Polyphenols and Maillard Reaction Products in Dried Prunus spinosa Fruits: Quality Aspects and Contribution to Anti-Inflammatory and Antioxidant Activity in Human Immune Cells Ex Vivo
Abstract
:1. Introduction
2. Results and Discussion
2.1. Fractionated Extraction and Phytochemical Composition of the Extract/Fractions
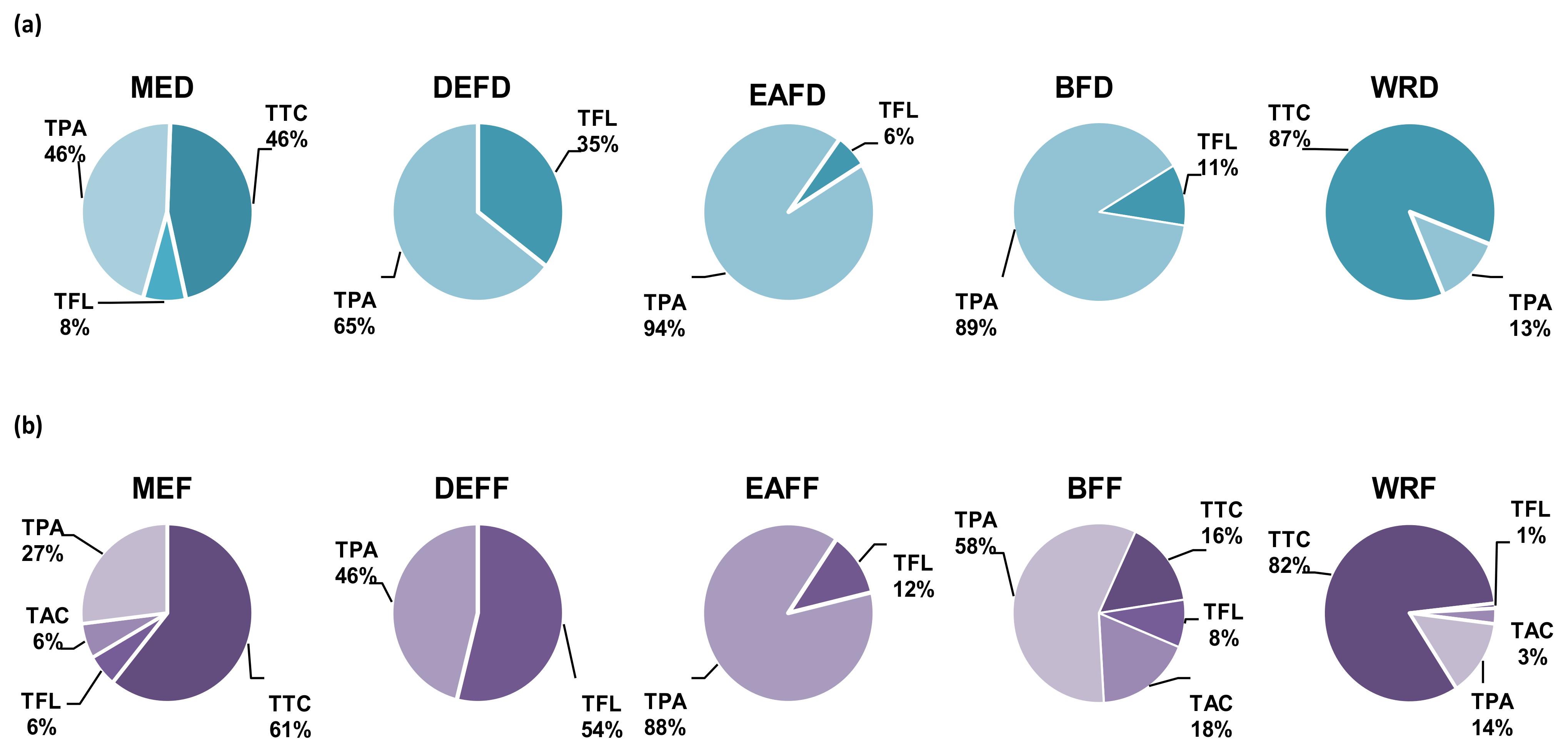
2.1.1. Polyphenolic Profile
2.1.2. Non-Phenolic Compounds
2.2. Bioactivity of the Extracts/Fractions and Model Compounds in Human Immune Cells Ex Vivo
2.2.1. Cellular Models
2.2.2. Effects on Viability of Neutrophils and PBMCs
2.2.3. Influence on Pro-Oxidant Functions of Human Neutrophils: Antioxidant Effect
2.2.4. Effect on the ELA-2 Release by Human Neutrophils
2.2.5. Influence on the Cytokine Secretion by Human Neutrophils and PBMCs
2.2.6. Drying-Related Differences in Biological Capacity of Dried and Fresh Fruits
2.2.7. Biological Effects of HMF
3. Materials and Methods
3.1. Plant Material and Extracts Preparation
3.2. Qualitative and Quantitative Analysis of Phenolic Compounds
3.3. Isolation and Structure Elucidation of MRPs
3.4. Biological Activity Studies in Cellular Models
3.4.1. Isolation of Neutrophils and PBMCs from Human Buffy Coats
3.4.2. Viability Assessment of Neutrophils and PBMCs
3.4.3. Evaluation of ROS, ELA-2, IL-8, IL-6, TNF-α and IL-10 Secretion by Human Immune Cells
3.5. Statistics and Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| BFD | n-butanol fraction of MED |
| BFF | n-butanol fraction of MEF |
| CHA | chlorogenic acid (5-O-caffeoylquinic acid) |
