Quercetin Reduces Inflammation and Protects Gut Microbiota in Broilers
Abstract
:1. Introduction
2. Results
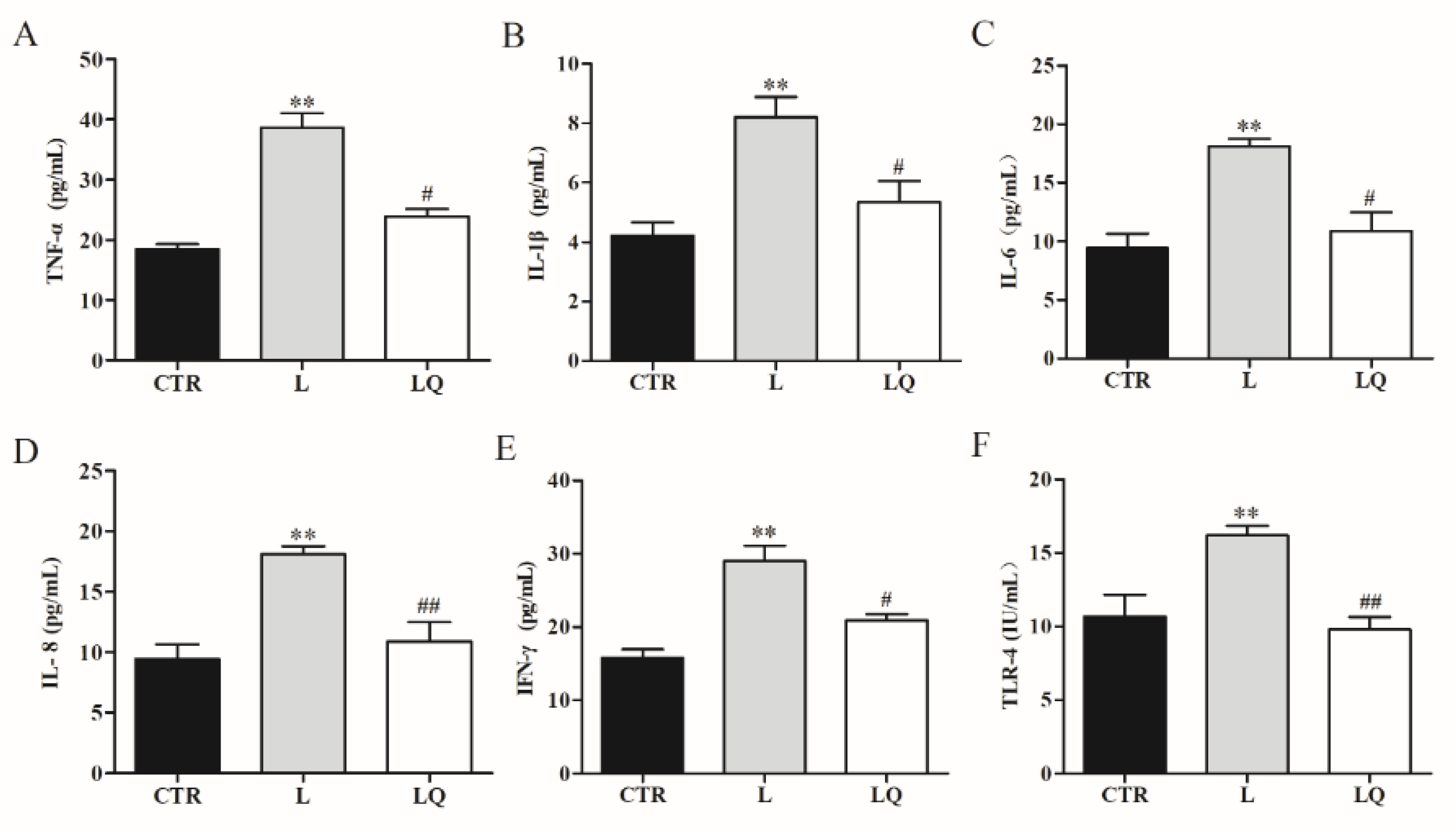
2.1. Effect of Dietary Quercetin on the Intestinal Inflammation in Broiler Chickens Challenged with LPS
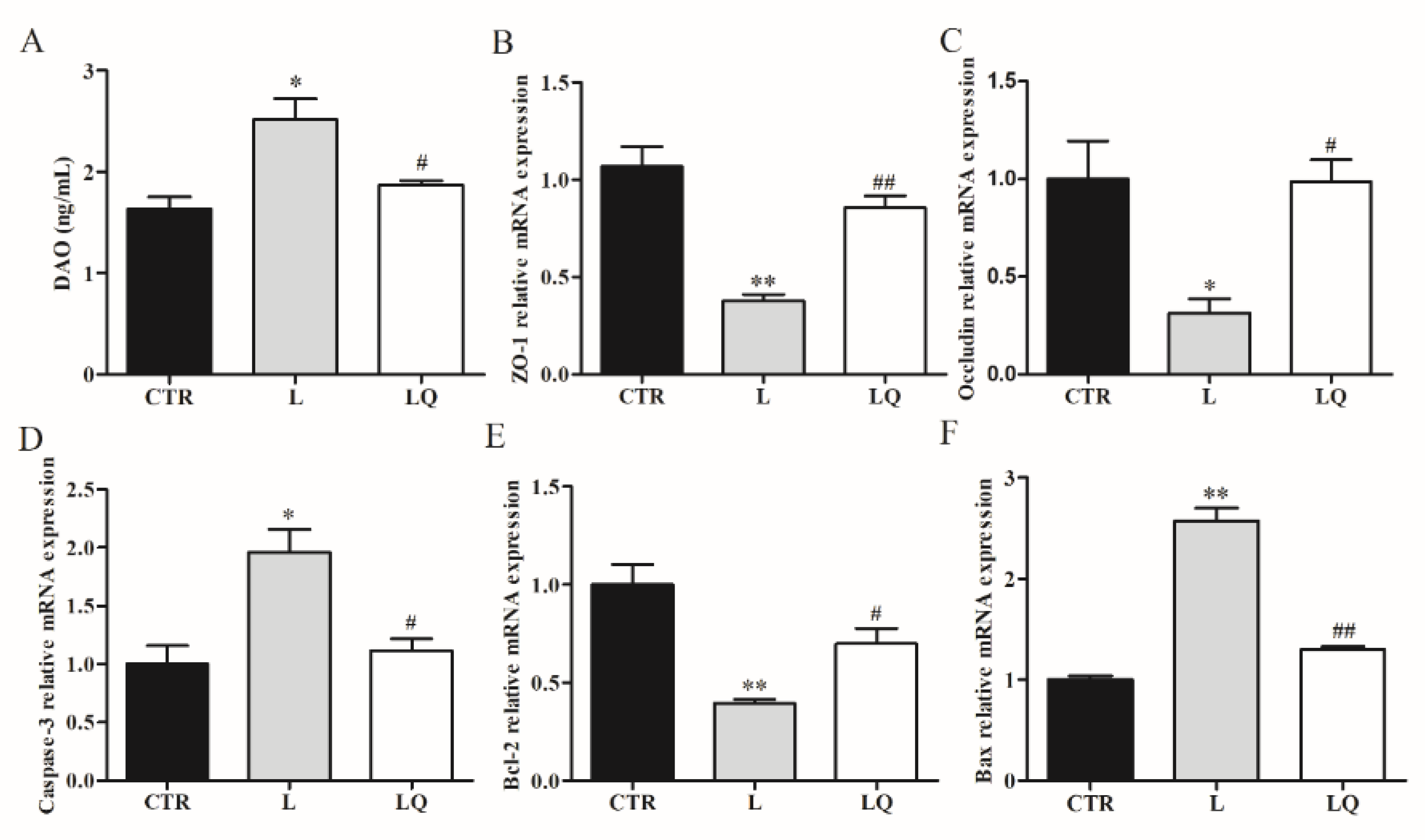
2.2. Effect of Dietary Quercetin on the Intestinal Barrier of Broiler Chickens Challenged with LPS
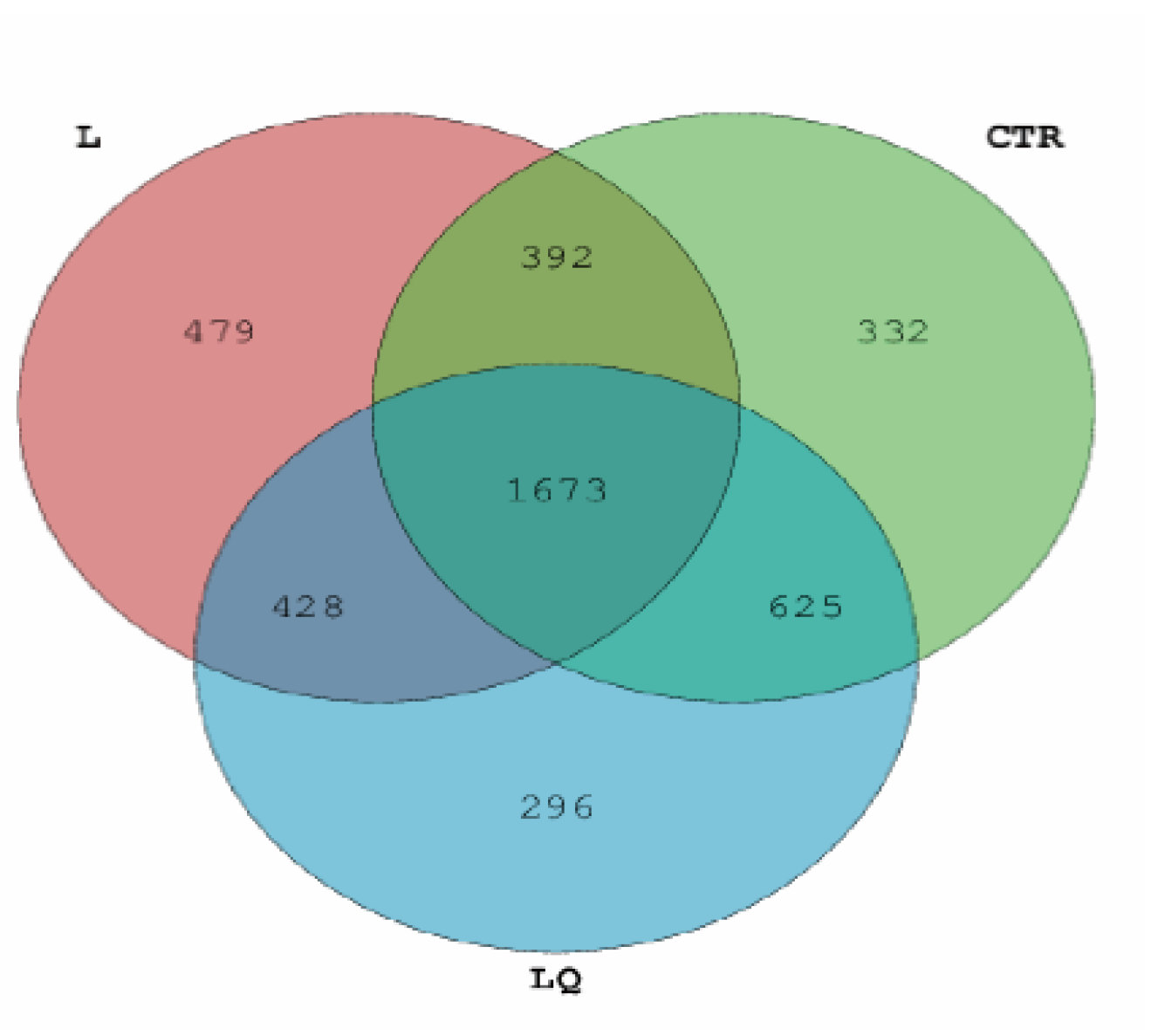
2.3. 16S rRNA Sequencing and Total out Analysis
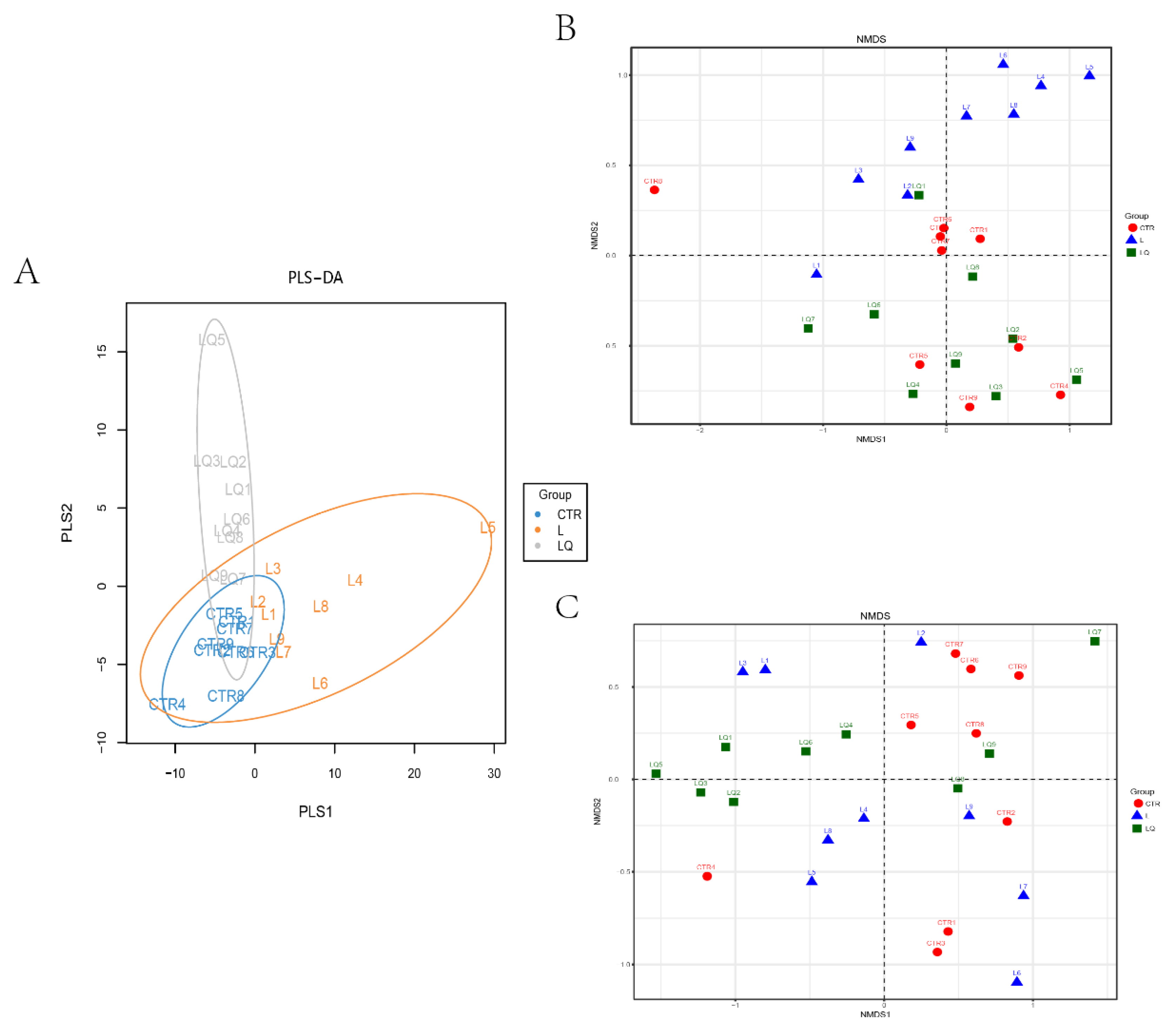
2.4. Diversity and Similarity of Microbial Community
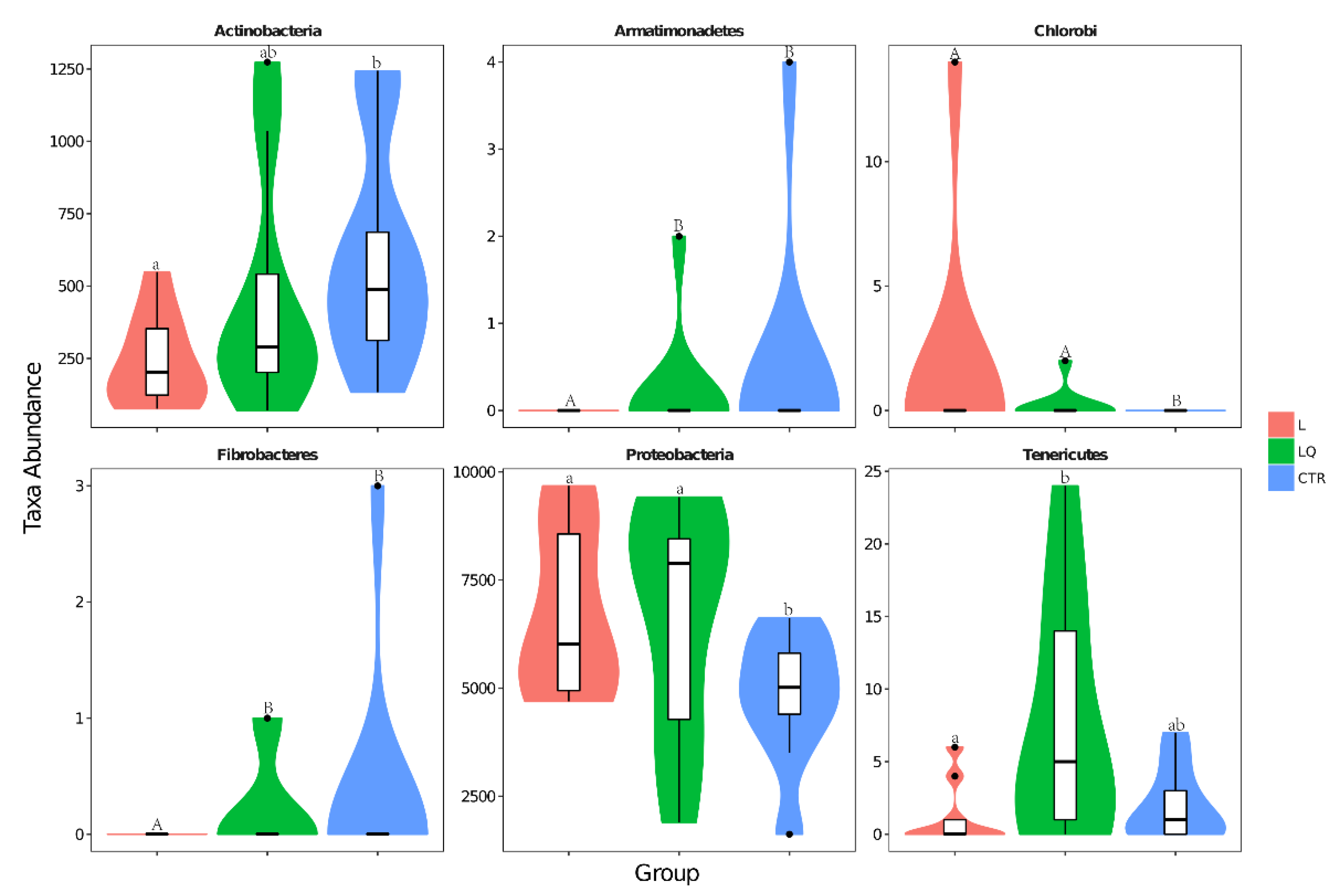
2.5. Phylum Level Abundance and Significance Difference of the Three Groups
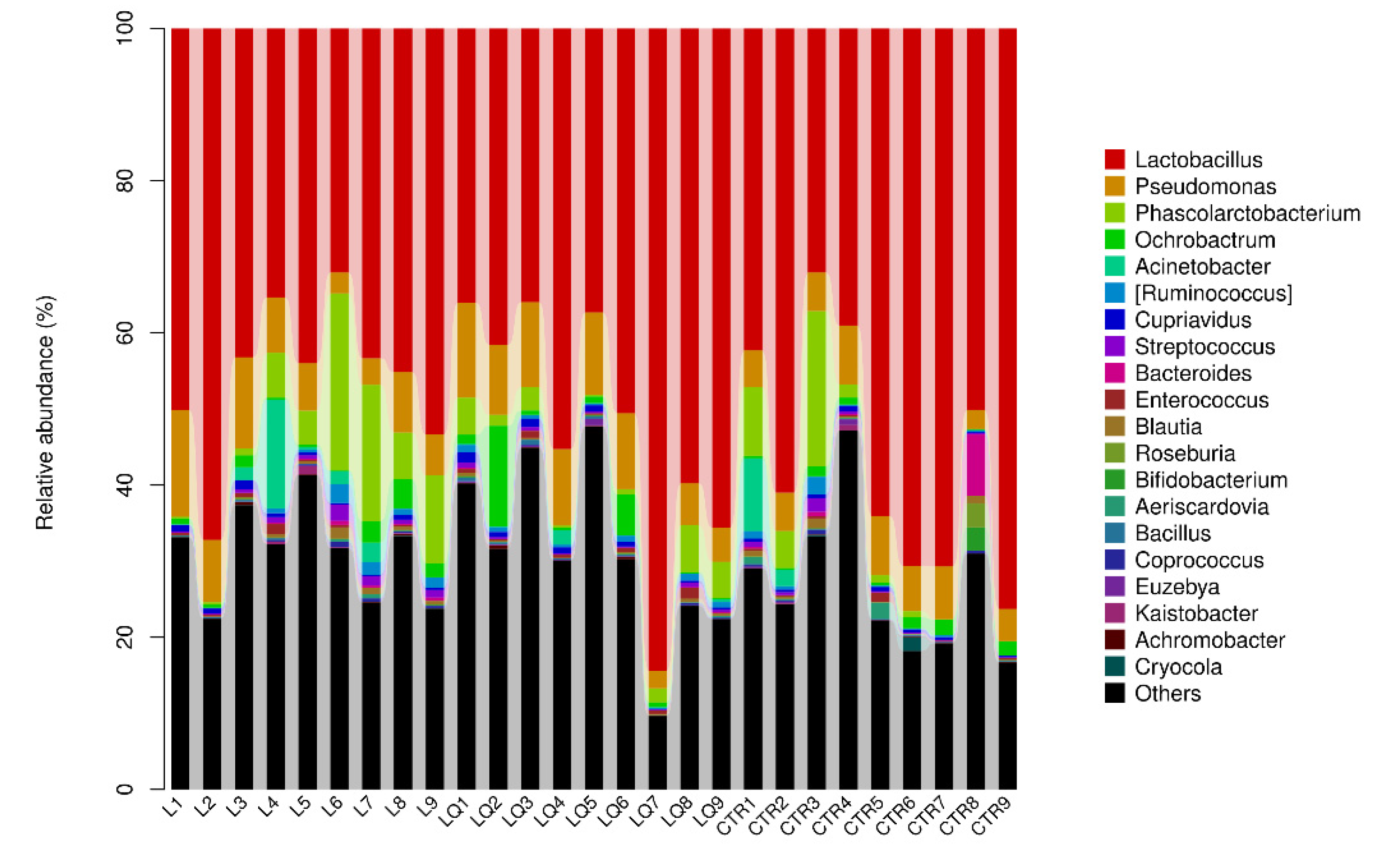
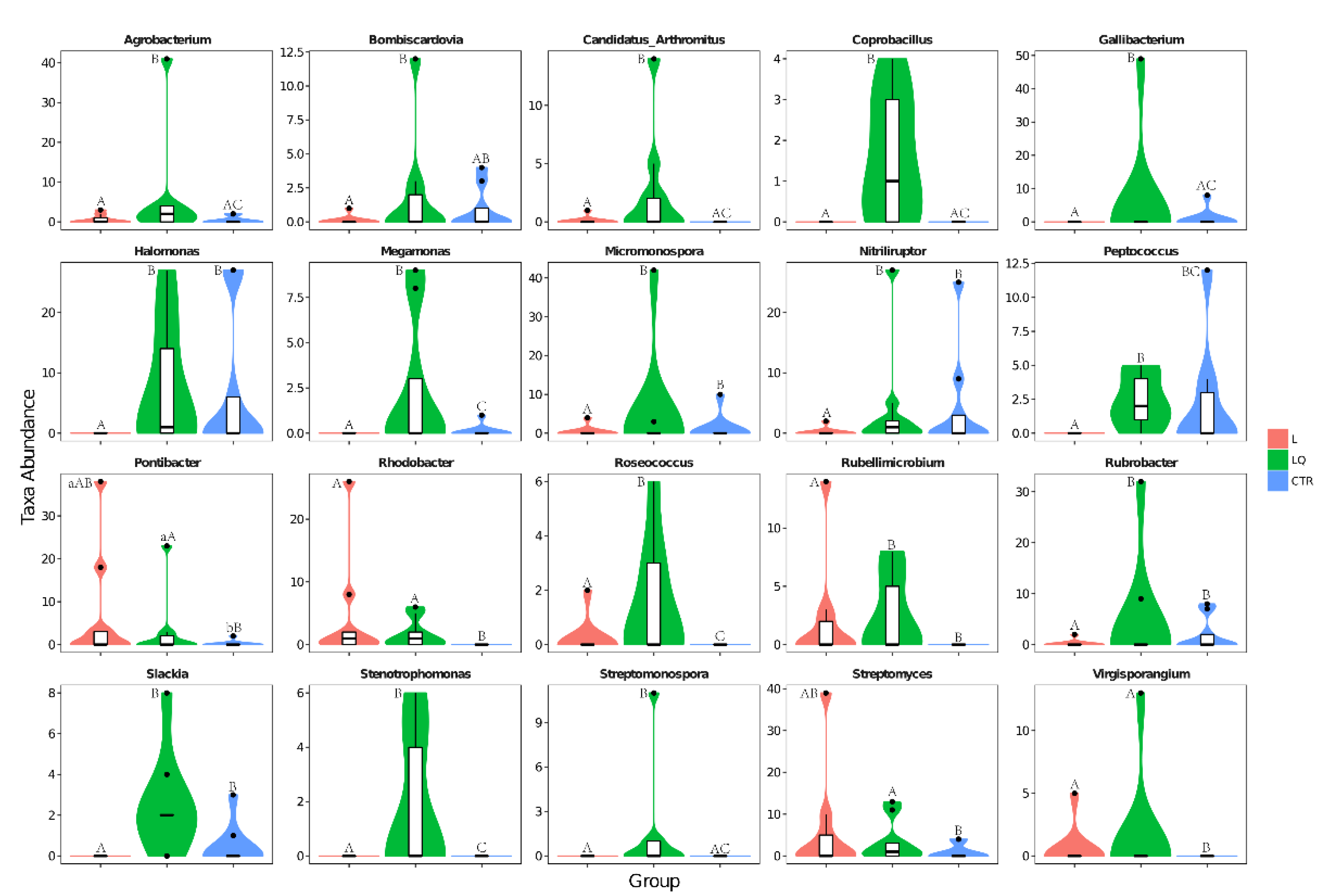
2.6. Genus Level Abundance and Significance Difference of the Three Groups
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Animal Treatment and Sample Preparation
5.2. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Analysis
5.3. Enzyme-Linked Immunosorbent Assay (ELISA)
5.4. Determination of Diamine Oxidase Activity (DAO) Level
5.5. Extraction of Total DNA and Sequencing of 16S rRNA
5.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kaldhusdal, M.; Benestad, S.L.; Løvland, A. Epidemiologic aspects of necrotic enteritis in broiler chickens-disease occurrence and production performance. Avian Pathol. 2016, 45, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Prescott, J.F.; Parreira, V.R.; Mehdizadeh Gohari, I.; Lepp, D.; Gong, J. The pathogenesis of necrotic enteritis in chickens: What we know and what we need to know: A review. Avian Pathol. 2016, 45, 288–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ateya, A.I.; Arafat, N.; Saleh, R.M.; Ghanem, H.M.; Naguib, D.; Radwan, H.A.; Elseady, Y.Y. Intestinal gene expressions in broiler chickens infected with Escherichia coli and dietary supplemented with probiotic, acidifier and synbiotic. Vet. Res. Commun. 2019, 43, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Kollef, M.H.; Bassetti, M.; Francois, B.; Burnham, J.; Dimopoulos, G.; Garnacho-Montero, J.; Lipman, J.; Luyt, C.-E.; Nicolau, D.P.; Postma, M.J.; et al. The intensive care medicine research agenda on multidrug-resistant bacteria, antibiotics, and stewardship. Intens Care Med. 2017, 43, 1187–1197. [Google Scholar] [CrossRef]
