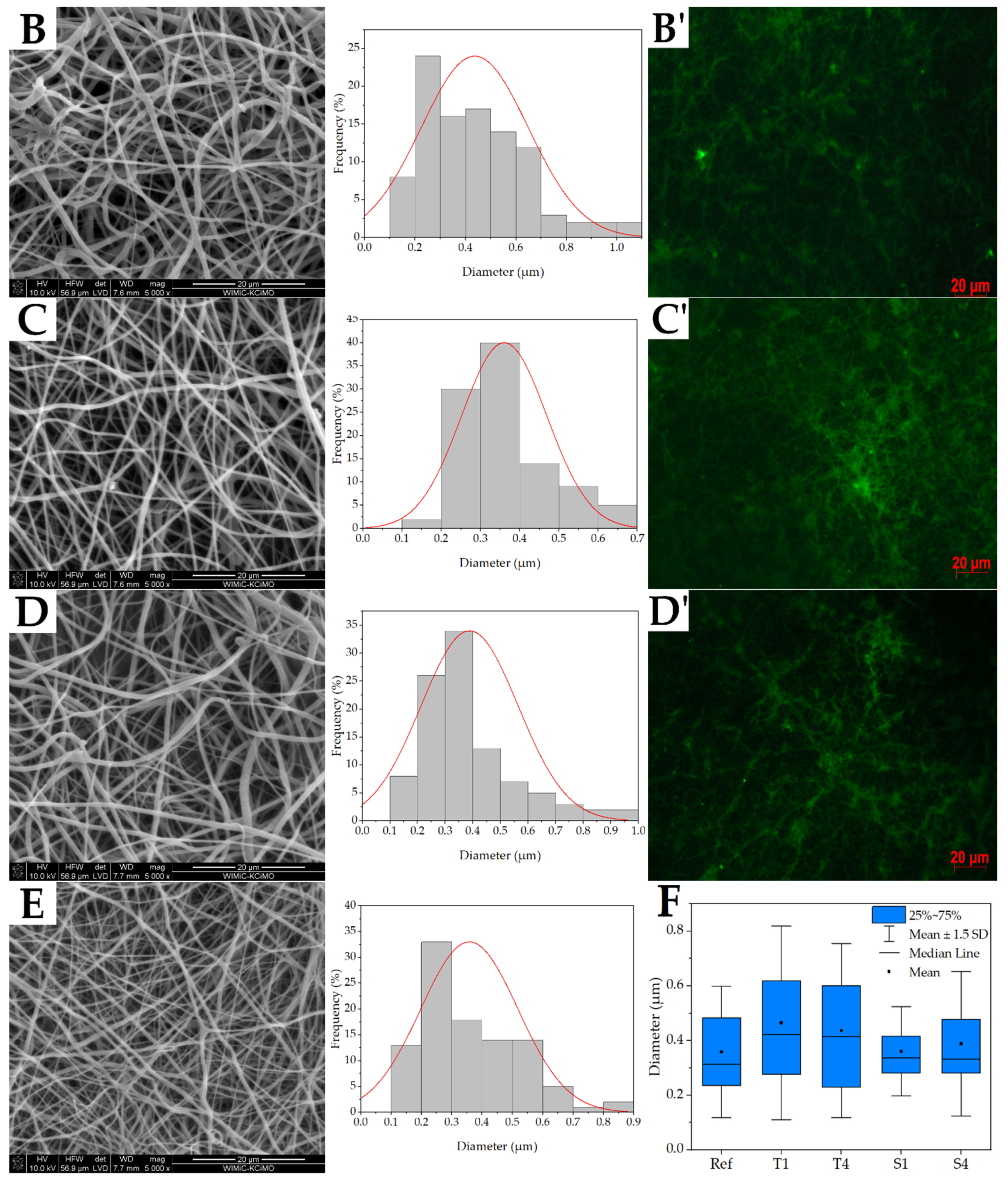
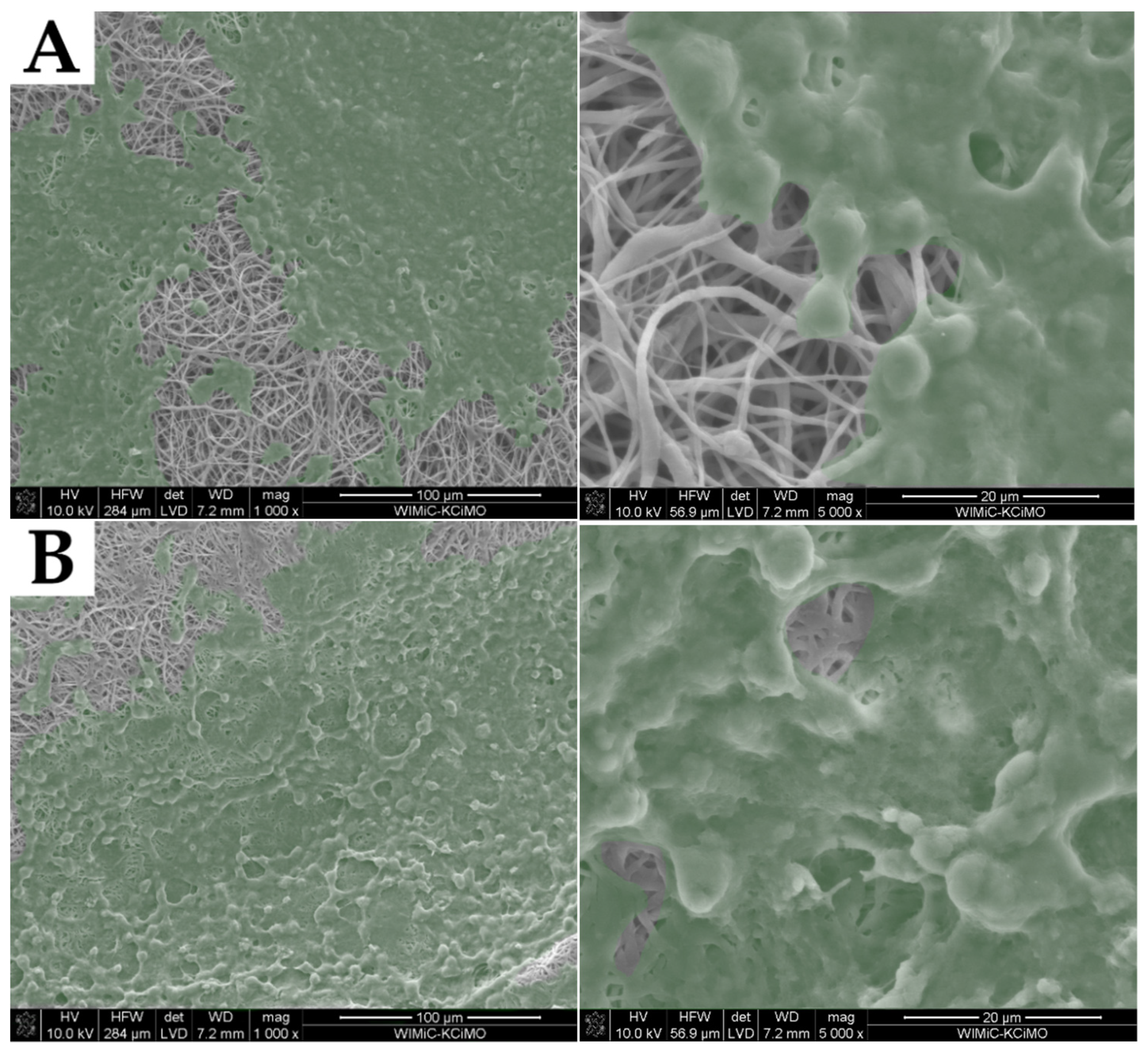
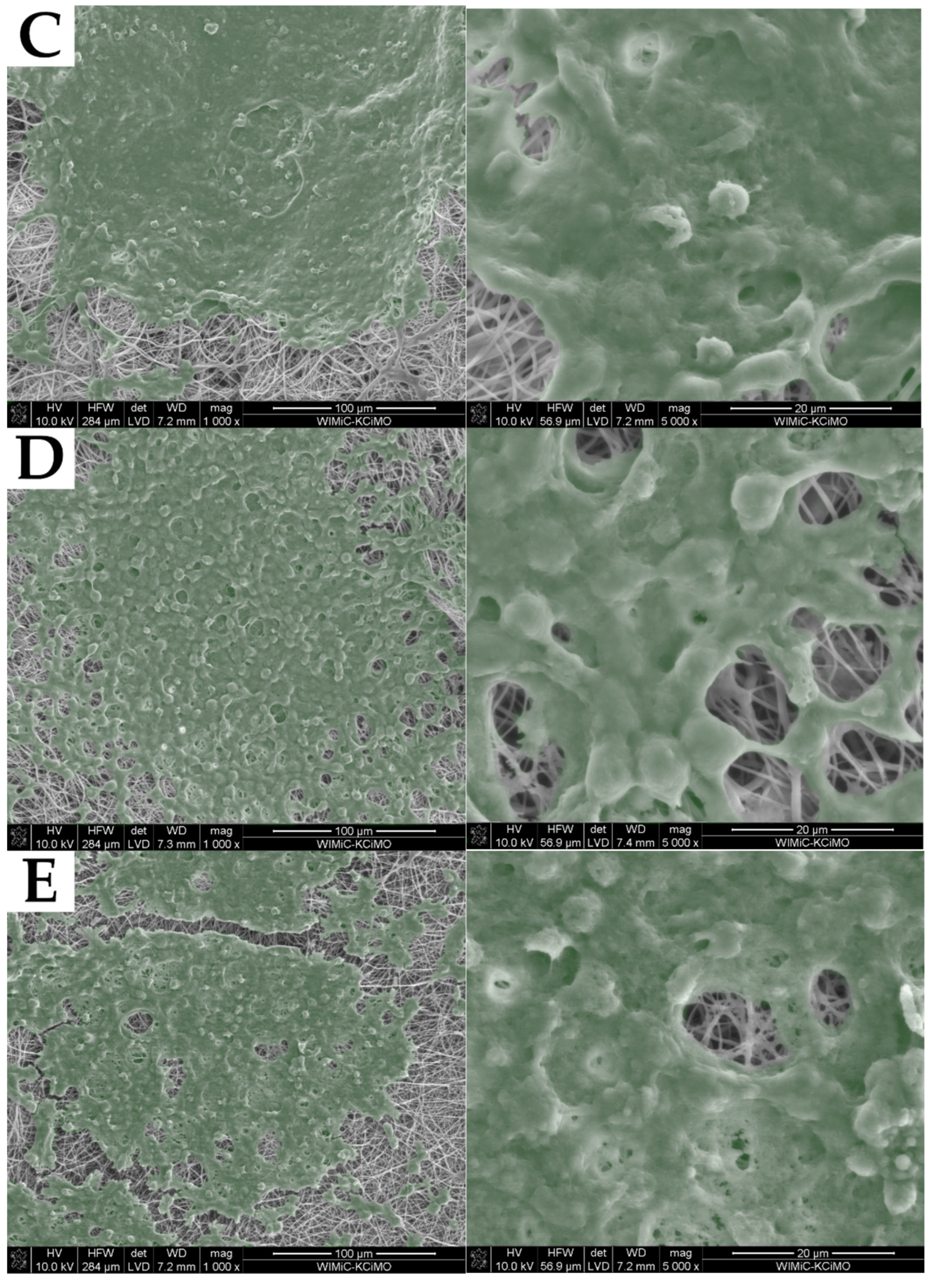
2.2. Fiber Morphology
2.3. Structure of Nonwovens
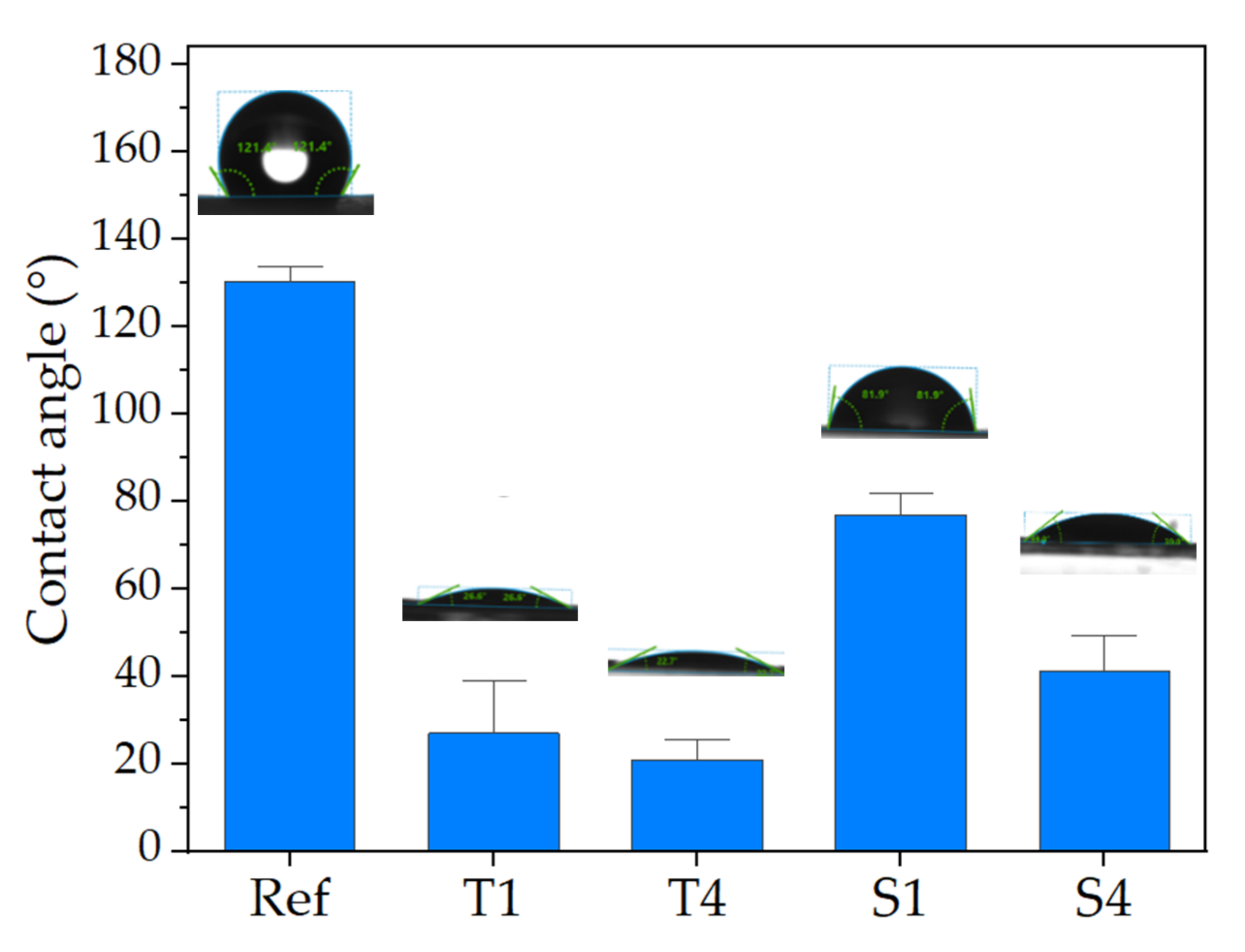
2.4. Wettability
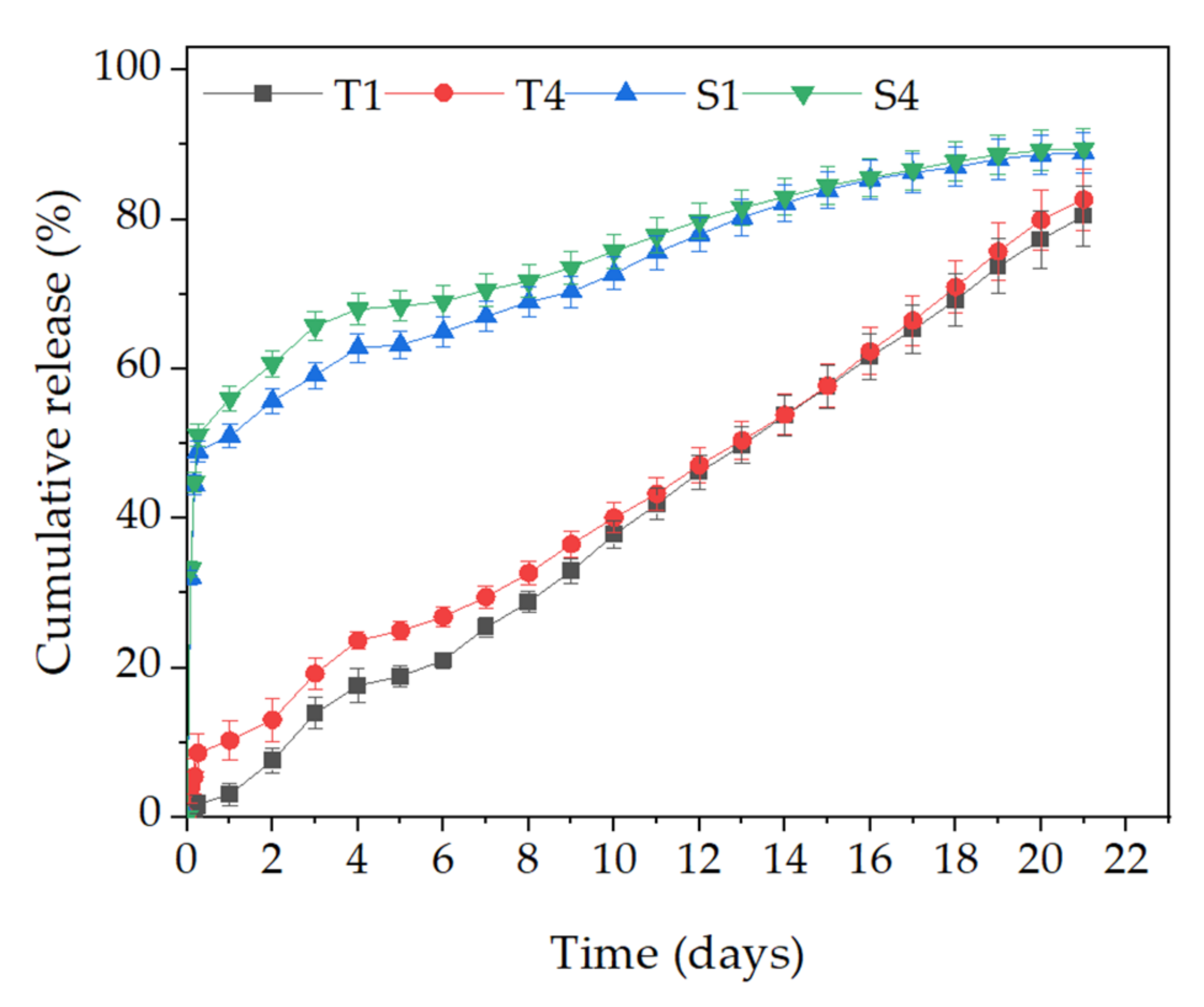
