Contemporary Developments and Emerging Trends in the Application of Spectroscopy Techniques: A Particular Reference to Coconut (Cocos nucifera L.)
Abstract
:1. Introduction
2. Methods
3. Results
3.1. Application of Spectroscopy Techniques for Quality Evaluation
3.1.1. Coconut Oil
3.1.2. Coconut Milk
3.1.3. Tender Coconut Water
3.1.4. Virgin Coconut Oil
3.1.5. Edible Coconut Products
3.1.6. Non-Edible Coconut Products
3.2. Application of Spectroscopy Techniques for Adulteration Detection/Authentication
3.2.1. Coconut Oil
3.2.2. Virgin Coconut Oil
3.2.3. Tender Coconut Water
3.3. Microbial Contaminants and Toxic Component (Heavy Metals) Detection in Coconut Products
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arunachalam, V. Genomics of Cultivated Palms; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Nampoothiri, K.; Krishnakumar, V.; Thampan, P.K.; Nair, M.A. The Coconut Palm (Cocos nucifera L.)—Research and Development Perspectives; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Naik, A.; Madhusudhan, M.C.; Raghavarao, K.; Subba, D. Downstream Processing for Production of Value Added Products from Coconut. Curr. Biochem. Eng. 2015, 2, 168–180. [Google Scholar] [CrossRef] [Green Version]
- Arancon, R.N. Market and Trade of Coconut Products; Wiley: Hoboken, NJ, USA, 2013. [Google Scholar]
- Roopan, S.M. An overview of phytoconstituents, biotechnological applications, and nutritive aspects of coconut (Cocos nucifera). Appl. Biochem. Biotechnol. 2016, 179, 1309–1324. [Google Scholar] [CrossRef]
- Yong, J.W.; Ge, L.; Ng, Y.F.; Tan, S.N.J.M. The chemical composition and biological properties of coconut (Cocos nucifera L.) water. Molecules 2009, 14, 5144–5164. [Google Scholar] [CrossRef] [Green Version]
- Vermeulen, K.; Strnad, M.; Kryštof, V.; Havlícěk, L.; Van der Aa, A.; Lenjou, M.; Nijs, G.; Rodrigus, I.; Stockman, B.; Van Onckelen, H. Antiproliferative effect of plant cytokinin analogues with an inhibitory activity on cyclin-dependent kinases. Leukemia 2002, 16, 299–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carandang, E. Health benefits of virgin coconut oil. Indian Coconut J. 2008, 38, 8. [Google Scholar]
- Alyaqoubi, S.; Abdullah, A.; Samudi, M.; Abdullah, N.; Addai, Z.R.; Musa, K.H. Study of antioxidant activity and physicochemical properties of coconut milk (Pati santan) in Malaysia. J. Chem. Pharm. Res. 2015, 7, 967–973. [Google Scholar]
- Seneviratne, K.; Kotuwegedara, R.; Ekanayake, S. Serum cholesterol and triglyceride levels of rats fed with consumer selected coconut oil blends. Int. Food Res. J. 2011, 18, 1303–1308. [Google Scholar]
- Liyanage, C.D.; Pieris, M. A physico-chemical analysis of coconut shell powder. Procedia Chem. 2015, 16, 222–228. [Google Scholar] [CrossRef] [Green Version]
- Deshpande, S.; Cheryan, M.; Gunasekaran, S.; Paulsen, M.R.; Salunkhe, D.; Clydesdale, F.M. Nondestructive optical methods of food quality evaluation. Crit. Rev. Food Sci. Nutr. 1984, 21, 323–379. [Google Scholar] [CrossRef]
- Pandiselvam, R.; Thirupathi, V.; Mohan, S.; Uma, D. Development of PLS Model for Rapid Estimation of Protein Content of Rice Using Fourier Transform-Near Infrared Spectroscopy; Agris: Rome, Italy, 2015. [Google Scholar]
- Pandiselvam, R.; Thirupathi, V.; Vennila, P. Fourier Transform–Near Infrared Spectroscopy for Rapid and Nondestructive Measurement of Amylose Content of Paddy; UDK: Berlin, Germany, 2016. [Google Scholar]
- Hassoun, A.; Cropotova, J.; Rustad, T.; Heia, K.; Lindberg, S.K.; Nilsen, H. Use of Spectroscopic Techniques for a Rapid and Non-Destructive Monitoring of Thermal Treatments and Storage Time of Sous-Vide Cooked Cod Fillets. Sensors 2020, 20, 2410. [Google Scholar] [CrossRef]
- Hassoun, A.; Mage, I.; Schmidt, W.F.; Temiz, H.T.; Li, L.; Kim, H.Y.; Nilsen, H.; Biancolillo, A.; Ait-Kaddour, A.; Sikorski, M.; et al. Fraud in Animal Origin Food Products: Advances in Emerging Spectroscopic Detection Methods over the Past Five Years. Foods 2020, 9, 1069. [Google Scholar] [CrossRef] [PubMed]
