Enhancement of Cholinesterase Inhibition of Alpinia galanga (L.) Willd. Essential Oil by Microemulsions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemicals and Enzymes
2.3. Extraction of AGO
2.4. GC-MS Analysis
2.5. Cholinesterase Activity Determination
2.6. Cytotoxicity
2.6.1. PBMC Isolation
2.6.2. Cell Viability Assay
2.7. Optimum HLB Value Determination
2.8. Photon Correlation Spectroscopy
2.9. Construction of Phase Diagrams
2.10. Development of Microemulsion
2.11. Statistical Analysis
3. Results
3.1. Yield, Density, and GC-MS Analysis of AGO
3.2. Cholinesterase Inhibition of AGO
3.3. Safety Profile of AGO on Normal Cells
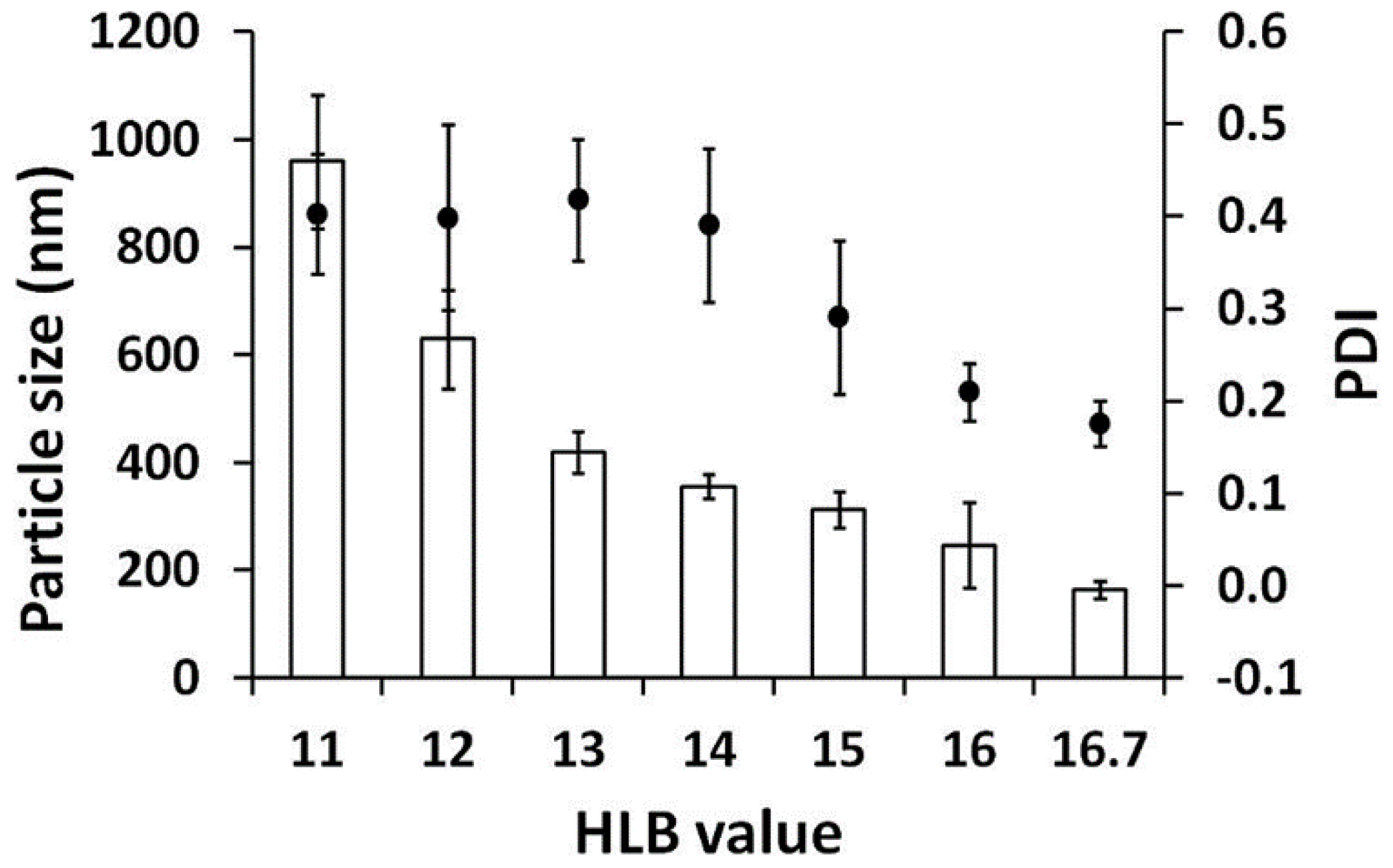
3.4. Optimum HLB Value Determination
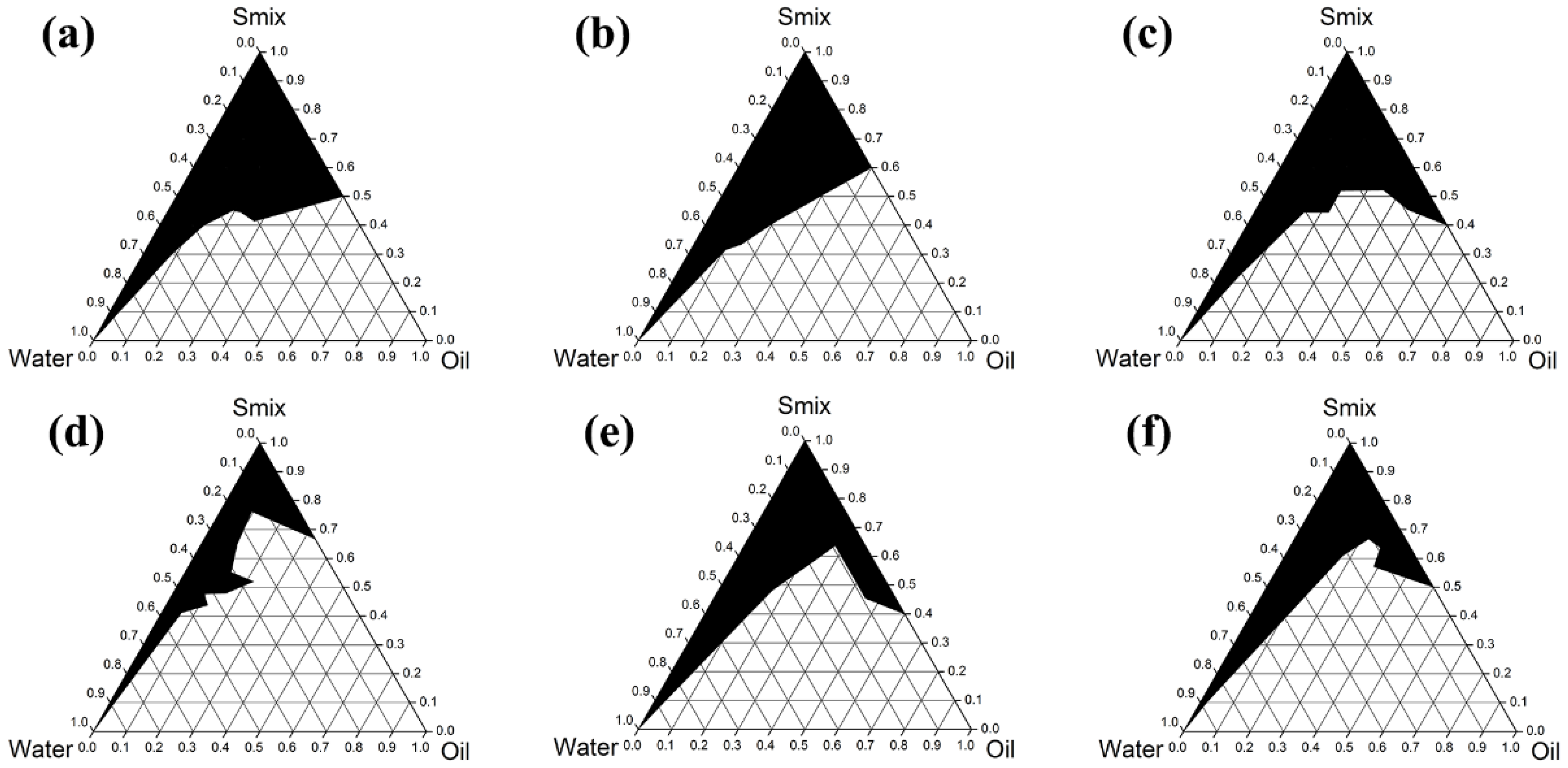
3.5. Developement of AGO Microemulsions
3.6. Cholinesterase Inhibition of AGO ME
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eigner, D.; Scholz, D. Ferula asa-foetida and Curcuma longa in traditional medical treatment and diet in Nepal. J. Ethnopharmacol. 1999, 67, 1–6. [Google Scholar] [CrossRef]
- Fransworth, N.R.; Bunyaprapraphatsara, N. Thai Medicinal Plants Recommended for Primary Health Care System; Prachancho: Bangkok, Thailand, 1992. [Google Scholar]
- Ikram, M.; Fazal, S. Compendium of Medicinal Plants; Pakistan Council of Scientific & Industrial Research: Peshawar, Pakistan, 1978.
- Kirtikar, K.R.; Basu, B.D. Indian Medicinal Plants; Deraduhun International Book Distributors: Uttarakhand, India, 1996. [Google Scholar]
- Latha, C.; Shriram, V.D.; Jahagirdar, S.S.; Dhakephalkar, P.K.; Rojatkar, S.R. Antiplasmid activity of 1’-acetoxychavicol acetate from Alpinia galanga against multi-drug resistant bacteria. J. Ethnopharmacol. 2009, 123, 522–525. [Google Scholar] [CrossRef] [PubMed]
- Nadkarni, K.M. The Indian Materia Medica; Popular Prakashan: Bombay, India, 1976. [Google Scholar]
- Matsuda, H.; Pongpiriyadacha, Y.; Morikawa, T.; Ochi, M.; Yoshikawa, M. Gastroprotective effects of phenylpropanoids from the rhizomes of Alpinia galanga in rats: Structural requirements and mode of action. Eur. J. Pharmacol. 2003, 471, 59–67. [Google Scholar] [CrossRef]