2.5. Efficiency of Encapsulation and Release Studies
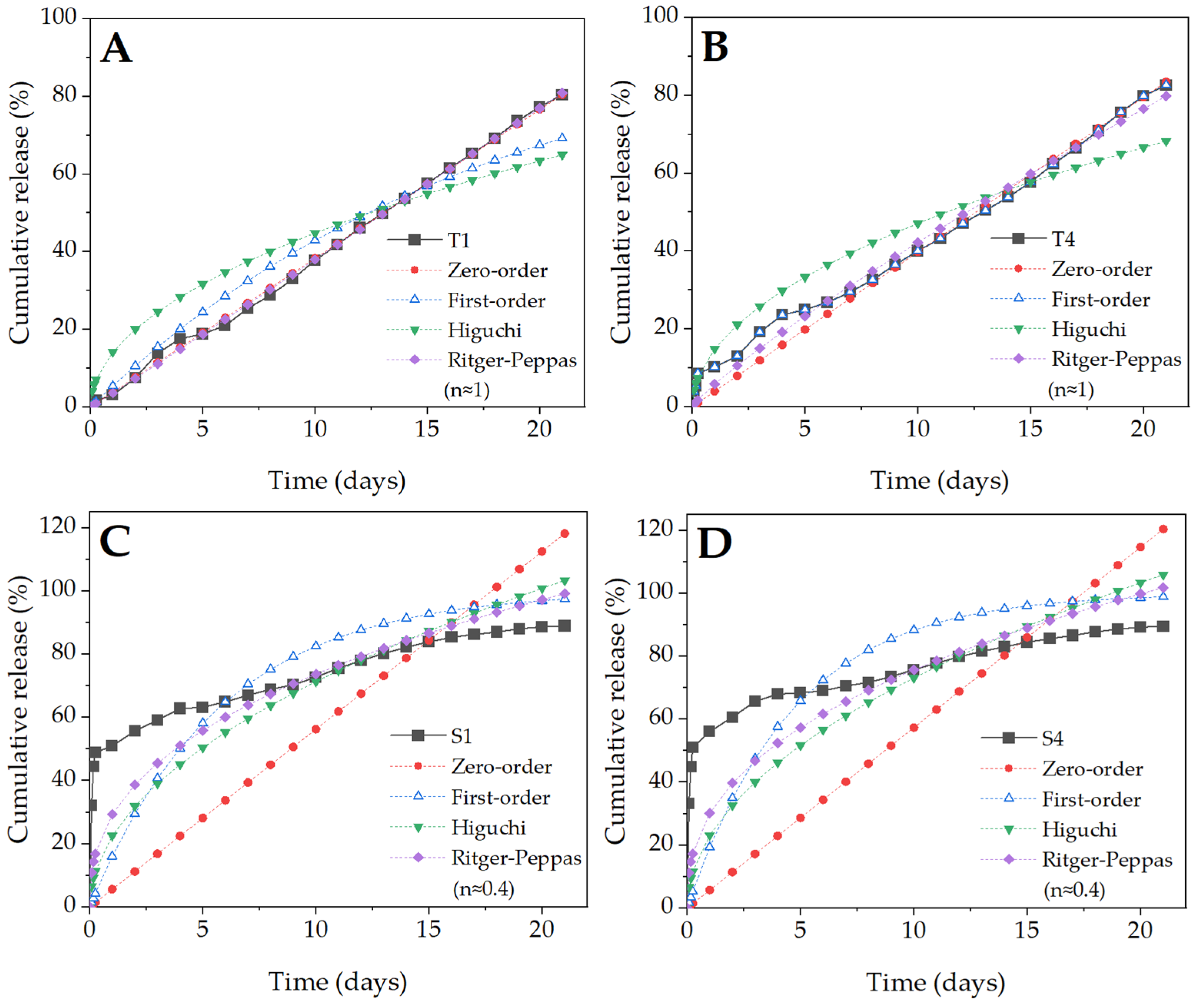
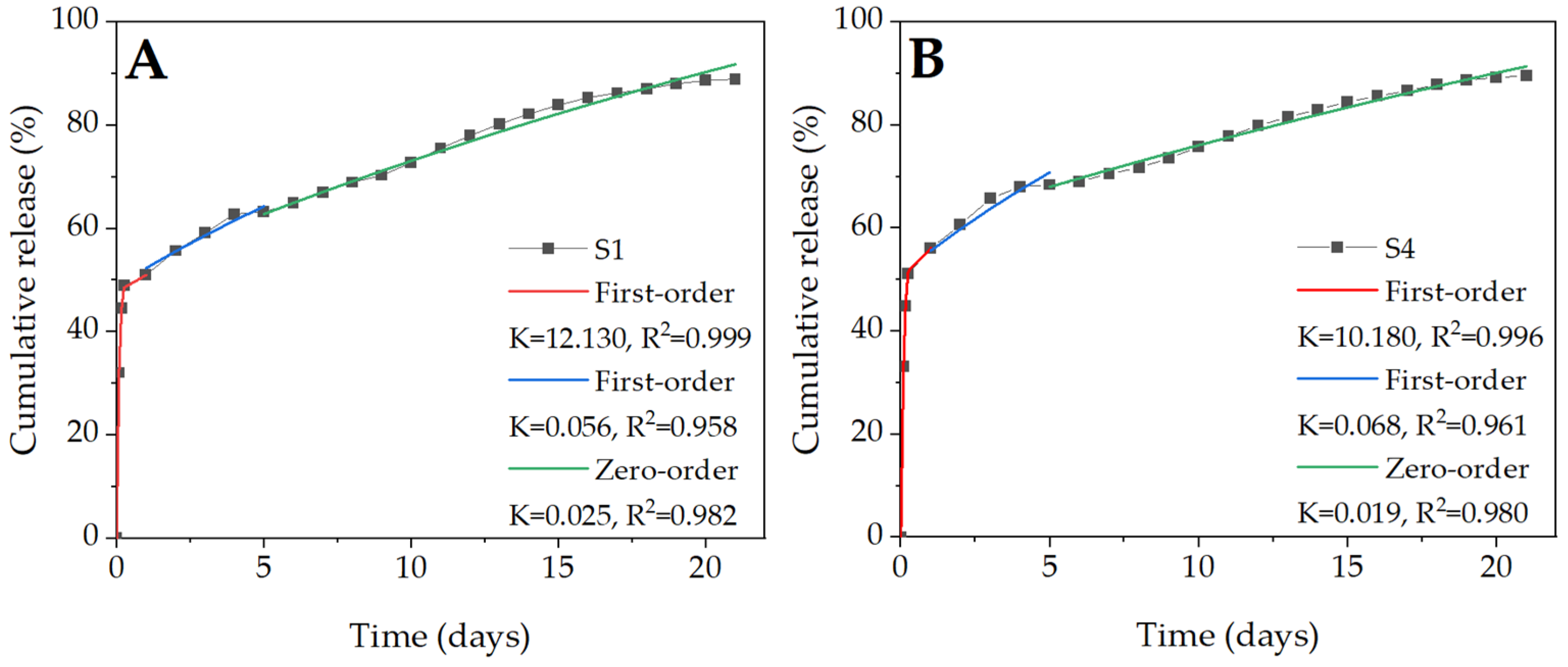
2.6. BSA Release Kinetics
2.7. In Vitro Study
3. Discussion
4. Materials and Methods
4.1. Fabrication of Nonwovens