| CYG | cyanidin 3-O-β-d-glucopyranoside |
| DEFD | diethyl ether fraction of MED |
| DEFF | diethyl ether fraction of MEF |
| DEX | dexamethasone |
| dw | dry weight |
| EAFD | ethyl acetate fraction of MED |
| EAFF | ethyl acetate fraction of MEF |
| ELA-2 | elastase 2 (neutrophils elastase) |
| fMLP | N-formyl-l-methionyl-l-leucyl-l-phenylalanine |
| fw | fresh weight |
| GAE | gallic acid equivalents |
| IL | interleukin |
| LOD | limits of detection |
| LOQ | limits of quantitation |
| LPS | bacterial lipopolysaccharide |
| MED | methanol-water (75:25, v/v) extract of dried fruits |
| MEF | methanol-water (75:25, v/v) extract of fresh fruits |
| MRPs | Maillard reaction products |
| MS3 | three-stage mass spectrometry |
| PB2 | procyanidin B2 |
| PBMCs | peripheral blood mononuclear cells |
| QU | quercetin |
| ROS | reactive oxygen species |
| TAC | total content of anthocyanins |
| TFL | total content of flavonoids |
| TNF-α | tumour necrosis factor α |
| TPA | total content of phenolic acids |
| TPC | total phenolic content (Folin–Ciocalteu assay) |
| TPH | total phenolic content (sum of individual phenolics by HPLC) |
| TTC | total content of tannin-type proanthocyanidins |
| WRD | water residue of MED after fractionated extraction |
| WRF | water residue of MEF after fractionated extraction |
References
- Popescu, I.; Caudullo, G. Prunus spinosa in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publications Office of the European Union: Luxembourg, 2016; p. e01aa69+. Available online: https://w3id.org/mtv/FISE-Comm/v01/e01aa69 (accessed on 10 December 2021).