- Burkill, I.H. A Dictionary of the Economic Products of the Malay Peninsula; Crown Agents for the Colonies: London, UK, 1966. [Google Scholar]
- Zhang, L.; Liang, X.; Ou, Z.; Ye, M.; Shi, Y.; Chen, Y.; Zhao, J.; Zheng, D.; Xiang, H. Screening of chemical composition, anti-arthritis, antitumor and antioxidant capacities of essential oils from four Zingiberaceae herbs. Ind. Crops. Prod. 2020, 149, 112342. [Google Scholar] [CrossRef]
- Khattak, S.; Shah, H.U.; Ahmad, W.; Ahmad, M. Biological effects of indigenous medicinal plants Curcuma longa and Alpinia galanga. Fitoterapia 2005, 76, 254–257. [Google Scholar] [CrossRef]
- Oonmetta-aree, J.; Suzuki, T.; Gasaluck, P.; Eumkeb, G. Antimicrobial properties and action of galangal (Alpinia galanga Linn.) on Staphylococcus aureus. LWT 2006, 39, 1214–1220. [Google Scholar] [CrossRef]
- Mitsui, S.; Kobayashi, S.; Nagahori, H.; Ogiso, A. Constituents from seeds of Alpinia galanga Wild, and their anti-ulcer activities. Chem. Pharm. Bull. 1976, 24, 2377–2382. [Google Scholar] [CrossRef] [Green Version]
- Khodavandi, A.; Tahzir, N.A.B.; Cheng, P.W.; Chen, P.Y.V.; Alizadeh, F.; Hrmal, N.S.; Pei, C.P. Antifungal activity of Rhizome coptidis and Alpinia galangal against Candida species. J. Pure Appl. Microbiol. 2013, 7, 1725–1730. [Google Scholar]
- Tamura, S.; Shiomi, A.; Kimura, T.; Murakami, N. Halogenated analogs of 1′-acetoxychavicol acetate, Rev-export inhibitor from Alpinia galanga, designed from mechanism of action. Bioorg. Med. Chem. Lett. 2010, 20, 2082–2085. [Google Scholar] [CrossRef]
- Kitphati, W.; Wattanakamolkul, K.; Lomarat, P.; Phanthong, P.; Anantachoke, N.; Nukoolkarn, V.; Thirapanmethee, K.; Bunyapraphatsara, N. Anticholinesterase of essential oils and their constituents on purified and cellular enzymes. JAASP 2012, 1, 58–67. [Google Scholar]
- Chaiyana, W.; Okonogi, S. Inhibition of cholinesterase by essential oil from food plant. Phytomedicine 2012, 19, 836–839. [Google Scholar] [CrossRef] [PubMed]
- Adewusi, E.; Moodley, N.; Steenkamp, V. Medicinal plants with cholinesterase inhibitory activity: A Review. Afr. J. Biotechnol. 2011, 9, 8257–8276. [Google Scholar]