- Hassoun, A.; Guðjónsdóttir, M.; Prieto, M.A.; Garcia-Oliveira, P.; Simal-Gandara, J.; Marini, F.; Di Donato, F.; D’Archivio, A.A.; Biancolillo, A. Application of Novel Techniques for Monitoring Quality Changes in Meat and Fish Products during Traditional Processing Processes: Reconciling Novelty and Tradition. Processes 2020, 8, 988. [Google Scholar] [CrossRef]
- Aït-Kaddour, A.; Hassoun, A.; Bord, C.; Schmidt-Filgueras, R.; Biancolillo, A.; Di Donato, F.; Temiz, H.T.; Cozzolino, D. Application of Spectroscopic Techniques to Evaluate Heat Treatments in Milk and Dairy Products: An Overview of the Last Decade. Food Bioprocess Technol. 2021, 14, 781–803. [Google Scholar] [CrossRef]
- Kucha, C.T.; Liu, L.; Ngadi, M.O. Non-destructive spectroscopic techniques and multivariate analysis for assessment of fat quality in pork and pork products: A review. Sensors 2018, 18, 377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scotter, C.N. Non-destructive spectroscopic techniques for the measurement of food quality. Trends Food Sci. Technol. 1997, 8, 285–292. [Google Scholar] [CrossRef]
- Penner, M.H. Basic principles of spectroscopy. In Food Analysis; Springer: Berlin/Heidelberg, Germany, 2017; pp. 79–88. [Google Scholar]
- Pandiselvam, R.; Manikantan, M.; Ramesh, S.; Beegum, S.; Mathew, A. Adulteration in Coconut and Virgin Coconut Oil: Implications and Detection Methods; ICAR-Central Plantation Crops Research Institute: Kasaragod, India, 2019. [Google Scholar]
- Kaavya, R.; Pandiselvam, R.; Mohammed, M.; Dakshayani, R.; Kothakota, A.; Ramesh, S.; Cozzolino, D.; Ashokkumar, C. Application of infrared spectroscopy techniques for the assessment of quality and safety in spices: A review. Appl. Spectrosc. Rev. 2020, 55, 593–611. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, C.; Ye, R.; Duan, Q. Advances on Water Quality Detection by UV-Vis Spectroscopy. J. Appl. Sci. 2020, 10, 6874. [Google Scholar] [CrossRef]
- Meyers, R.A. Encyclopedia of Analytical Chemistry; Univerza v Novi Gorici: Nova Gorica, Slovenia, 2006. [Google Scholar]
- Rodrigues, F.T.; Marchioni, E.; Lordel-Madeleine, S.; Kuntz, F.; Villavicencio, A.L.C.H.; Julien-David, D. Degradation of profenofos in aqueous solution and in vegetable sample by electron beam radiation. Radiat. Phys. Chem. 2020, 166, 108441. [Google Scholar] [CrossRef]
- Khan, M.; Soylak, M. Magnetic solid phase extraction of lead, cadmium, and cobalt on magnetic carboxyl-modified nanodiamonds (MCNDs) from natural water samples and their determination by flame atomic absorption spectrometry. At. Spectrosc. 2018, 39, 81–89. [Google Scholar] [CrossRef]
- Van de Voort, F.; Ismail, A.; Sedman, J. A rapid, automated method for the determination of cis and trans content of fats and oils by Fourier transform infrared spectroscopy. J. Am. Oil Chem. Soc. 1995, 72, 873–880. [Google Scholar] [CrossRef]
- Grundas, S.; Stępniewski, A. Advances in Agrophysical Research; BoD–Books on Demand: Norderstedt, Germany, 2013. [Google Scholar]
- Li-Chan, E.; Ismail, A.; Sedman, J.; Van de Voort, F. Vibrational spectroscopy of food and food products. In Handbook of Vibrational Spectroscopy; Wiley: Hoboken, NJ, USA, 2006. [Google Scholar]
- Shurvell, H. Spectra–structure correlations in the mid-and far-infrared. In Handbook of Vibrational Spectroscopy; Wiley: Hoboken, NJ, USA, 2006. [Google Scholar]
- Torović, L.; Dimitrov, N.; Lopes, A.; Martins, C.; Alvito, P.; Assunção, R. Patulin in fruit juices: Occurrence, bioaccessibility, and risk assessment for Serbian population. Food Addit. Contam. A 2018, 35, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies (Tables and Charts); John Wiley & Sons, Ltd.: London, UK; New York, NY, USA, 2001. [Google Scholar]
- Thygesen, L.G.; Løkke, M.M.; Micklander, E.; Engelsen, S.B. Vibrational microspectroscopy of food. Raman vs. FT-IR. Trends Food Sci. Technol. 2003, 14, 50–57. [Google Scholar] [CrossRef]