- Dou, W.; Zhang, J.; Sun, A.; Zhang, E.; Ding, L.; Mukherjee, S.; Wei, X.; Chou, G.; Wang, Z.-T.; Mani, S. Protective effect of naringenin against experimental colitis via suppression of Toll-like receptor 4/NF-κB signalling. Br. J. Nutr. 2013, 110, 599–608. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.S.; Jobin, C. The flavonoid luteolin prevents lipopolysaccharide-induced NF-kappaB signalling and gene expression by blocking IkappaB kinase activity in intestinal epithelial cells and bone-marrow derived dendritic cells. Immunology 2005, 115, 375–387. [Google Scholar] [CrossRef]
- Amasheh, M.; Grotjohann, I.; Amasheh, S.; Fromm, A.; Söderholm, J.D.; Zeitz, M.; Fromm, M.; Schulzke, J.-D. Regulation of mucosal structure and barrier function in rat colon exposed to tumor necrosis factor alpha and interferon gamma in vitro: A novel model for studying the pathomechanisms of inflammatory bowel disease cytokines. Scand. J. Gastroenterol. 2009, 44, 1226–1235. [Google Scholar] [CrossRef]
- Endale, M.; Park, S.-C.; Kim, S.; Kim, S.-H.; Yang, Y.; Cho, J.Y.; Rhee, M.H. Quercetin disrupts tyrosine-phosphorylated phosphatidylinositol 3-kinase and myeloid differentiation factor-88 association, and inhibits MAPK/AP-1 and IKK/NF-κB-induced inflammatory mediators production in RAW 264.7 cells. Immunobiology 2013, 218, 1452–1467. [Google Scholar] [CrossRef]
- Gil-Cardoso, K.; Ginés, I.; Pinent, M.; Ardévol, A.; Blay, M.; Terra, X. Effects of flavonoids on intestinal inflammation, barrier integrity and changes in gut microbiota during diet-induced obesity. Nutr. Res. Rev. 2016, 29, 234–248. [Google Scholar] [CrossRef]
- Suzuki, T.; Hara, H. Quercetin enhances intestinal barrier function through the assembly of zonula [corrected] occludens-2, occludin, and claudin-1 and the expression of claudin-4 in Caco-2 cells. J. Nutr. 2009, 139, 965–974. [Google Scholar] [CrossRef]