- Farfara, D.; Lifshitz, V.; Frenkel, D. Neuroprotective and neurotoxic properties of glial cells in the pathogenesis of Alzheimer’s disease. J. Cell Mol. Med. 2008, 12, 762–780. [Google Scholar] [CrossRef] [PubMed]
- Quinn, D.M. Acetylcholinesterase: Enzyme structure, reaction dynamics, and virtual transition states. Chem. Rev. 1987, 87, 955–979. [Google Scholar] [CrossRef]
- Çokuğraş, A.N.; Cengiz, D.; Tezcan, E.F. The Effects of Ni2+, Co2+, and Mn2+ on Human Serum Butyrylcholinesterase. J. Protein. Chem. 2003, 22, 585–589. [Google Scholar] [CrossRef]
- Chatonnet, A.; Lockridge, O. Comparison of butyrylcholinesterase and acetylcholinesterase. Biochem. J. 1989, 260, 625–634. [Google Scholar] [CrossRef]
- Raveh, L.; Grauer, E.; Grunwald, J.; Cohen, E.; Ashani, Y. The Stoichiometry of Protection against Soman and VX Toxicity in Monkeys Pretreated with Human Butyrylcholinesterase. Toxicol. Appl. Pharmacol. 1997, 145, 43–53. [Google Scholar] [CrossRef]
- Dave, K.R.; Syal, A.R.; Katyare, S.S. Tissue cholinesterases. A comparative study of their kinetic properties. Z. Naturforsch. C J. Biosci. 2000, 55, 100–108. [Google Scholar] [CrossRef]
- Prody, C.A.; Zevin-Sonkin, D.; Gnatt, A.; Goldberg, O.; Soreq, H. Isolation and characterization of full-length cDNA clones coding for cholinesterase from fetal human tissues. Proc. Natl. Acad. Sci. USA 1987, 84, 3555–3559. [Google Scholar] [CrossRef] [Green Version]
- Perry, E.K.; Tomlinson, B.E.; Blessed, G.; Bergmann, K.; Gibson, P.H.; Perry, R.H. Correlation of cholinergic abnormalities with senile plaques and mental test scores in senile dementia. Br. Med. J. 1978, 2, 1457–1459. [Google Scholar] [CrossRef] [Green Version]
- Hawkins, B.T.; Davis, T.P. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol. Rev. 2005, 57, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Kassem, L.M.; Ibrahim, N.A.; Farhana, S.A. Nanoparticle therapy is a promising approach in the management and prevention of many diseases: Does it help in curing Alzheimer disease? J. Nanotechnol. 2020, 2020, 8147080. [Google Scholar] [CrossRef]