- Xu, Y.; Zhong, P.; Jiang, A.; Shen, X.; Li, X.; Xu, Z.; Shen, Y.; Sun, Y.; Lei, H. Raman spectroscopy coupled with chemometrics for food authentication: A review. TrAC Trends Anal. Chem. 2020, 131, 116017. [Google Scholar] [CrossRef]
- Esteban, M.; Ariño-Blasco, M.C.; Díaz-Cruz, J.M. Chemometrics in Electrochemistry. In Comprehensive Chemometrics; MDPI: Basel, Switzerland, 2020; pp. 1–31. [Google Scholar]
- Costa, H.B.; Souza, L.M.; Soprani, L.C.; Oliveira, B.G.; Ogawa, E.M.; Korres, A.M.; Ventura, J.A.; Romão, W. Monitoring the physicochemical degradation of coconut water using ESI-FT-ICR MS. Food Chem. 2015, 174, 139–146. [Google Scholar] [CrossRef] [Green Version]
- Purkayastha, M.D.; Kalita, D.; Das, V.K.; Mahanta, C.L.; Thakur, A.J.; Chaudhuri, M.K. Effects of L-ascorbic acid addition on micro-filtered coconut water: Preliminary quality prediction study using 1H-NMR, FTIR and GC-MS. Innov. Food Sci. Emerg. Technol. 2012, 13, 184–199. [Google Scholar] [CrossRef]
- Sucupira, N.; Alves Filho, E.; Silva, L.; De Brito, E.; Wurlitzer, N.; Sousa, P. NMR spectroscopy and chemometrics to evaluate different processing of coconut water. Food Chem. 2017, 216, 217–224. [Google Scholar] [CrossRef]
- Prades, A.; Assa, R.R.; Dornier, M.; Pain, J.P. Near Infrared Spectroscopy: A Tool for On-Line Monitoring of Beverage Quality; FAO: Roma, Italy, 2008. [Google Scholar]
- Psomiadis, D.; Zisi, N.; Koger, C.; Horvath, B.; Bodiselitsch, B. Sugar-specific carbon isotope ratio analysis of coconut waters for authentication purposes. J. Food Sci. Technol. 2018, 55, 2994–3000. [Google Scholar] [CrossRef] [PubMed]
- Richardson, P.I.; Muhamadali, H.; Ellis, D.I.; Goodacre, R. Rapid quantification of the adulteration of fresh coconut water by dilution and sugars using Raman spectroscopy and chemometrics. Food Chem. 2019, 272, 157–164. [Google Scholar] [CrossRef]
- Warsakoon, W. Preliminary Study on Heavy Metals in Coconut and Coconut Products. CORD 2010, 26, 7. [Google Scholar] [CrossRef]
- Cheevitsopon, E.; Sirisomboon, P. Rapid evaluation of fat content in curry soup containing coconut milk by using near infrared spectroscopy. J. Near Infrared Spectrosc. 2018, 26, 16–25. [Google Scholar] [CrossRef] [Green Version]
- Thitibunjan, N.; Sirisomboon, P. Evaluation of pH of Curry Soup Containing Coconut Milk by Near Infrared Spectroscopy. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2019; p. 012061. [Google Scholar]
- Rohman, A.; Che Man, Y.B.; Hashim, P.; Ismail, A. FTIR spectroscopy combined with chemometrics for analysis of lard adulteration in some vegetable oils Espectroscopia FTIR combinada con quimiometría para el análisis de adulteración con grasa de cerdo de aceites vegetales. CyTA—J. Food 2011, 9, 96–101. [Google Scholar] [CrossRef]
- Marina, A.; Rosli, W.; Noorhidayah, M. Quantitative Analysis of Peroxide Value in Virgin Coconut Oil by ATRFTIR Spectroscopy. Open Conf. Proc. J. 2013, 4, 53–56. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, Y.; Semwal, A.D. A study on monitoring of frying performance and oxidative stability of virgin coconut oil (VCO) during continuous/prolonged deep fat frying process using chemical and FTIR spectroscopy. J. Food Sci. Technol. 2015, 52, 984–991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zicker, M.C.; Craig, A.P.; de Oliveira Ramiro, D.; Franca, A.S.; Labanca, R.A.; Ferreira, A.V. Quantitative analysis of acidity level in virgin coconut oils by Fourier transform infrared spectroscopy and chemometrics. Eur. J. Lipid Sci. Technol. 2016, 118, 1350–1357. [Google Scholar] [CrossRef]
- Alvarenga, B.R.; Xavier, F.A.N.; Soares, F.L.F.; Carneiro, R.L. Thermal stability assessment of vegetable oils by Raman spectroscopy and chemometrics. Food Anal. Methods 2018, 11, 1969–1976. [Google Scholar] [CrossRef]
- Noypitak, S.; Imsabai, W.; Noknoi, W.; Karoojee, S.; Terdwongworakul, A.; Kobori, H. Detection of cracked shell in intact aromatic young coconut using near infrared spectroscopy and acoustic response methods. J. Food Meas. Charact. 2019, 13, 1991–1999. [Google Scholar] [CrossRef]