- Bian, Y.; Liu, P.; Zhong, J.; Hu, Y.; Zhuang, S.; Fan, K.; Liu, Z. Quercetin Attenuates Adhesion Molecule Expression in Intestinal Microvascular Endothelial Cells by Modulating Multiple Pathways. Dig. Dis. Sci. 2018, 63, 3297–3304. [Google Scholar] [CrossRef] [PubMed]
- Pisani, A.; Rausch, P.; Bang, C.; Ellul, S.; Tabone, T.; Marantidis Cordina, C.; Zahra, G.; Franke, A.; Ellul, P. Dysbiosis in the Gut Microbiota in Patients with Inflammatory Bowel Disease during Remission. Microbiol. Spectr. 2022, e0061622. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, L.; Chen, J.; Xiao, A. Response of Intestinal Bacterial Flora to the Long-term Feeding of Aflatoxin B1 (AFB1) in Mice. Toxins 2017, 9, 317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giloteaux, L.; Goodrich, J.K.; Walters, W.A.; Levine, S.M.; Ley, R.E.; Hanson, M.R. Reduced diversity and altered composition of the gut microbiome in individuals with myalgic encephalomyelitis/chronic fatigue syndrome. Microbiome 2016, 4, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Wei, S.; Liu, B.; Wang, F.; Lu, Z.; Jin, M.; Wang, Y. Maternal consumption of a fermented diet protects offspring against intestinal inflammation by regulating the gut microbiota. Gut Microbes 2022, 14, 2057779. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, L.; Sun, Y.; Qing, D.-G.; Xu, X.-Q.; Wang, J.-C.; Si, J.-Y.; Li, N. The Regulatory Effects of Licochalcone A on the Intestinal Epithelium and Gut Microbiota in Murine Colitis. Molecules 2021, 26, 4149. [Google Scholar] [CrossRef]
- McClave, S.A. Can feeding strategies alter immune signaling and gut sepsis in critical illness? JPEN J. Parenter. Enter. Nutr. 2021, 45 (Suppl. 2), 66–73. [Google Scholar] [CrossRef]
- Caglayan Sozmen, S.; Karaman, M.; Cilaker Micili, S.; Isik, S.; Bagriyanik, A.; Arikan Ayyildiz, Z.; Uzuner, N.; Anal, O.; Karaman, O. Effects of Quercetin Treatment on Epithelium-derived Cytokines and Epithelial Cell Apoptosis in Allergic Airway Inflammation Mice Model. Iran. J. Allergy Asthma Immunol. 2016, 15, 487–497. [Google Scholar]
- Zhu, Q.; Liu, M.; He, Y.; Yang, B. Quercetin protect cigarette smoke extracts induced inflammation and apoptosis in RPE cells. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2010–2015. [Google Scholar] [CrossRef] [Green Version]