- Jogani, V.V.; Shah, P.J.; Mishra, P.; Mishra, A.K.; Misra, A.R. Intranasal mucoadhesive microemulsion of tacrine to improve brain targeting. Alzheimer Dis. Assoc. Disord. 2008, 22, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, L.C.; Vacacela, M.; Clares, B.; Garcia, M.L.; Fabrega, M.J.; Calpena, A.C. Development of a nasal donepezil-loaded microemulsion for the treatment of Alzheimer’s disease: In vitro and ex vivo characterization. CNS Neurol. Disord. Drug Targets 2018, 17, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Etman, S.M.; Elnaggar, Y.S.; Abdelmonsif, D.A.; Abdallah, O.Y. Oral brain-targeted microemulsion for enhanced piperine delivery in Alzheimer’s disease therapy: In vitro appraisal, in vivo activity, and nanotoxicity. AAPS PharmSciTech 2018, 19, 3698–3711. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Cong, W.; Wang, Y.; Liu, Q.; Luo, G. Microemulsion-based patch for transdermal delivery of huperzine A and ligustrazine phosphate in treatment of Alzheimer’s disease. Drug Dev. Ind. Pharm. 2012, 38, 752–761. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Singh, M.; Kumar, P.; Vikram, V.; Mishra, N. Development and characterization of morin hydrate loaded microemulsion for the management of Alzheimer’s disease. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1620–1630. [Google Scholar] [CrossRef] [Green Version]
- Oertel, W.; Ross, J.S.; Eggert, K.; Adler, G. Rationale for transdermal drug administration in Alzheimer disease. Neurology 2007, 69, S4–S9. [Google Scholar] [CrossRef]
- Kováts, E. Gas-chromatographische Charakterisierung organischer Verbindungen. Teil 1: Retentionsindices aliphatischer Halogenide, Alkohole, Aldehyde und Ketone. Helv. Chim. Acta 1958, 41, 1915–1932. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Feather-Stone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Chaiyana, W.; Saeio, K.; Hennink, W.E.; Okonogi, S. Characterization of potent anticholinesterase plant oil based microemulsion. Int. J. Pharm. 2010, 401, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Chaiyana, W.; Phongpradist, R.; Leelapornpisid, P. Characterization of hydrodistillated pomelo peel oil and the enhancement of biological activities using microemulsion formulations. Int. J. Pharm. Pharm. Sci. 2014, 6, 596–602. [Google Scholar]