- Rambo, M.; Alves, A.; Garcia, W.; Ferreira, M. Multivariate analysis of coconut residues by near infrared spectroscopy. Talanta 2015, 138, 263–272. [Google Scholar] [CrossRef]
- Morbeck, F.L.; Lelis, R.C.C.; Schueler, M.V.E.; Santos, W.A.; Sampaio, D.A.; de Silva, B.C.; Morais, R.d.M.; Santana, G.M. Extraction and evaluation of tannin from green coconut mesocarp. Matéria 2019, 24, e748. [Google Scholar] [CrossRef]
- Brígida, A.; Calado, V.; Gonçalves, L.; Coelho, M. Effect of chemical treatments on properties of green coconut fiber. Carbohydr. Polym. 2010, 79, 832–838. [Google Scholar] [CrossRef]
- Ndife, J. Functional Foods: Basics, Ingredients and Applications; Amotees Link Services and Publishers: Kaduna, Nigeria, 2016; p. 156. [Google Scholar]
- Wardlaw, G.M.; Insel, P.M. Perspectives in Nutrition; McGraw Hil: New York, NY, USA, 1996. [Google Scholar]
- Ndife, J.; Obot, D.; Abasiekong, K. Quality Evaluation of Coconut (Cocos nucifera L.) Oils Produced by Different Extraction Methods. Asian Food Sci. J. 2019, 8, 1–10. [Google Scholar] [CrossRef]
- Szmatoła, M.; Chrobak, J.; Grabowski, R.; Iłowska, J.; Woch, J.; Szwach, I.; Semeniuk, I.; Drabik, J.; Wrona, M.; Kozdrach, R. Spectroscopic methods in the evaluation of modified vegetable base oils from Crambe abyssinica. Molecules 2018, 23, 3243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Irudayaraj, J.; Paradkar, M.M. Discriminant analysis of edible oils and fats by FTIR, FT-NIR and FT-Raman spectroscopy. Food Chem. 2005, 93, 25–32. [Google Scholar] [CrossRef]
- Dayrit, F.M.; Buenafe, O.E.M.; Chainani, E.T.; De Vera, I.M.S. Analysis of monoglycerides, diglycerides, sterols, and free fatty acids in coconut (Cocos nucifera L.) oil by 31P NMR spectroscopy. J. Agric. 2008, 56, 5765–5769. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.A.; Santos, J.M.; Breitkreitz, M.C.; Ferreira, J.M.S.; Lins, P.M.P.; Farias, S.C.; de Morais, D.R.; Eberlin, M.N.; Bottoli, C.B.G. Characterization of the lipid profile from coconut (Cocos nucifera L.) oil of different varieties by electrospray ionization mass spectrometry associated with principal component analysis and independent component analysis. Food Res. Int. 2019, 123, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Satheesh, N.; Prasad, N. Optimization of parameters for fermentative production of virgin coconut oil by Lactobacillus sp. Food Sci. Technol. 2013, 14, 312–317. [Google Scholar]
- Zhu, X.; Zhao, Z.; Wang, L.; Zhang, L. A new method to measure fat content in coconut milk based on Y-type optic fiber system. Optik 2014, 125, 6172–6178. [Google Scholar] [CrossRef]
- Wattanapahu, S.; Suwonsichon, T.; Jirapakkul, W.; Kasermsumran, S. Categorization of coconut milk products by their sensory characteristics. Agric. Nat. Resour. 2012, 46, 944–954. [Google Scholar]
- Sirisomboon, P.; Nawayon, J. Evaluation of total solids of curry soup containing coconut milk by near infrared spectroscopy. J. Near Infrared Spectrosc. 2016, 24, 191–198. [Google Scholar] [CrossRef]
- Dupuis, A.; Hennekinne, J.A.; Garin, J.; Brun, V. Protein Standard Absolute Quantification (PSAQ) for improved investigation of staphylococcal food poisoning outbreaks. Proteomics 2008, 8, 4633–4636. [Google Scholar] [CrossRef]
- Rohman, A.; Che Man, Y.B.; Ismail, A.; Hashim, P. Application of FTIR spectroscopy for the determination of virgin coconut oil in binary mixtures with olive oil and palm oil. J. Am. Oil Chem. Soc. 2010, 87, 601–606. [Google Scholar] [CrossRef]
- Kulp, K.; Ponte, J.G.; D’Appolonia, B.L. Staling of white pan bread: Fundamental causes. C R C Crit. Rev. Food Sci. Nutr. 2009, 15, 1–48. [Google Scholar] [CrossRef]
- Gu, H.; Liu, K.; Huang, X.; Chen, Q.; Sun, Y.; Tan, C.P. Feasibility study for the analysis of coconut water using fluorescence spectroscopy coupled with PARAFAC and SVM methods. Br. Food J. 2020, 122, 3203–3212. [Google Scholar] [CrossRef]
- Cunha, A.G.; Alves Filho, E.G.; Silva, L.M.A.; Ribeiro, P.R.V.; Rodrigues, T.H.S.; de Brito, E.S.; de Miranda, M.R.A. Chemical composition of thermally processed coconut water evaluated by GC–MS, UPLC-HRMS, and NMR. Food Chem. 2020, 324, 126874. [Google Scholar] [CrossRef] [PubMed]