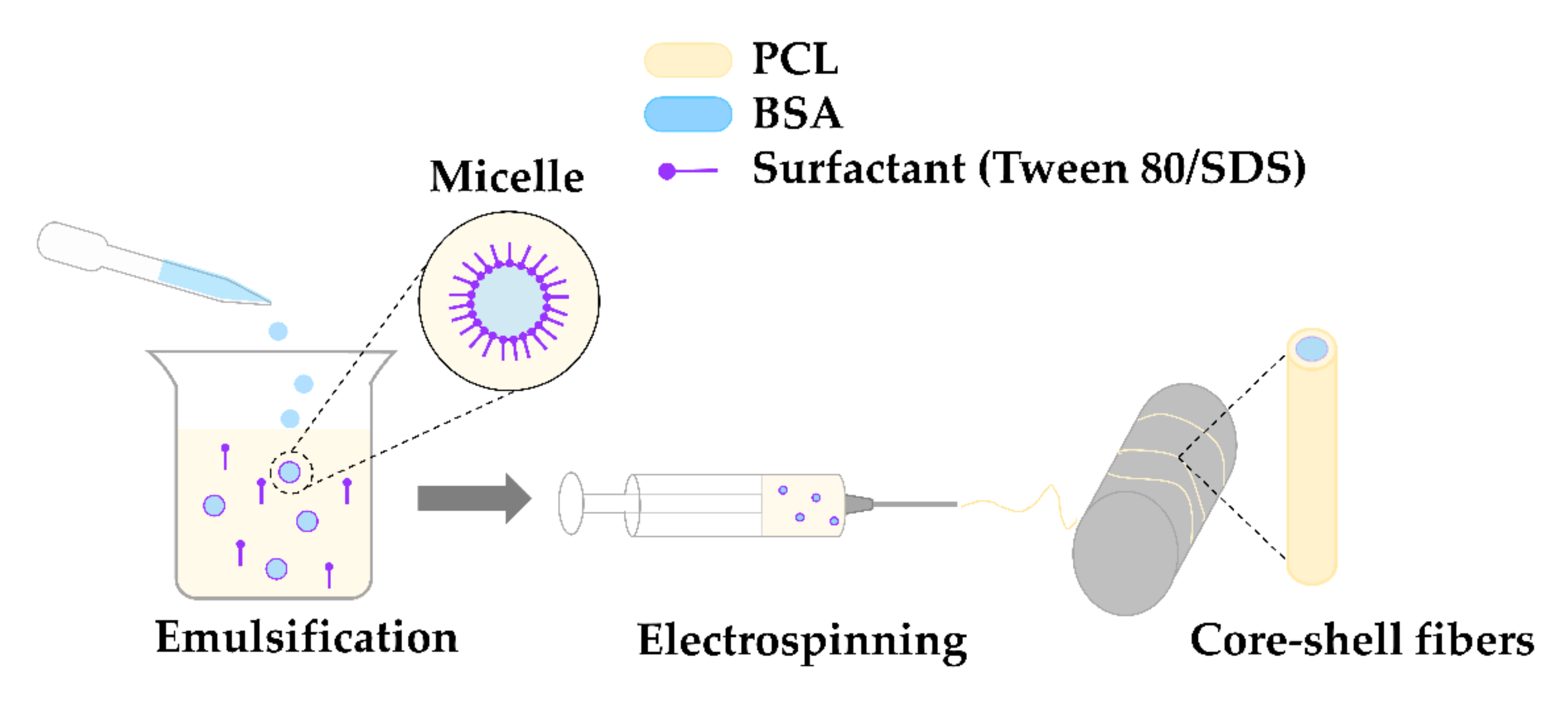
4.1.1. Preparation of the Emulsion
4.1.2. Electrospinning Process
4.2. Methods
4.2.1. Characterization of the Electrospinning Solution
4.2.2. Fibers Morphology
4.2.3. Structural Analysis
4.2.4. Wettability
4.2.5. Efficiency of Encapsulation and Release Studies
4.2.6. Mathematical Modeling of BSA Release
- Zero-order
- First-order
- Higuchi
- Ritger-Peppas
- Mn—cumulative amount of the drug released over time t,
- M∞—initial amount of the drug,
- K, n—constants.