- Singh, P.K.; Iqubal, M.K.; Shukla, V.K.; Shuaib, M. Microemulsions: Current trends in novel drug delivery systems. J. Pharm. Chem. Biol. Sci. 2014, 1, 39–51. [Google Scholar]
- Mancini, E.; Arnold, N.A.; De Martino, L.; De Feo, V.; Formisano, C.; Rigano, D.; Senatore, F. Chemical composition and phytotoxic effects of essential oils of Salvia hierosolymitana Boiss. and Salvia multicaulis Vahl. var. simplicifolia Boiss. growing wild in Lebanon. Molecules 2009, 14, 4725–4736. [Google Scholar] [PubMed] [Green Version]
- Maggio, A.; Rosselli, S.; Bruno, M.; Spadaro, V.; Raimondo, F.M.; Senatore, F. Chemical composition of essential oil from Italian populations of Artemisia alba Turra (Asteraceae). Molecules 2012, 17, 10232–10241. [Google Scholar] [CrossRef] [PubMed]
- Ben Taarit, M.; Msaada, K.; Hosni, K.; Marzouk, B. Changes in fatty acid and essential oil composition of sage (Salvia officinalis L.) leaves under NaCl stress. Food. Chem. 2010, 119, 951–956. [Google Scholar] [CrossRef]
- Coffi, K.; Soleymane, K.; Harisolo, R.; Balo, T.; Claude, C.; Pierre, C.; Gilles, F.; Antoine, A. Monoterpene hydrocarbons, major components of the dried leaves essential oils of five species of the genus Eucalyptus from Cote d’Ivoire. Nat. Sci. 2012, 4, 106–111. [Google Scholar] [CrossRef] [Green Version]
- Mehrabani, M.; Asadipour, A.; Saber Amoli, S.; Daru, J. Chemical constituents of the essential oil of Nepeta depauperata Benth. from Iran. Pharm. Sci. 2004, 12, 98–100. [Google Scholar]
- Yassa, N.; Akhani, H.; Aghaahmadi, M.; Salimian, M. Essential Oils from Two Endemic Species of Apiaceae from Iran. Z. Naturforsch. C 2003, 58, 459–463. [Google Scholar] [CrossRef]
- Bernotienė, G.; Nivinskienė, O.; Butkienė, R.; Mockutė, D. Essential oil composition variability in sage (Salvia officinalis L.). Chemija 2007, 18, 38–43. [Google Scholar]