- Amevor, F.K.; Cui, Z.; Du, X.; Ning, Z.; Deng, X.; Xu, D.; Shu, G.; Wu, Y.; Cao, X.; Shuo, W.; et al. Supplementation of Dietary Quercetin and Vitamin E Promotes the Intestinal Structure and Immune Barrier Integrity in Aged Breeder Hens. Front. Immunol. 2022, 13, 860889. [Google Scholar] [CrossRef]
- Ma, F.; Sun, M.; Song, Y.; Wang, A.; Jiang, S.; Qian, F.; Mu, G.; Tuo, Y. Lactiplantibacillus plantarum-12 Alleviates Inflammation and Colon Cancer Symptoms in AOM/DSS-Treated Mice through Modulating the Intestinal Microbiome and Metabolome. Nutrients 2022, 14, 1916. [Google Scholar] [CrossRef] [PubMed]
- Salemi, R.; Tomasello, B.; Gattuso, G.; Signorelli, S.S.; Candido, S. Overactivation of IL6 cis-signaling in leukocytes is an inflammatory hallmark of deep vein thrombosis. Mol. Med. Rep. 2022, 25, 136. [Google Scholar] [CrossRef] [PubMed]
- Farré, R.; Fiorani, M.; Abdu Rahiman, S.; Matteoli, G. Intestinal Permeability, Inflammation and the Role of Nutrients. Nutrients 2020, 12, 1185. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liang, L.; Yang, C.; Zhou, Y.; Chen, Y. Extracellular vesicles of Fusobacterium nucleatum compromise intestinal barrier through targeting RIPK1-mediated cell death pathway. Gut Microbes 2021, 13, 1902718. [Google Scholar] [CrossRef]
- Li, M.; Kong, Y.; Wu, X.; Yin, Z.; Niu, X.; Wang, G. Dietary α-lipoic acid can alleviate the bioaccumulation, oxidative stress, cell apoptosis, and inflammation induced by lead (Pb) in Channa argus. Fish Shellfish Immunol. 2021, 119, 249–261. [Google Scholar] [CrossRef]
- Arslan, A.S.; Seven, I.; Mutlu, S.I.; Arkali, G.; Birben, N.; Seven, P.T. Potential ameliorative effect of dietary quercetin against lead-induced oxidative stress, biochemical changes, and apoptosis in laying Japanese quails. Ecotoxicol. Environ. Saf. 2022, 231, 113200. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, W.; Kim, I.H.; Yang, Y. Dietary hydrolyzed wheat gluten supplementation ameliorated intestinal barrier dysfunctions of broilers challenged with Escherichia coli O78. Poult. Sci. 2022, 101, 101615. [Google Scholar] [CrossRef]