4.2.7. Biological Studies
4.2.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Wang, C.; Wang, M. Formation of core-shell structures in emulsion electrospun fibres: A comparative study. Aust. J. Chem. 2014, 67, 1403–1413. [Google Scholar] [CrossRef]
- Shahriar, S.M.S.; Mondal, J.; Hasan, M.N.; Revuri, V.; Lee, D.Y.; Lee, Y.K. Electrospinning Nanofibers for Therapeutics Delivery. Nanomaterials 2019, 9, 532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, H.; Yang, X.; Che, X.; Yang, M.; Zhai, G. Biomedical application and controlled drug release of electrospun fibrous materials. Mater. Sci. Eng. C 2018, 90, 750–763. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Huang, J.; Yu, G.; Cardenas, R.; Wei, S.; Wujcik, E.K.; Guo, Z. Coaxial electrospun fibers: Applications in drug delivery and tissue engineering. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 654–677. [Google Scholar] [CrossRef]
- Naskar, A.; Lee, S.; Ko, D.; Kim, S.; Kim, K.S. Bovine serum albumin-immobilized black phosphorus-based γ-Fe2O3 nanocomposites: A promising biocompatible nanoplatform. Biomedicines 2021, 9, 858. [Google Scholar] [CrossRef]
- Sun, Y.; Lu, R.; Liu, J.; Wang, X.; Dong, H.; Chen, S. The early adhesion effects of human gingival fibroblasts on bovine serum albumin loaded hydrogenated titanium nanotube surface. Molecules 2021, 26, 5229. [Google Scholar] [CrossRef]
- Mishra, V.; Heath, R. Structural and Biochemical Features of Human Serum Albumin Essential for Eukaryotic Cell Culture. Int. J. Mol. Sci. 2021, 22, 8411. [Google Scholar] [CrossRef]
- Bolaños, K.; Kogan, M.J.; Araya, E. Capping gold nanoparticles with albumin to improve their biomedical properties. Int. J. Nanomed. 2019, 14, 6387–6406. [Google Scholar] [CrossRef] [Green Version]
- Belinskaia, D.A.; Voronina, P.A.; Shmurak, V.I.; Jenkins, R.O.; Goncharov, N.V. Serum Albumin in Health and Disease: Esterase, Antioxidant, Transporting and Signaling Properties. Int. J. Mol. Sci. 2021, 22, 10318. [Google Scholar] [CrossRef]
- McClellan, P.; Landis, W.J. Recent Applications of Coaxial and Emulsion Electrospinning Methods in the Field of Tissue Engineering. Biores. Open Access 2016, 5, 212–227. [Google Scholar] [CrossRef] [Green Version]
- Yıldız, A.; Kara, A.A.; Acartürk, F. Peptide-protein based nanofibers in pharmaceutical and biomedical applications. Int. J. Biol. Macromol. 2020, 148, 1084–1097. [Google Scholar] [CrossRef] [PubMed]
- Baskapan, B.; Callanan, A. Electrospinning Fabrication Methods to Incorporate Laminin in Polycaprolactone for Kidney Tissue Engineering. Tissue Eng. Regen. Med. 2021, 19, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Tian, L.; Prabhakaran, M.P.; Ding, X.; Ramakrishna, S. Fabrication of Nerve Growth Factor Encapsulated Aligned Poly(ε-Caprolactone) Nanofibers and Their Assessment as a Potential Neural Tissue Engineering Scaffold. Polymers 2016, 8, 54. [Google Scholar] [CrossRef] [PubMed]
- Parham, S.; Kharazi, A.Z.; Bakhsheshi-Rad, H.R.; Ghayour, H.; Ismail, A.F.; Nur, H.; Berto, F. Electrospun Nano-fibers for biomedical and tissue engineering applications: A comprehensive review. Materials 2020, 13, 2153. [Google Scholar] [CrossRef]
- Sanchez, M.A.; Rodriguez, A.P.; Monsalve, L.N.; Georgiadou, S. Emulsion Electrospinning for Drug Delivery : Two Encapsulation Methods. Austin J. Pharmacol. Ther. 2020, 8, 1117. [Google Scholar]
- Hameed, M.M.A.; Khan, S.A.P.M.; Thamer, B.M.; Al-Enizi, A.; Aldalbahi, A.; El-Hamshary, H.; El-Newehy, M.H. Core-shell nanofibers from poly(vinyl alcohol) based biopolymers using emulsion electrospinning as drug delivery system for cephalexin drug. J. Macromol. Sci. Part A Pure Appl. Chem. 2020, 58, 130–144. [Google Scholar] [CrossRef]
- Shibata, T.; Yoshimura, N.; Kobayashi, A.; Ito, T.; Hara, K.; Tahara, K. Emulsion-electrospun polyvinyl alcohol nanofibers as a solid dispersion system to improve solubility and control the release of probucol, a poorly water-soluble drug. J. Drug Deliv. Sci. Technol. 2022, 67, 102953. [Google Scholar] [CrossRef]
- Wang, L.; Wang, C.; Wang, L.; Zhang, Q.; Wang, Y.; Xia, X. Emulsion electrospun polylactic acid/Apocynum venetum nanocellulose nanofiber membranes with controlled sea buckthorn extract release as a drug delivery system. Text. Res. J. 2020, 91, 1046–1055. [Google Scholar] [CrossRef]
- Luraghi, A.; Peri, F.; Moroni, L. Electrospinning for drug delivery applications: A review. J. Control. Release 2021, 334, 463–484. [Google Scholar] [CrossRef]
- Ng, N.; Rogers, M.A. Surfactants. In Encyclopedia of Food Chemistry; Melton, L., Shahidi, F., Varelis, P., Eds.; Academic Press: Oxford, UK, 2019; pp. 276–282. [Google Scholar] [CrossRef]
- Hu, J.; Prabhakaran, M.P.; Ding, X.; Ramakrishna, S. Emulsion electrospinning of polycaprolactone: Influence of surfactant type towards the scaffold properties. J. Biomater. Sci. Polym. Ed. 2015, 26, 57–75. [Google Scholar] [CrossRef]
- Pal, J.; Wu, D.; Hakkarainen, M.; Srivastava, R.K. The viscoelastic interaction between dispersed and continuous phase of PCL/HA-PVA oil-in-water emulsion uncovers the theoretical and experimental basis for fiber formation during emulsion electrospinning. Eur. Polym. J. 2017, 96, 44–54. [Google Scholar] [CrossRef]
- Dave, N.; Joshi, T. A Concise Review on Surfactants and Its Significance. Int. J. Appl. Chem. 2017, 13, 663–672. [Google Scholar]
- Mahmood, M.E.; Al-koofee, D.A.F. Effect of Temperature Changes on Critical Micelle Concentration for Tween Series Surfactant. Glob. J. Sci. Front. Res. Chem. 2013, 13, 1–7. [Google Scholar]
- Atanase, L.I.; Riess, G. Poly(vinyl alcohol-co-vinyl acetate) complex formation with anionic surfactants particle size of nanogels and their disaggregation with sodium dodecyl sulfate, Colloids Surfaces A Physicochem. Eng. Asp. 2010, 355, 29–36. [Google Scholar] [CrossRef]
- Yang, J.; Pal, R. Investigation of Surfactant-Polymer Interactions Using Rheology and Surface Tension Measurements. Polymers 2020, 12, 2302. [Google Scholar] [CrossRef] [PubMed]
- Dal-Bó, A.G.; Laus, R.; Felippe, A.C.; Zanette, D.; Minatti, E. Association of anionic surfactant mixed micelles with hydrophobically modified ethyl(hydroxyethyl)cellulose, Colloids Surfaces A Physicochem. Eng. Asp. 2011, 380, 100–106. [Google Scholar] [CrossRef]
- Zhang, C.; Feng, F.; Zhang, H. Emulsion Electrospinning: Fundamentals, Food Applications and Prospects. Trends Food Sci. Technol. 2018, 80, 175–186. [Google Scholar] [CrossRef]
- Yazgan, G.; Popa, A.; Rossi, R.; Maniura-Weber, K.; Puigmartí-Luis, J.; Crespy, D.; Fortunato, G. Tunable release of hydrophilic compounds from hydrophobic nanostructured fibers prepared by emulsion electrospinning. Polymer 2015, 66, 268–276. [Google Scholar] [CrossRef]