- Safaei-Ghomi, J.; Batooli, H. Determination of bioactive molecules from flowers, leaves, stems and roots of Perovskia Abrotanoides karel growing in Central Iran by nano scale injection. Dig. J. Nanomater. Biostruct. 2010, 5, 551–556. [Google Scholar]
- De Castro, O.; Senatore, F.; Rigano, D.; Formisano, C.; Cennamo, P.; Gianguzzi, L. Composition of the essential oil of Petagnaea gussonei (Sprengel) Rauschert, a relict species from Sicily (Southern Italy). Flavour Fragr. J. 2008, 23, 172–177. [Google Scholar] [CrossRef]
- Jirovetz, L.; Buchbauer, G.; Shafi, M.P.; Leela, N.K. Analysis of the essential oils of the leaves, stems, rhizomes and roots of the medicinal plant Alpinia galanga from southern India. Acta. Pharm. 2003, 53, 73–81. [Google Scholar] [PubMed]
- Raina, V.K.; Srivastava, S.K.; Syamasunder, K.V. The essential oil of ‘greater galangal’ [Alpinia galanga (L.) Willd.] from the lower Himalayan region of India. Flavour Fragr. J. 2002, 17, 358–360. [Google Scholar] [CrossRef]
- Ivanović, M.; Makoter, K.; Islamčević Razboršek, M. Comparative study of chemical composition and antioxidant activity of essential oils and crude extracts of four characteristic Zingiberaceae herbs. Plants 2021, 10, 501. [Google Scholar] [CrossRef]
- Zhao, T.; Ding, K.M.; Zhang, L.; Cheng, X.M.; Wang, C.H.; Zheng, W.T. Acetylcholinesterase and butyrylcholinesterase inhibitory activities of β-carboline and quinoline alkaloids derivatives from the plants of genus Peganum. J. Chem. 2013, 2013, 717232. [Google Scholar] [CrossRef] [Green Version]
- Onor, M.L.; Trevisiol, M.; Aguglia, E. Rivastigmine in the treatment of Alzheimer’s disease: An update. Clin. Interv. Aging 2007, 2, 17–32. [Google Scholar] [CrossRef]
- Lock, A.; Cornish, J.; Musson, D.S. The role of in vitro immune response assessment for biomaterials. J. Funct. Biomater. 2019, 10, 31. [Google Scholar] [CrossRef] [Green Version]
- Mahdi, E.S.; Sakeena, M.H.; Abdulkarim, M.F.; Abdullah, G.Z.; Sattar, M.A.; Noor, A.M. Effect of surfactant and surfactant blends on pseudoternary phase diagram behavior of newly synthesized palm kernel oil esters. Drug Des. Dev. Ther. 2011, 5, 311–323. [Google Scholar] [CrossRef] [Green Version]