- Spraul, M.; Schütz, B.; Rinke, P.; Koswig, S.; Humpfer, E.; Schäfer, H.; Mörtter, M.; Fang, F.; Marx, U.C.; Minoja, A. NMR-based multi parametric quality control of fruit juices: SGF profiling. Nutrients 2009, 1, 148–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuny, M.; Vigneau, E.; Le Gall, G.; Colquhoun, I.; Lees, M.; Rutledge, D. Fruit juice authentication by 1 H NMR spectroscopy in combination with different chemometrics tools. Anal. Bioanal. Chem. 2008, 390, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, Y.; Semwal, A.D.; Sajeevkumar, V.A.; Sharma, G. Melting, crystallization and storage stability of virgin coconut oil and its blends by differential scanning calorimetry (DSC) and Fourier transform infrared spectroscopy (FTIR). J. Food Sci. Technol. 2017, 54, 45–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villarino, B.J.; Dy, L.M.; Lizada, M.C.C. Descriptive sensory evaluation of virgin coconut oil and refined, bleached and deodorized coconut oil. LWT—Food Sci. Technol. 2007, 40, 193–199. [Google Scholar] [CrossRef]
- Rohman, A.; Man, Y.B.C. The use of Fourier transform mid infrared (FT-MIR) spectroscopy for detection and quantification of adulteration in virgin coconut oil. Food Chem. 2011, 129, 583–588. [Google Scholar] [CrossRef]
- Richards, A.; Wijesundera, C.; Salisbury, P. Evaluation of oxidative stability of canola oils by headspace analysis. J. Am. Oil Chem. Soc. 2005, 82, 869–874. [Google Scholar] [CrossRef]
- Muik, B.; Lendl, B.; Molina-Díaz, A.; Ayora-Cañada, M.J. Direct monitoring of lipid oxidation in edible oils by Fourier transform Raman spectroscopy. Chem. Phys. Lipids 2005, 134, 173–182. [Google Scholar] [CrossRef]
- Rohman, A.; Che Man, Y.; Ismail, A.; Hashim, P. Monitoring the oxidative stability of virgin coconut oil during oven test using chemical indexes and FTIR spectroscopy. Int. Food Res. J. 2011, 18, 303–310. [Google Scholar]
- Rohman, A. The use of infrared spectroscopy in combination with chemometrics for quality control and authentication of edible fats and oils: A review. Appl. Spectrosc. Rev. 2017, 52, 589–604. [Google Scholar] [CrossRef]
- Rohman, A.; Man, Y.C. Application of Fourier transform infrared spectroscopy for authentication of functional food oils. Appl. Spectrosc. Rev. 2012, 47, 1–13. [Google Scholar] [CrossRef]
- Kardash, E.; Tur’yan, Y. Acid value determination in vegetable oils by indirect titration in aqueous-alcohol media. Croat. Chem. Acta 2005, 78, 99–103. [Google Scholar]
- D’Amato, A.; Fasoli, E.; Righetti, P.G. Harry Belafonte and the secret proteome of coconut milk. J. Proteom. 2012, 75, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Lakshmanan, M.K.; Chinnu, T.; Arvamuthan, G. Near-infrared reflectance spectroscopy based online moisture measurement in copra. J. Food Process Eng. 2020, 43, e13383. [Google Scholar] [CrossRef]
- Shenderey, C.; Shmulevich, I.; Alchanatis, V.; Egozi, H.; Hoffman, A.; Ostrovsky, V.; Lurie, S.; Arie, R.B.; Schmilovitch, Z. NIRS detection of moldy core in apples. Food Bioprocess Technol. 2010, 3, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Magwaza, L.S.; Opara, U.L.; Nieuwoudt, H.; Cronje, P.J.; Saeys, W.; Nicolaï, B. NIR spectroscopy applications for internal and external quality analysis of citrus fruit—A review. Food Bioprocess Technol. 2012, 5, 425–444. [Google Scholar] [CrossRef]
- Fan, S.; Li, C.; Huang, W.; Chen, L. Detection of blueberry internal bruising over time using NIR hyperspectral reflectance imaging with optimum wavelengths. Postharvest Biol. Technol. 2017, 134, 55–66. [Google Scholar] [CrossRef]
- Vaithanomsat, P.; Apiwatanapiwat, W.; Chumchuent, N.; Kongtud, W.; Sundhrarajun, S. The potential of coconut husk utilization for bioethanol production. Agric. Nat. Resour. 2011, 45, 159–164. [Google Scholar]