- Johnson, P.M.; Knewtson, K.E.; Hodge, J.G.; Lehtinen, J.M.; Trofimoff, A.S.; Fritz, D.J.; Robinson, J.L. Surfactant location and internal phase volume fraction dictate emulsion electrospun fiber morphology and modulate drug release and cell response. Biomater. Sci. 2021, 9, 1397–1408. [Google Scholar] [CrossRef]
- Roy, T.; Maity, P.P.; Rameshbabu, A.P.; Das, B.; John, A.; Dutta, A.; Ghorai, S.K.; Chattopadhyay, S.; Dhara, S. Core-shell nanofibrous scaffold based on polycaprolactone-silk fibroin emulsion electrospinning for tissue engineering applications. Bioengineering 2018, 5, 68. [Google Scholar] [CrossRef] [Green Version]
- Burlaga, N.; Bartolewska, M.; Buzgo, M.; Simaite, A. Optimization of the Emulsion Electrospinning for Increased Activity of Biopharmaceuticals. Proceedings 2021, 78, 39. [Google Scholar] [CrossRef]
- Liu, M.; Hao, X.; Wang, Y.; Jiang, Z.; Zhang, H. A biodegradable core-sheath nanofibrous 3D hierarchy prepared by emulsion electrospinning for sustained drug release. J. Mater. Sci. 2020, 55, 16730–16743. [Google Scholar] [CrossRef]
- Mouro, C.; Gomes, A.; Ahonen, M.; Fangueiro, R.; Gouveia, I.C. Chelidonium majus l Incorporated emulsion electrospun pcl/pva_pec nanofibrous meshes for antibacterial wound dressing applications. Nanomaterials 2021, 11, 1785. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Su, Y.; Liu, S.; Tan, L.; Mo, X.; Ramakrishna, S. Encapsulation of proteins in poly(l-lactide-co-caprolactone) fibers by emulsion electrospinning. Colloids Surfaces B Biointerfaces 2010, 75, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Skinner, S.J.M.; Geaney, S.; Lin, H. Incorporation and release of dual growth factors for nerve tissue engineering using nanofibrous bicomponent scaffolds. Biomed. Mater. 2018, 13, 044107. [Google Scholar] [CrossRef] [Green Version]
- Giannetti, R.; Abraham, G.; Rivero, G. The role of emulsion parameters in tramadol sustained-release from electrospun mats. Mater. Sci. Eng. C 2019, 99, 1493–1501. [Google Scholar] [CrossRef]
- Zhao, X.; Lui, Y.S.; Toh, P.W.J.; Loo, S.C.J. Sustained release of hydrophilic L-ascorbic acid 2-phosphate magnesium from electrospun polycaprolactone scaffold-A study across blend, coaxial, and emulsion electrospinning techniques. Materials 2014, 7, 7398–7408. [Google Scholar] [CrossRef] [Green Version]
- Morris, A.H.; Mahal, R.; Udell, J.; Wu, M.; Kyriakides, T.R. Multicompartment Drug Release System for Dynamic Modulation of Tissue Responses. Adv. Healthc. Mater. 2017, 6, 1700370. [Google Scholar] [CrossRef]
- Hammouda, B. Temperature Effect on the Nanostructure of SDS Micelles in Water. J. Res. Natl. Inst. Stand. Technol. 2013, 118, 151. [Google Scholar] [CrossRef]
- Bhatt, S.; Pulpytel, J.; Mirshahi, M.; Arefi-Khonsari, F. Catalyst-free plasma-assisted copolymerization of poly(ε-caprolactone) -poly(ethylene glycol) for biomedical applications. ACS Macro Lett. 2012, 1, 764–767. [Google Scholar] [CrossRef]
- Basar, A.; Castro, S.; Torres-Giner, S.; Lagaron, J.; Sasmazel, H.T. Novel poly(ε-caprolactone)/gelatin wound dressings prepared by emulsion electrospinning with controlled release capacity of Ketoprofen anti-inflammatory drug. Mater. Sci. Eng. C 2017, 81, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Belatik, A.; Hotchandani, S.; Carpentier, R.; Tajmir-Riahi, H.-A. Locating the binding sites of pb(II) ion with human and bovine serum albumins. PLoS ONE 2012, 7, e36723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nalwa, H.S. Conductive Polymers: Spectroscopy and Physical Properties. Handb. Org. Conduct. Mol. Polym. 1997, 3, 497–588. [Google Scholar]
- Saffarionpour, S. Preparation of Food Flavor Nanoemulsions by High- and Low-Energy Emulsification Approaches. Food Eng. Rev. 2019, 11, 259–289. [Google Scholar] [CrossRef]
- Wang, S.-C.; Wei, T.-C.; Chen, W.-B.; Tsao, H.-K. Effects of surfactant micelles on viscosity and conductivity of poly(ethylene glycol) solutions. J. Chem. Phys. 2004, 120, 4980–4988. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, M.; Pezeshki-Modaress, M.; Zandi, M. Biphasic, tough composite core/shell PCL/PVA-GEL nanofibers for biomedical application. J. Appl. Polym. Sci. 2019, 137, 48713. [Google Scholar] [CrossRef]
- Chanamaia, R.; Horna, G.; McClements, D. Influence of oil polarity on droplet growth in oil-in-water emulsions stabilized by a weakly adsorbing biopolymer or a nonionic surfactant. J. Colloid Interface Sci. 2002, 247, 167–176. [Google Scholar] [CrossRef]
- Posadowska, U.; Brzychczy-Włoch, M.; Pamuła, E. Gentamicin loaded PLGA nanoparticles as local drug delivery system for the osteomyelitis treatment. Acta Bioeng. Biomech. 2015, 17, 41–47. [Google Scholar] [CrossRef]
- Abdullah, M.F.; Nuge, T.; Andriyana, A.; Ang, B.C.; Muhamad, F. Core–Shell Fibers: Design, Roles, and Controllable Release Strategies in Tissue Engineering and Drug Delivery. Polymers 2019, 11, 2008. [Google Scholar] [CrossRef] [Green Version]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable controlled-release polymers and polymeric nanoparticles: Mechanisms of controlling drug release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Chen, X.; Liu, Y.; Gao, Y.; Liu, P. Electrospun Coaxial Fibers to Optimize the Release of Poorly Water-Soluble Drug. Polymers 2022, 14, 469. [Google Scholar] [CrossRef] [PubMed]
- Medda, L.; Monduzzi, M.; Salis, A. The molecular motion of bovine serum albumin under physiological conditions is ion specific. Chem. Commun. 2015, 51, 6663–6666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arechabala, B.; Coiffard, C.; Rivalland, P.; Coiffard, L.J.M.; De Roeck-Holtzhauer, Y. Comparison of cytotoxicity of various surfactants tested on normal human fibroblast cultures using the neutral red test, MTT assay and LDH release. J. Appl. Toxicol. 1999, 19, 163–165. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, Z.; Gu, J.; Zhou, W.; Liang, X.; Zhou, G.; Han, C.C.; Xu, S.; Liu, Y. Mechanism of a long-term controlled drug release system based on simple blended electrospun fibers. J. Control. Release 2020, 320, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Lonza, ViaLight Plus Kit Cell Viability Assay. 2011, pp. 1–5. Available online: https://bioscience.lonza.com/lonza_bs/CH/en/Cell-analysis/p/000000000000186469/ViaLight™-Plus-Cell-Proliferation-and-Cytotoxicity-BioAssay-Kit%2C-500-Test (accessed on 6 March 2022).
- Lonza, ToxiLightTM Bioassay Kit. 2011, pp. 3–7. Available online: https://bioscience.lonza.com/lonza_bs/CH/en/Cell-analysis/p/000000000000186486/ToxiLight™-Non-Destructive-Cytotoxicity-BioAssay-Kit-with-Plates%2C-500-Test (accessed on 6 March 2022).