- Palkovits, R.; Althues, H.; Rumplecker, A.; Tesche, B.; Dreier, A.; Holle, U.; Fink, G.; Cheng, C.H.; Shantz, D.F.; Kaskel, S. Polymerization of w/o microemulsions for the preparation of transparent SiO2/PMMA nanocomposites. Langmuir 2005, 21, 6048–6053. [Google Scholar] [CrossRef]
- Ban, S.; Kitahara, M.; Yamasaki, A. Preparation of O/W Emulsions with Poly(oxyethylene) Hydrogenated Castor Oil by using SPG Membrane Emulsification Method. Nippon Kagaku Kaishi 1994, 1994, 737–742. [Google Scholar] [CrossRef] [Green Version]
- Rhee, Y.S.; Park, C.W.; Nam, T.Y.; Shin, Y.S.; Chi, S.C.; Park, E.S. Formulation of parenteral microemulsion containing itraconazole. Arch. Pharm. Res 2007, 30, 114–123. [Google Scholar] [CrossRef] [PubMed]


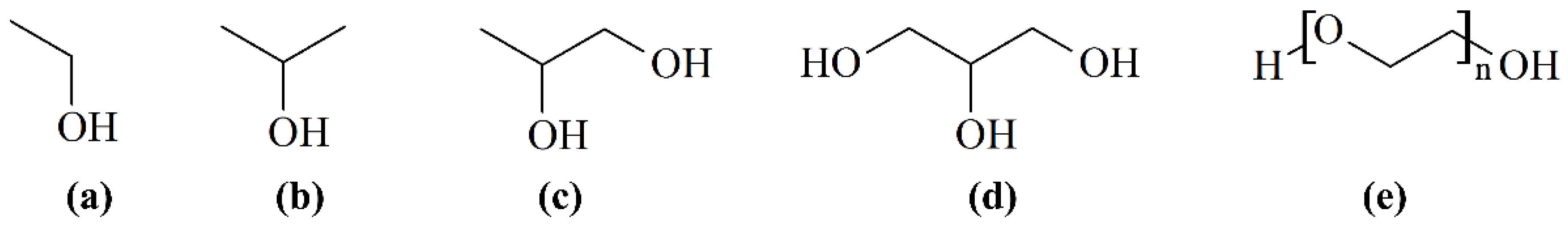


| Peak | RT | Compound | %Area | Identification | RIcalc | RIliterature [Ref] |
|---|---|---|---|---|---|---|
| 1 | 3.32 | 1,8-Cineole | 29.64 | MS, RI | 1034 | 1034 [39,40,41] |
| 2 | 3.56 | γ-Terpinene | 1.22 | MS, RI | 1057 | 1057 [42] |
| 3 | 3.99 | α-Terpinolene | 0.44 | MS, RI | 1093 | 1088 [41,43,44] |
| 4 | 4.09 | 1-Undecene | 0.20 | MS, RI | 1100 | 1100 [45] |
| 5 | 5.48 | (-)-Borneol | 0.72 | MS, RI | 1160 | 1165 [43,44] |
| 6 | 6.03 | p-Cymen-8-ol | 3.06 | MS, RI | 1179 | 1179 [42] |
| 7 | 6.25 | α-Terpineol | 0.20 | MS, RI | 1186 | 1189 [39,40,43,44] |
| 8 | 7.5 | Z-Citral | 1.23 | MS, RI | 1240 | 1240 [39] |
| 9 | 8.51 | (-)-Bornyl acetate | 0.39 | MS, RI | 1284 | 1284 [39,46] |
| 10 | 10.56 | Methyl cinnamate | 33.31 | MS, RI | 1347 | 1352 [47] |
| 11 | 10.78 | α-Cubebene | 0.15 | MS, RI | 1352 | 1352 [39,40,42] |
| 12 | 11.55 | Decaoic acid | 1.31 | MS, RI | 1371 | 1367 [45] |
| 13 | 12.3 | β-Elemene | 1.91 | MS, RI | 1388 | 1387 [39,40,42] |
| 14 | 12.83 | α-Gurjunene | 0.20 | MS, RI | 1400 | 1405 [47] |
| 15 | 13.39 | β-Caryophyllene | 3.38 | MS, RI | 1416 | 1415 [39,40] |
| 16 | 15.44 | β-Farnesene | 0.42 | MS, RI | 1469 | 1463 [47,48] |
| 17 | 15.56 | β-Selinene | 0.46 | MS, RI | 1472 | 1475 [39,42] |
| 18 | 15.71 | δ-Selinene | 0.31 | MS, RI | 1476 | 1491 [41] |
| 19 | 15.95 | Germacrene D | 5.62 | MS, RI | 1482 | 1483 [42,47] |