- Munir, F.; Musharraf, S.G.; Sherazi, S.T.H.; Malik, M.I.; Bhanger, M.I. Detection of lard contamination in five different edible oils by FT-IR spectroscopy using a partial least squares calibration model. Turk. J. Chem. 2019, 43, 1098–1108. [Google Scholar] [CrossRef]
- Neves, M.D.G.; Poppi, R.J. Monitoring of adulteration and purity in coconut oil using raman spectroscopy and multivariate curve resolution. Food Anal. Methods 2018, 11, 1897–1905. [Google Scholar] [CrossRef]
- Jamwal, R.; Kumari, S.; Dhaulaniya, A.S.; Balan, B.; Singh, D.K. Application of ATR-FTIR spectroscopy along with regression modelling for the detection of adulteration of virgin coconut oil with paraffin oil. LWT 2020, 118, 108754. [Google Scholar]
- Man, Y.B.C. Analysis of canola oil in virgin coconut oil using FTIR spectroscopy and chemometrics. J. Food Pharm. Sci. 2013, 1, 1. [Google Scholar]
- Manaf, M.A.; Man, Y.B.C.; Hamid, N.S.A.; Ismail, A.; Abidin, S.Z. Analysis of adulteration of virgin coconut oil by palm kernel olein using Fourier transform infrared spectroscopy. J. Food Lipids 2007, 14, 111–121. [Google Scholar] [CrossRef]
- Mansor, T.S.T.; Man, Y.B.C.; Rohman, A. Application of fast gas chromatography and Fourier transform infrared spectroscopy for analysis of lard adulteration in virgin coconut oil. Appl. Spectrosc. Rev. 2011, 4, 365–372. [Google Scholar]
- Richardson, P. The Development of Spectroscopic Methods for the Detection of Adulteration in Coconut Water. Ph.D. Thesis, The University of Manchester, Manchester, UK, 2018. [Google Scholar]
- Richardson, P.I.; Muhamadali, H.; Lei, Y.; Golovanov, A.P.; Ellis, D.I.; Goodacre, R. Detection of the adulteration of fresh coconut water via NMR spectroscopy and chemometrics. Analyst 2019, 144, 1401–1408. [Google Scholar] [CrossRef]
- Imaizumi, V.M.; Sartori, M.M.P.; Ducatti, C.; Venturini Filho, W.G. Use of stable isotopes of carbon to detect coconut water adulteration. Sci. Agric. 2019, 76, 261–265. [Google Scholar] [CrossRef] [Green Version]
- Dayrit, F.M.; Dimzon, I.K.D.; Valde, M.F.; Santos, J.E.R.; Garrovillas, M.J.M.; Villarino, B.J. Quality characteristics of virgin coconut oil: Comparisons with refined coconut oil. J. Pure Appl. Chem. 2011, 83, 1789–1799. [Google Scholar] [CrossRef]
- Vijayakumar, M.; Vasudevan, D.; Sundaram, K.; Krishnan, S.; Vaidyanathan, K.; Nandakumar, S.; Chandrasekhar, R.; Mathew, N. A randomized study of coconut oil versus sunflower oil on cardiovascular risk factors in patients with stable coronary heart disease. Indian Heart J. 2016, 68, 498–506. [Google Scholar] [CrossRef] [Green Version]
- Hamsi, M.A.; Othman, F.; Das, S.; Kamisah, Y.; Thent, Z.C.; Qodriyah, H.M.S.; Zakaria, Z.; Emran, A.; Subermaniam, K.; Jaarin, K. Effect of consumption of fresh and heated virgin coconut oil on the blood pressure and inflammatory biomarkers: An experimental study in Sprague Dawley rats. Alex. J. Med. 2015, 51, 53–63. [Google Scholar] [CrossRef] [Green Version]
- Rohman, A.; Man, Y.B.C. Potential use of FTIR-ATR spectroscopic method for determination of virgin coconut oil and extra virgin olive oil in ternary mixture systems. Food Anal. Methods 2011, 4, 155–162. [Google Scholar] [CrossRef]
- Rohman, A.; Che Man, Y.; Ali, M. The authentication of virgin coconut oil from grape seed oil and soybean oil using ftir spectroscopy and chemometrics. Int. J. Appl. Pharm. 2019, 11, 259–263. [Google Scholar] [CrossRef] [Green Version]
- Glotz, J. Infographic: The UK Coconut Water Market at a Glance; William Reed Publisher: Crawley, UK, 2016; p. 24. [Google Scholar]
- Rolle, R.S. Good Practice for the Small-Scale Production of Bottled Coconut Water; Food & Agriculture Org.: Roma, Italy, 2007; Volume 1. [Google Scholar]
- Sharma, R.K.; Agrawal, M. Biological effects of heavy metals: An overview. J. Environ. Biol. 2005, 26, 301–313. [Google Scholar]
- Leggett, M.J.; McDonnell, G.; Denyer, S.P.; Setlow, P.; Maillard, J.Y. Bacterial spore structures and their protective role in biocide resistance. J. Appl. Microbiol. 2012, 113, 485–498. [Google Scholar] [CrossRef]