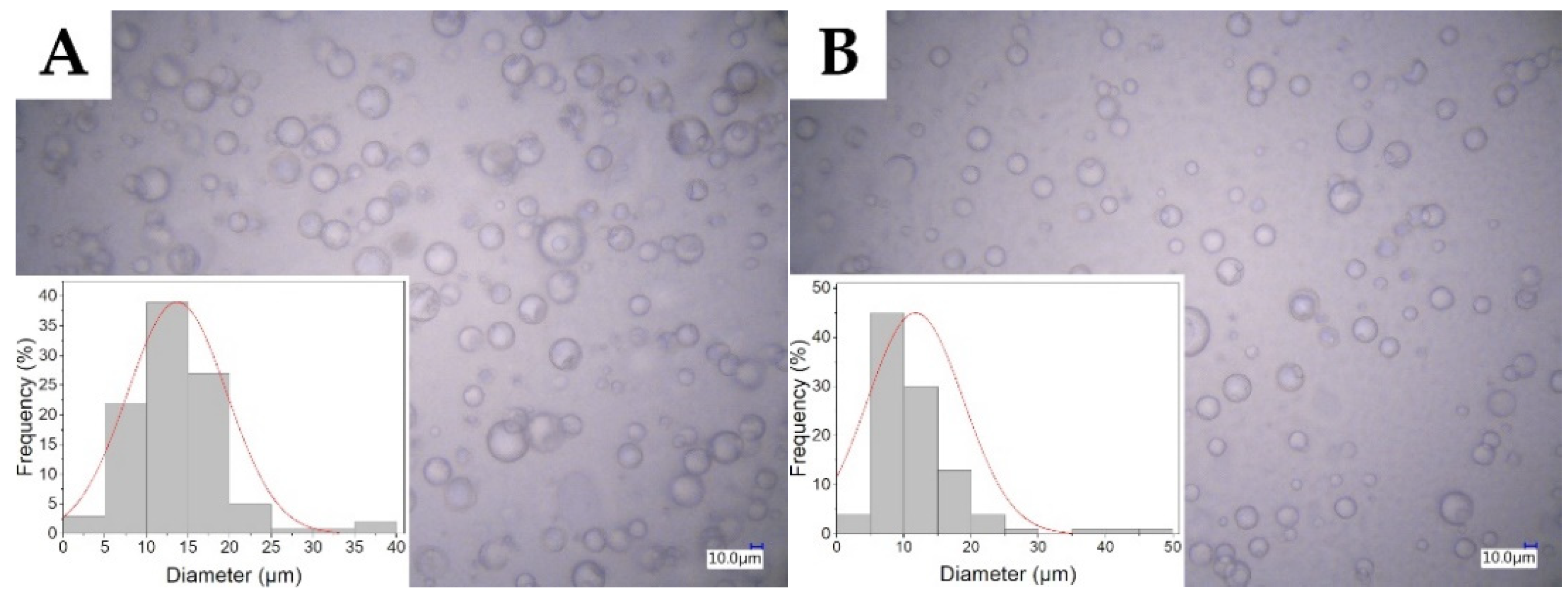
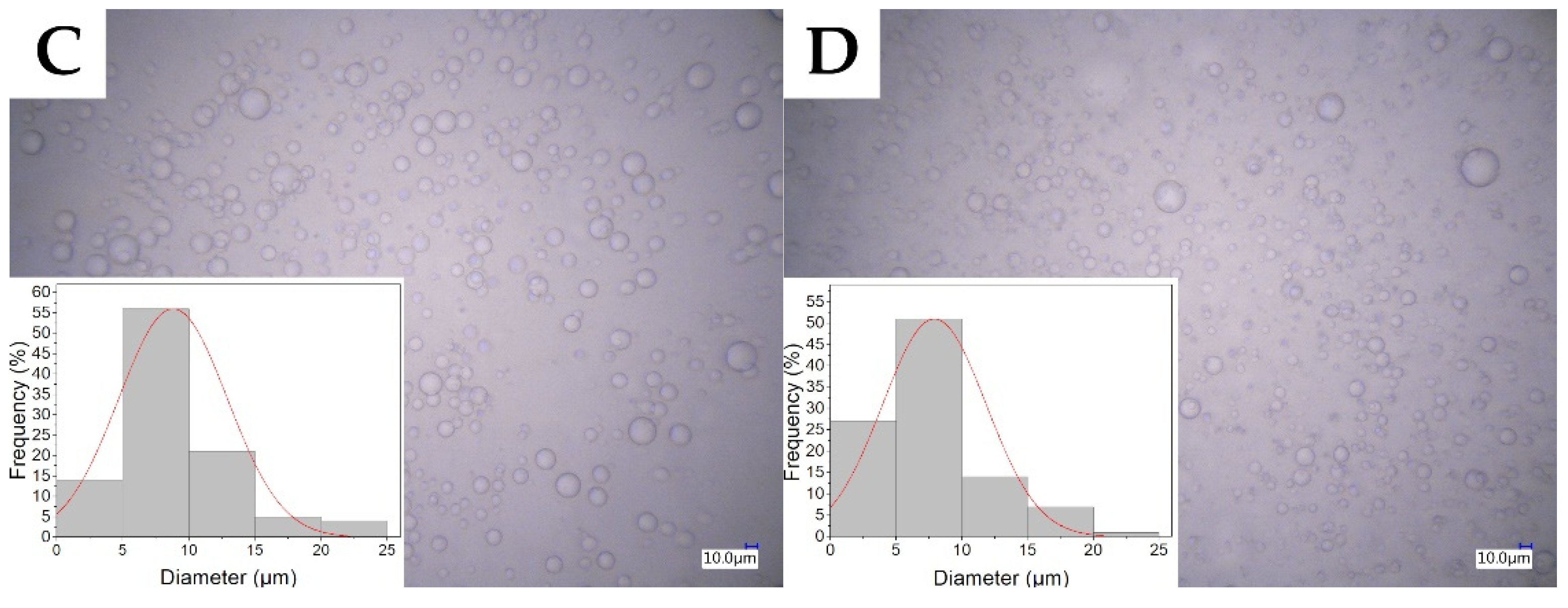
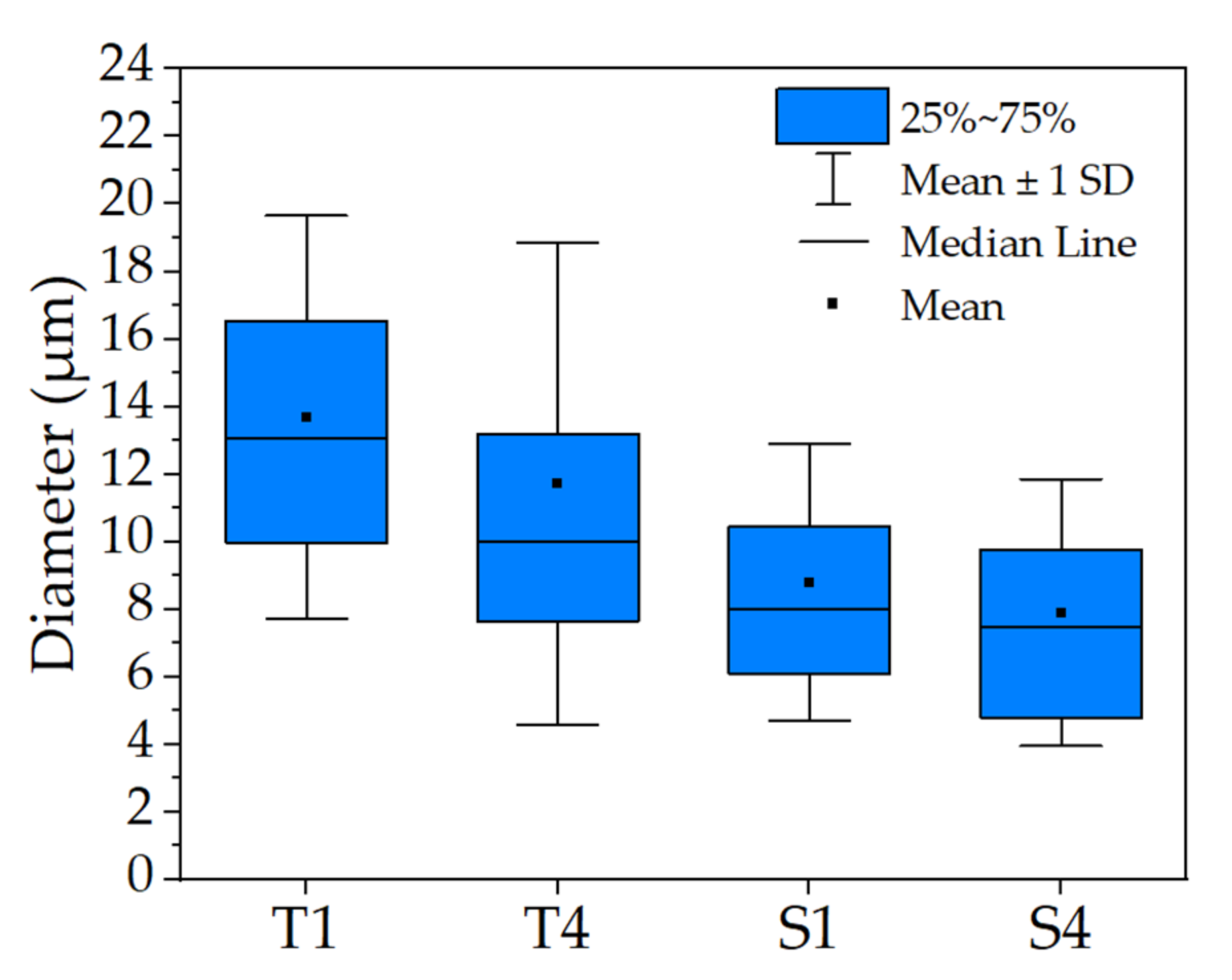
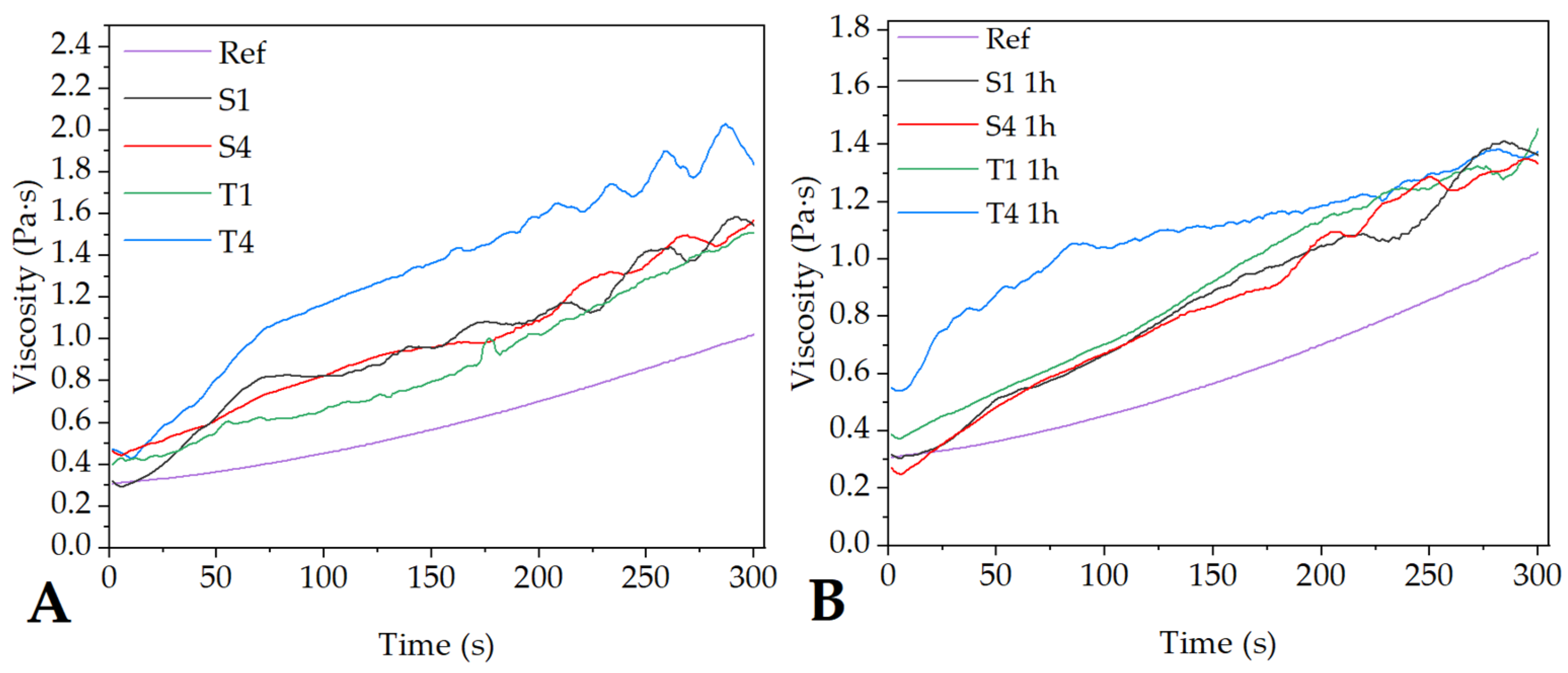




| Sample | Conductance (µS/cm) | Mean Diameter of Micelle (µm) |
|---|---|---|
| Ref * | 0.3 ± 0.02 | - |
| T1 | 1.9 ± 0.02 | 13.68 ± 5.97 |
| T4 | 5.4 ± 0.07 | 11.72 ± 7.13 |
| S1 | 140.5 ± 0.04 | 8.80 ± 4.11 |
| S4 | 183 ± 0.06 | 7.91 ± 3.95 |
| Sample | Loading Efficiency (%) | Encapsulation Efficiency (%) | |
|---|---|---|---|
| Theoretical | Experimental | ||
| T1 | 9.38 | 8.38 | 89.36 |
| T4 | 9.38 | 8.57 | 91.39 |
| S1 | 18.75 | 14.51 | 77.37 |
| S4 | 18.75 | 16.38 | 87.36 |
| Model | T1 | T4 | S1 | S4 | ||||
|---|---|---|---|---|---|---|---|---|
| K | R2 | K | R2 | K | R2 | K | R2 | |
| Zero-order | 0.038 | 0.999 | 0.040 | 0.993 | 0.056 | 0.749 | 0.057 | 0.682 |
| First-order | 0.056 | 0.971 | 0.056 | 0.965 | 0.174 | 0.837 | 0.215 | 0.815 |
| Higuchi | 0.141 | 0.930 | 0.149 | 0.934 | 0.226 | 0.874 | 0.231 | 0.835 |
| Ritger–Peppas | 0.036 | 0.999 | 0.058 | 0.987 | 0.293 | 0.719 | 0.301 | 0.774 |
| Sample | Description |
|---|---|
| Ref | Reference (neat PCL fibers). |
| T1 | PCL/BSA fibers with addition of 0.1% (v/v) Tween 80. |
| T4 | PCL/BSA fibers with addition of 0.4% (v/v) Tween 80. |
| S1 | PCL/BSA fibers with addition of 0.1% (w/v) SDS. |