- Yang, L.; Liu, G.; Lian, K.; Qiao, Y.; Zhang, B.; Zhu, X.; Luo, Y.; Shang, Y.; Gu, X.-L. Dietary leonurine hydrochloride supplementation attenuates lipopolysaccharide challenge-induced intestinal inflammation and barrier dysfunction by inhibiting the NF-κB/MAPK signaling pathway in broilers. J. Anim. Sci. 2019, 97, 1679–1692. [Google Scholar] [CrossRef]
- Cheng, S.-C.; Huang, W.-C.; S Pang, J.-H.; Wu, Y.-H.; Cheng, C.-Y. Quercetin Inhibits the Production of IL-1β-Induced Inflammatory Cytokines and Chemokines in ARPE-19 Cells via the MAPK and NF-κB Signaling Pathways. Int. J. Mol. Sci. 2019, 20, 2957. [Google Scholar] [CrossRef] [Green Version]
- Lin, R.; Piao, M.; Song, Y. Dietary Quercetin Increases Colonic Microbial Diversity and Attenuates Colitis Severity in Citrobacter rodentium-Infected Mice. Front. Microbiol. 2019, 10, 1092. [Google Scholar] [CrossRef]
- Hong, Z.; Piao, M. Effect of Quercetin Monoglycosides on Oxidative Stress and Gut Microbiota Diversity in Mice with Dextran Sodium Sulphate-Induced Colitis. Biomed. Res. Int. 2018, 2018, 8343052. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yuan, Q.; Xu, G.; Chen, H.; Lei, H.; Su, J. Effects of Quercetin on Proliferation and H2O2-Induced Apoptosis of Intestinal Porcine Enterocyte Cells. Molecules 2018, 23, 2012. [Google Scholar] [CrossRef] [Green Version]
- Junyuan, Z.; Hui, X.; Chunlan, H.; Junjie, F.; Qixiang, M.; Yingying, L.; Lihong, L.; Xingpeng, W.; Yue, Z. Quercetin protects against intestinal barrier disruption and inflammation in acute necrotizing pancreatitis through TLR4/MyD88/p38 MAPK and ERS inhibition. Pancreatology 2018, 18, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Joardar, N.; Sengupta, S.; Sinha Babu, S.P. Gut microbes as future therapeutics in treating inflammatory and infectious diseases: Lessons from recent findings. J. Nutr. Biochem. 2018, 61, 111–128. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Qu, C.; Liang, S.; Wang, G.; Han, H.; Chen, N.; Wang, X.; Luo, Z.; Zhong, C.; Chen, Y.; et al. Estrogen inhibits the overgrowth of Escherichia coli in the rat intestine under simulated microgravity. Mol. Med. Rep. 2018, 17, 2313–2320. [Google Scholar] [PubMed] [Green Version]