- Prasad, E.; Raghul, V. Trace elements in coconut water—A preliminary study. Environ. Geochem. Health 1994, 16, 76–78. [Google Scholar] [CrossRef]
- De Sousa, R.A.; Baccan, N.; Cadore, S. Determination of metals in Brazilian coconut water using an inductively coupled plasma optical emission spectrometer. J. Braz. Chem. Soc. 2005, 16, 540–544. [Google Scholar] [CrossRef]
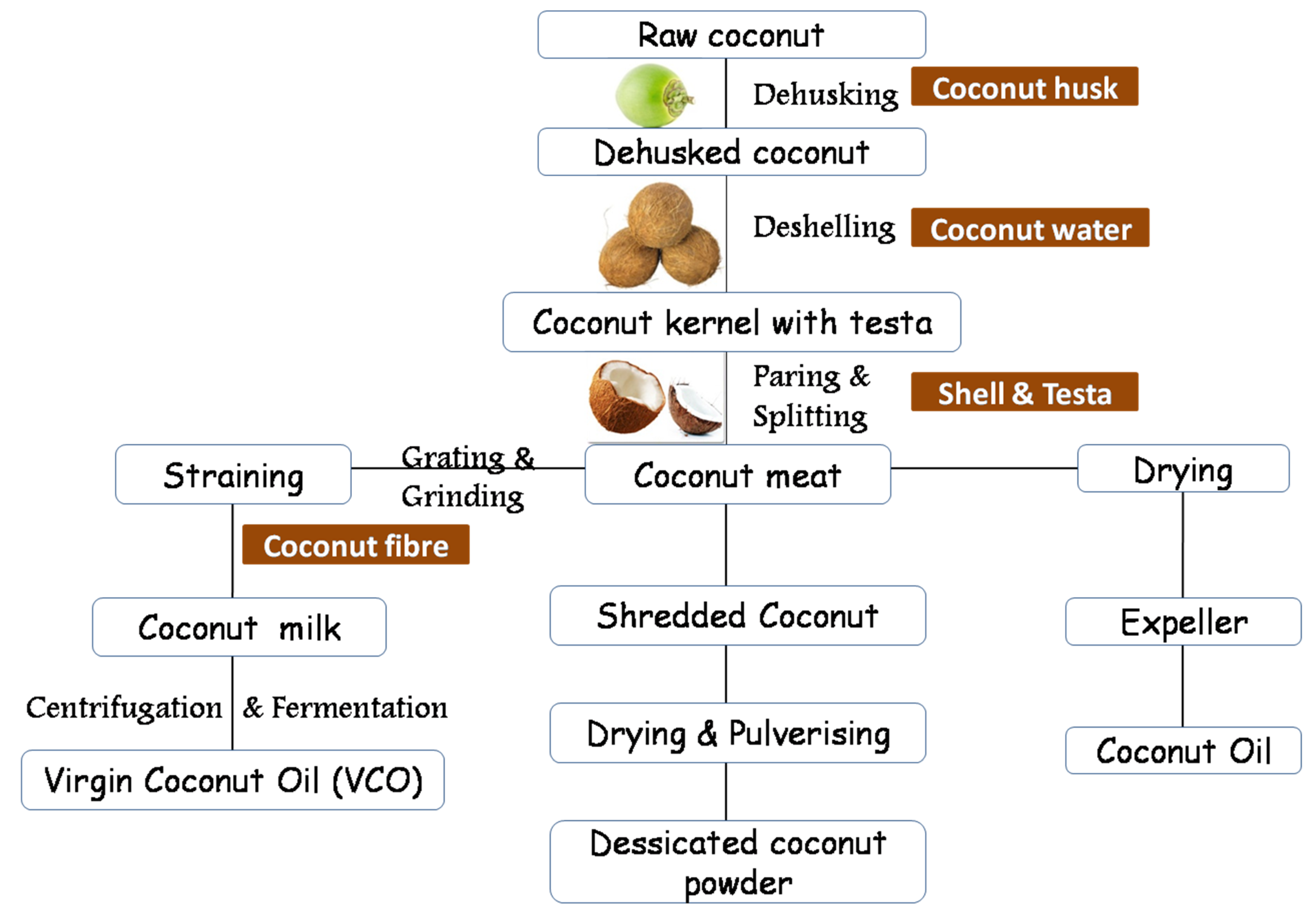

| Coconut Product | No. of Samples | Spectral Analysis | Major Findings | Reference |
|---|---|---|---|---|
| Water from fresh and aged coconuts | 7 | FT-ICR mass spectrometry | Detection and identification of chemical compounds that are synthesized when coconut water undergoes natural aging. | [37] |
| Water from green coconuts with amaturation age of 5–7 months | 8 | 1H NMR, FTIR (4000 to 400 cm−1) and GC-MS along with PCA statistical analysis | All these techniques were identified as rapid screening methods for the quantitative detection of micro filtered coconut water during storage. | [38] |
| Coconut water | 12 | NMR spectroscopy with chemometrics | Provide quick and non-destructive quantitative information about primary metabolites present in both processed and unprocessed coconut water. | [39] |
| Coconut water | 192 | NIR | Found to be a highly sensitive tool that helped to monitor coconut water deterioration during different stages of maturity. | [40] |
| Coconut water | 54 | Stable isotope ratio mass spectrometry | Detected the presence of added C-4 plant sugars such as cane sugars and maize syrups. | [41] |
| Fresh coconut water | 155 | RS with chemometrics | Accurate analytical method for the detection of added sugars in coconut water. | [42] |
| Coconut kernel, milk, milk powder and cream | 44 | FAAS | Detected the presence of heavy metals in the different coconut products. | [43] |
| Coconut curry soup | 12 | NIR (3600–12,500 cm−1) | NIR spectroscopy can be considered for use in factories producing coconut curry soups. | [44] |
| Coconut curry soup | 73 | NIR (3600–12,500 cm−1) | NIR spectroscopy can be used as an alternative method to evaluate the pH of curry soups. | [45] |
| Coconut curry soup | 12 | NIR (3600–12,500 cm−1) | NIR spectroscopy could be applied for the quality assurance of instant curry soups. | [44] |
| Virgin coconut oil | 36 | FTIR (4000–400 cm−1) | FTIR spectroscopy was able to detect carbonylic compounds from hydroperoxide decompositions. | [46] |
| Virgin coconut oil | 30 | ATR-FTIR (4000–650 cm−1) | Detection of peroxide values in virgin coconut oil. | [47] |
| Virgin coconut oil | 8 | FTIR (4000–500 cm−1) | FTIR spectra found the thermo-stability of virgin coconut oil samples even after 8h of frying. | [48] |
| Virgin coconut oil | 72 | FTIR (3100–680 cm−1) with PLSR | FTIR was found to be superior to the acid–base titration method for determining free fatty acids. | [49] |
| Virgin coconut oil | 8 | RS with chemometrics | RS could be used in restaurants for monitoring the quality of different oils. | [50] |
| Young coconut fruits | 202 | NIR (11,100–3996 cm−1) with acoustic response | Identified cracked shells in young coconuts remain in bunches. | [51] |
| Coconut husks | 54 | NIR with chemometrics | Evaluated the lignocellulosic components of coconut husks. | [52] |
| Coconut husks | 4 | FTIR spectroscopy (4000–400 cm−1) | Determined the adhesive capacity of coconut husks and their application in tannin extraction. | [53] |
| Coconut fibers | 4 | FTIR spectroscopy (4000–500 cm−1) | Evaluated modifications in the chemical composition of coconut fibers. | [54] |
| Spectral Analysis | Statistical Tool | Salient Findings | Reference |
|---|---|---|---|
| ESI-MS | PCA |
| [37] |
| ICP-OES | Factorial and Doehlert design |
| [66] |
| FT-IR | PLS |
| [67] |
| FT-NIR | PLS |
| [44] |
| FT-NIR | PLS |
| [44] |
| Product | Adulterant | Spectral Analysis | Statistical Tool and Accuracy | Reference |
|---|---|---|---|---|
| Coconut oil | Lard | FT-IR (4000–400 cm−1) | PLS R2: 0.9577, RMSEC: 0.0488 | [88] |