| S4 | PCL/BSA fibers with addition of 0.4% (w/v) SDS. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurpanik, R.; Lechowska-Liszka, A.; Mastalska-Popławska, J.; Nocuń, M.; Rapacz-Kmita, A.; Ścisłowska-Czarnecka, A.; Stodolak-Zych, E. Effect of Ionic and Non-Ionic Surfactant on Bovine Serum Albumin Encapsulation and Biological Properties of Emulsion-Electrospun Fibers. Molecules 2022, 27, 3232. https://doi.org/10.3390/molecules27103232
Kurpanik R, Lechowska-Liszka A, Mastalska-Popławska J, Nocuń M, Rapacz-Kmita A, Ścisłowska-Czarnecka A, Stodolak-Zych E. Effect of Ionic and Non-Ionic Surfactant on Bovine Serum Albumin Encapsulation and Biological Properties of Emulsion-Electrospun Fibers. Molecules. 2022; 27(10):3232. https://doi.org/10.3390/molecules27103232
Chicago/Turabian StyleKurpanik, Roksana, Agnieszka Lechowska-Liszka, Joanna Mastalska-Popławska, Marek Nocuń, Alicja Rapacz-Kmita, Anna Ścisłowska-Czarnecka, and Ewa Stodolak-Zych. 2022. "Effect of Ionic and Non-Ionic Surfactant on Bovine Serum Albumin Encapsulation and Biological Properties of Emulsion-Electrospun Fibers" Molecules 27, no. 10: 3232. https://doi.org/10.3390/molecules27103232
APA StyleKurpanik, R., Lechowska-Liszka, A., Mastalska-Popławska, J., Nocuń, M., Rapacz-Kmita, A., Ścisłowska-Czarnecka, A., & Stodolak-Zych, E. (2022). Effect of Ionic and Non-Ionic Surfactant on Bovine Serum Albumin Encapsulation and Biological Properties of Emulsion-Electrospun Fibers. Molecules, 27(10), 3232. https://doi.org/10.3390/molecules27103232