- Song, B.; Li, P.; Yan, S.; Liu, Y.; Gao, M.; Lv, H.; Lv, Z.; Guo, Y. Effects of Dietary Astragalus Polysaccharide Supplementation on the Th17/Treg Balance and the Gut Microbiota of Broiler Chickens Challenged With Necrotic Enteritis. Front. Immunol. 2022, 13, 781934. [Google Scholar] [CrossRef]
- Franco-de-Moraes, A.C.; de Almeida-Pititto, B.; da Rocha Fernandes, G.; Gomes, E.P.; da Costa Pereira, A.; Ferreira, S.R.G. Worse inflammatory profile in omnivores than in vegetarians associates with the gut microbiota composition. Diabetol. Metab. Syndr. 2017, 9, 62. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Zhu, X.; Qiu, Y.; Wang, L.; Shang, X.; Gao, K.; Yang, X.; Jiang, Z. Effect of Niacin on Growth Performance, Intestinal Morphology, Mucosal Immunity and Microbiota Composition in Weaned Piglets. Animals 2021, 11, 2186. [Google Scholar] [CrossRef]
- Nimal Christhudas, I.V.S.; Praveen Kumar, P.; Agastian, P. In vitro α-glucosidase inhibition and antioxidative potential of an endophyte species (Streptomyces sp. loyola UGC) isolated from Datura stramonium L. Curr. Microbiol. 2013, 67, 69–76. [Google Scholar] [CrossRef]
- Karthik, L.; Kumar, G.; Bhaskara Rao, K.V. Antioxidant activity of newly discovered lineage of marine actinobacteria. Asian Pac. J. Trop. Med. 2013, 6, 325–332. [Google Scholar] [CrossRef] [Green Version]
- Mohammadipanah, F.; Momenilandi, M. Potential of rare actinomycetes in the production of metabolites against multiple oxidant agents. Pharm. Biol. 2018, 56, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, S.; Agrawal-Rajput, R. Lipopolysaccharide from Rhodobacter sphaeroides Attenuates Microglia-Mediated Inflammation and Phagocytosis and Directs Regulatory T Cell Response. Int. J. Inflamm. 2015, 2015, 361326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Yao, J.; Zhou, B.; Yang, J.; Chaudry, M.T.; Wang, M.; Xiao, F.; Li, Y.; Yin, W. Bacteriostatic Effect of Quercetin as an Antibiotic Alternative In Vivo and Its Antibacterial Mechanism In Vitro. J. Food Prot. 2018, 81, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Etxeberria, U.; Arias, N.; Boqué, N.; Macarulla, M.T.; Portillo, M.P.; Martínez, J.A.; Milagro, F.I. Reshaping faecal gut microbiota composition by the intake of trans-resveratrol and quercetin in high-fat sucrose diet-fed rats. J. Nutr. Biochem. 2015, 26, 651–660. [Google Scholar] [CrossRef]