| Coconut oil | Sunflower, soybean, canola, sesame, corn, castor bean, peanut, palm kernel, babassu, mineral, and Vaseline oils | RS (3200–200 cm−1) | Multivariate curve resolution–alternating least squares (MCR-ALS) R2: 0.962–0.992, RMSEC: 916–2.944 | [89] |
| VCO | Paraffin oil | FTIR-ATR (4000–400 cm−1) | Qualitative analysis: LDA, PCA Quantitative analysis: PCR, PLSR PLSR-R2: 0.999, RMSEC: 0.142 PCR-R2: 0.998, RMSEC: 0.204 | [90] |
| VCO | Corn oil and sunflower oil | FTMIR (4000–650 cm−1) | PLS Corn oil-R2: 0.999, RMSEC: 0.866 Sunflower oil-R2: 0.999, RMSEC: 0.374 | [75] |
| VCO | Canola oil | FTIR (4000–650 cm−1) | PLS, PCR and DA PLS-R2: 0.998, RMSEC: 0.392 PCR-R2: 0.990, RMSEC: 1.37 | [91] |
| VCO | Palm kernel olein | FTIR (4000–650 cm−1) | PLS and DS R2: 0.9875, RMSECV: 1.70 | [92] |
| VCO | Olive oil and palm oil (binary mixture) | FTIR (4000–650 cm−1) | PLS and PCR PLS: VCO in OO-R2: 0.9992, RMSEC: 0.765 VCO in PO-R2: 0.9996, RMSEC: 0.494 PCR: VCO in OO-R2: 0.9991, RMSEC: 0.768 VCO in PO-R2: 0.9742, RMSEC: 3.86 | [46] |
| VCO | Palm oil and olive oil (ternary mixture) | FTIR (4000–650 cm−1) | PLS (2nd derivative) R2: 0.999, RMSEC: 0.200 PCR: R2: 0.999, RMSEC: 1.30 | [75] |
| VCO | Lard | FTIR (4000–650 cm−1) | PLS and DA PLS: R2: 0.9990, RMSEC:0.722 | [93] |
| VCO | Grape seed oil, soybean oil | FTIR (4000–650 cm−1) | PLS: GSO in VCO-R2: 0.998, RMSEC:0.007 VCO in SO-R2: 0.999, RMSEC: 0.268 PCR: GSO in VCO-R2: 0.998, RMSEC:0.622 VCO in SO-R2: 0.999, RMSEC:0.208 | [67] |
| Coconut water | Sucrose, glucoseand fructose | 1D proton NMR spectroscopy | PLSR (combined region) R2: 0.999, RMSEC:0.5889 | [94] |
| Coconut water | Sucrose, glucose, fructose and high-fructose corn syrup (HFCS) | RS | PLSR Sucrose: R2: 0.9997, RMSEC:1.1551 Glucose: R2: 0.9997 RMSEC:1.3020 fructose: R2: 0.9996 RMSEC:1.3105 HFCS: R2: 0.9998 RMSEC:4.2641 | [95] |
| Coconut water | sugar | IRMS coupled with an elemental analyzer | [96] |
| Coconut Product | Spectral Analysis | Salient Findings | Reference |
|---|---|---|---|
| Fresh coconut Coconut milk, cream, and milk powder | AAS |
| [43] |
| Coconut water Coconut milk | AAS |
| [96] |
| VCO | AAS |
| [78] |
| Fermented coconut oil | AAS |
| [105] |
| Coconut water | ICP-AAS |
| [105] |
| Coconut water Coconut milk | High-resolution continuum source graphite FAAS |
| [102,103] |
| Coconut water | AAS |
| [96] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandiselvam, R.; Kaavya, R.; Martinez Monteagudo, S.I.; Divya, V.; Jain, S.; Khanashyam, A.C.; Kothakota, A.; Prasath, V.A.; Ramesh, S.V.; Sruthi, N.U.; et al. Contemporary Developments and Emerging Trends in the Application of Spectroscopy Techniques: A Particular Reference to Coconut (Cocos nucifera L.). Molecules 2022, 27, 3250. https://doi.org/10.3390/molecules27103250
Pandiselvam R, Kaavya R, Martinez Monteagudo SI, Divya V, Jain S, Khanashyam AC, Kothakota A, Prasath VA, Ramesh SV, Sruthi NU, et al. Contemporary Developments and Emerging Trends in the Application of Spectroscopy Techniques: A Particular Reference to Coconut (Cocos nucifera L.). Molecules. 2022; 27(10):3250. https://doi.org/10.3390/molecules27103250
Chicago/Turabian StylePandiselvam, Ravi, Rathnakumar Kaavya, Sergio I. Martinez Monteagudo, V. Divya, Surangna Jain, Anandu Chandra Khanashyam, Anjineyulu Kothakota, V. Arun Prasath, S. V. Ramesh, N. U. Sruthi, and et al. 2022. "Contemporary Developments and Emerging Trends in the Application of Spectroscopy Techniques: A Particular Reference to Coconut (Cocos nucifera L.)" Molecules 27, no. 10: 3250. https://doi.org/10.3390/molecules27103250
APA StylePandiselvam, R., Kaavya, R., Martinez Monteagudo, S. I., Divya, V., Jain, S., Khanashyam, A. C., Kothakota, A., Prasath, V. A., Ramesh, S. V., Sruthi, N. U., Kumar, M., Manikantan, M. R., Kumar, C. A., Khaneghah, A. M., & Cozzolino, D. (2022). Contemporary Developments and Emerging Trends in the Application of Spectroscopy Techniques: A Particular Reference to Coconut (Cocos nucifera L.). Molecules, 27(10), 3250. https://doi.org/10.3390/molecules27103250