| 20 | 16.5 | α-Amorphene | 3.01 | MS, RI | 1494 | 1487 [40] |
| 21 | 16.65 | α-Selinene | 0.83 | MS, RI | 1498 | 1498 [39] |
| 22 | 16.79 | γ-Bisabolene | 2.25 | MS, RI | 1511 | 1510 [40] |
| 23 | 21.08 | α-Cadinol | 0.61 | MS, RI | 1640 | 1640 [39,40] |
| 24 | 21.62 | τ-Muurolol | 0.40 | MS, RI | 1645 | 1641 [42] |
| 25 | 22.21 | nor-Copaanone | 0.84 | MS, RI | 1650 | 1622 [42] |
| 26 | 22.45 | Dillapiol | 0.65 | MS, RI | 1652 | 1628 [46] |
| 27 | 27.46 | α-Bergamotol | 0.30 | MS, RI | 1691 | 1693 [44] |
| Co-Surfactants | Dielectric Constant |
|---|---|
| Propylene glycol | 32.1 |
| Ethanol | 25 |
| Propan-2-ol | 22 |
| Polyethylene glycol 400 | 12.4 |
| Polyethylene glycol 600 | 10.2 |
| Glycerin | 46 |
| Characterizations | Stability Test | |
|---|---|---|
| Before | After | |
| Internal droplet size (nm) | 71.6 ± 3.4 | 74.1 ± 2.8 |
| PDI | 0.217 ± 0.012 | 0.224 ± 0.014 |
| Formulation/Component | % Inhibition | |
|---|---|---|
| AChE | BChE | |
| AGO (100 µg/mL)/Tween® 20/PG/water | 95.63 ± 7.10 | 14.07 ± 1.44 |
| AGO (100 µg/mL) | 34.31 ± 8.08 | 1.40 ± 2.43 |
| Tween® 20 (0.1 mg/mL) | −0.03 ± 0.48 | −0.04 ± 0.20 |
| PG (0.1 mg/mL) | −1.65 ± 2.77 | −0.35 ± 1.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaiyana, W.; Sriyab, S.; Okonogi, S. Enhancement of Cholinesterase Inhibition of Alpinia galanga (L.) Willd. Essential Oil by Microemulsions. Molecules 2022, 27, 3275. https://doi.org/10.3390/molecules27103275
Chaiyana W, Sriyab S, Okonogi S. Enhancement of Cholinesterase Inhibition of Alpinia galanga (L.) Willd. Essential Oil by Microemulsions. Molecules. 2022; 27(10):3275. https://doi.org/10.3390/molecules27103275
Chicago/Turabian StyleChaiyana, Wantida, Suwannee Sriyab, and Siriporn Okonogi. 2022. "Enhancement of Cholinesterase Inhibition of Alpinia galanga (L.) Willd. Essential Oil by Microemulsions" Molecules 27, no. 10: 3275. https://doi.org/10.3390/molecules27103275
APA StyleChaiyana, W., Sriyab, S., & Okonogi, S. (2022). Enhancement of Cholinesterase Inhibition of Alpinia galanga (L.) Willd. Essential Oil by Microemulsions. Molecules, 27(10), 3275. https://doi.org/10.3390/molecules27103275