- Blaxter, M.; Mann, J.; Chapman, T.; Thomas, F.; Whitton, C.; Floyd, R.; Abebe, E. Defining operational taxonomic units using DNA barcode data. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005, 360, 1935–1943. [Google Scholar] [CrossRef]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef] [Green Version]
- Bokulich, N.A.; Subramanian, S.; Faith, J.J.; Gevers, D.; Gordon, J.I.; Knight, R.; Mills, D.A.; Caporaso, J.G. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 2013, 10, 57–59. [Google Scholar] [CrossRef]
- Lozupone, C.; Knight, R. UniFrac: A new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 2005, 71, 8228–8235. [Google Scholar] [CrossRef] [Green Version]
- Lozupone, C.A.; Hamady, M.; Kelley, S.T.; Knight, R. Quantitative and qualitative beta diversity measures lead to different insights into factors that structure microbial communities. Appl. Environ. Microbiol. 2007, 73, 1576–1585. [Google Scholar] [CrossRef] [Green Version]


| Group * | Chaol | STD | Simpson | STD | Shannon | STD |
|---|---|---|---|---|---|---|
| Mean | Mean | Mean | ||||
| L | 1187.048 | 199.8716 | 0.950465 | 0.026915 | 6.39 | 0.682486 |
| LQ | 1287.091 | 181.8062 | 0.967144 | 0.010034 | 7.2 | 0.229627 |
| CTR | 1098.337 | 188.4453 | 0.930107 | 0.062601 | 6.26 | 0.773747 |
| Ingredient (%) | 1–21 Days | Nutritional Index (g/kg) | 1–21 Days |
|---|---|---|---|
| Corn | 58.63 | Crude protein | 21 |
| Soybean meal | 32.07 | Metabolism energy (MJ/kg) | 3050 |
| Soybean oil | 3.53 | Moisture | 12.38 |
| Corn gluten meal | 2 | Dry matter | 87.62 |
| Limestone powder | 1.17 | Crude fat | 6.1 |
| DL-methionine (98%) | 0.23 | Crude fiber | 3.17 |
| Lysine (98%) | 0.38 | Crude ash | 5.55 |
| L-threonine | 0.05 | Calcium | 0.9 |
| Choline chloride | 0.1 | Total phosphorus | 0.64 |
| Calcium hydrogen phosphate | 1.03 | Available phosphorus | 0.41 |
| Sodium chloride | 0.33 | Sodium | 0.15 |
| Broiler chickens vitamins * | 0.2 | Chlorine | 0.23 |
| Broiler chickens trace elements † | 0.15 | Total lysine | 1.35 |
| Complex enzyme | 0.02 | Total methionine | 0.55 |
| Phytase (10,000 units) | 0.01 | Total sulfur-containing amino acid | 0.88 |
| L-tryptophan (20%) | 0.1 | Total threonine | 0.83 |
| Total | 100 | Total tryptophan | 0.25 |
| Genes | Primer Sequence (5′–3′) | Genebank No. | Size (bp) |
|---|---|---|---|
| TNF-α | F: TGGGAAGGGAATGAACCCTC | NM_204267.1 | 118 |
| R: AACTGGGCGGTCATAGAACA | |||
| IL-1β | F: AGCAGCCTCAGCGAAGAGACC | NM_204524.1 | 90 |
| R: GTCCACTGTGGTGTGCTCAGAATC | |||
| IL-6 | F: TTCACCGTGTGCGAGAACAGC | NM_204628.1 | 80 |
| R: CAGCCGTCCTCCTCCGTCAC | |||
| IL-8 | F: AGGATGGAAGAGAGGTGTGC | NM_205498.1 | 80 |
| R: CTGAGCCTTGGCCATAAGTG | |||
| TLR4 | F: AGGCACCTGAGCTTTTCCTC | NM_001030693.1 | 96 |
| R: TACCAACGTGAGGTTGAGCC | |||
| IFN-γ | F: CACTGACAAGTCAAAGCCGC | NM_205149.1 | 87 |
| R: ACCTTCTTCACGCCATCAGG | |||
| BCL-2 | F: GAGTTCGGCGGCGTGATGTG | NM_205339.2 | 92 |
| R: TTCAGGTACTCGGTCATCCAGGTG | |||
| BAX | F: TCCATTCAGGTTCTCTTGACC | XM_001235092.4 | 119 |
| R: GCCAAACATCCAAACACAGA | |||
| Caspase-3 | F: TACCGGACTGTCATCTCGTTCAGG | XM_015276122.2 | 166 |
| R: ACTGCTTCGCTTGCTGTGATCTTC | |||
| ZO-1 | F: ACACTGTGACCCCAAAACCT | XM_015278981.2 | 81 |
| R: ACTGAGACACAGTTTGCTCCA | |||
| Occludin | F: CATCGCCTCCATCGTCTACA | NM_205128.1 | 117 |
| R: TTGAGGTAGGTGCTGCCGTA | |||
| β-actin | F: GTGCTATGTTGCTCTAGACTTCG | NM_007393.5 | 174 |
| R: ATGCCACAGGATTCCATACC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, L.; Guo, L.; Xu, G.; Li, Z.; Appiah, M.O.; Yang, L.; Lu, W. Quercetin Reduces Inflammation and Protects Gut Microbiota in Broilers. Molecules 2022, 27, 3269. https://doi.org/10.3390/molecules27103269
Sun L, Guo L, Xu G, Li Z, Appiah MO, Yang L, Lu W. Quercetin Reduces Inflammation and Protects Gut Microbiota in Broilers. Molecules. 2022; 27(10):3269. https://doi.org/10.3390/molecules27103269
Chicago/Turabian StyleSun, Lei, Lewei Guo, Gaoqing Xu, Zhiqiang Li, Michael Osei Appiah, Lianyu Yang, and Wenfa Lu. 2022. "Quercetin Reduces Inflammation and Protects Gut Microbiota in Broilers" Molecules 27, no. 10: 3269. https://doi.org/10.3390/molecules27103269