- Facciola, S. Cornucopia II: A Source Book of Edible Plants, 2nd ed.; Facciola, S., Ed.; Kampong Publications: Vista, CA, USA, 1998; p. 204. [Google Scholar]
- Alarcόn, R.; Pardo-de-Santayana, M.; Priestley, C.; Morales, R.; Heinrich, M. Medicinal and local food plants in the south of Alava (Basque Country, Spain). J. Ethnopharmacol. 2015, 176, 207–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarić, S.; Mačukanović-Jocić, M.; Djurdjević, L.; Mitrović, M.; Kostić, O.; Karadžić, B.; Pavlović, P. An ethnobotanical survey of traditionally used plants on Suva planina mountain (south-eastern Serbia). J. Ethnopharmacol. 2015, 175, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Kültür, S. Medicinal plants used in Kirklareli Province (Turkey). J. Ethnopharmacol. 2007, 111, 341–364. [Google Scholar] [CrossRef]
- Zlatković, B.K.; Bogosavljević, S.S.; Radivojević, A.R.; Pavlović, M.A. Traditional use of the native medicinal plant resource of Mt. Rtanj (Eastern Serbia): Ethnobotanical evaluation and comparison. J. Ethnopharmacol. 2014, 151, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Fraternale, D.; Giamperi, L.; Bucchini, A.; Sestili, P.; Paolillo, M.; Ricci, D. Prunus spinosa fresh fruit juice: Antioxidant activity in cell-free and cellular systems. Nat. Prod. Commun. 2009, 4, 1665–1670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barros, L.; Carvalho, A.M.; Morais, J.S.; Ferreira, I.C.F.R. Strawberry tree, blackthorn and rose fruits: Detailed characterisation in nutrients and phytochemicals with antioxidant properties. Food Chem. 2010, 120, 247–254. [Google Scholar] [CrossRef]
- Guimarães, R.; Barros, L.; Dueñas, M.; Carvalho, A.M.; Queiroz, M.J.; Santos-Buelga, C.; Ferreira, I.C. Characterisation of phenolic compounds in wild fruits from Northeastern Portugal. Food Chem. 2013, 141, 3721–3730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morales, P.; Ferreira, I.C.F.R.; Carvalho, A.M.; Fernández-Ruiz, V.; Cortes Sánchez-Mata, M.; Cámara, M.; Morales, R.; Tardío, J. Wild edible fruits as a potential source of phytochemicals with capacity to inhibit lipid peroxidation. Eur. J. Lipid Sci. Technol. 2013, 115, 176–185. [Google Scholar] [CrossRef]
- Ruiz-Rodríguez, B.M.; De Ancos, B.; Sánchez-Moreno, B.; Fernández-Ruiz, V.; De Cortes Sánchez-Mata, M.; Cámara, M.; Tardío, J. Wild blackthorn (Prunus spinosa L.) and hawthorn (Crataegus monogyna Jacq.) fruits as valuable sources of antioxidants. Fruits 2014, 69, 61–73. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Stampar, F.; Veberic, R.; Sircelj, H. Wild Prunus fruit species as a rich source of bioactive compounds. J. Food Sci. 2016, 81, C1928–C1937. [Google Scholar] [CrossRef]
- Magiera, A.; Czerwińska, M.E.; Owczarek, A.; Marchelak, A.; Granica, S.; Olszewska, M.A. Polyphenol-enriched extracts of Prunus spinosa fruits: Anti-Inflammatory and antioxidant effects in human immune cells ex vivo in relation to phytochemical profile. Molecules 2022, 27, 1691. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, L.; Fraternale, D.; Di Giacomo, B.; Mari, M.; Albertini, M.C.; Gordillo, B.; Rocchi, M.B.L.; Sisti, D.; Coppari, S.; Semprucci, F.; et al. Chemical composition, antioxidant, antimicrobial and anti-inflammatory activity of Prunus spinosa L. fruit ethanol extract. J. Funct. Foods 2020, 67, 103885. [Google Scholar] [CrossRef]
- Arsic, B.; Kostic, D.; Randjelovic, S.; Radovanović, B.; Sunaric, S.; Ilic, S. Chemometric analysis of selected medicinal plants from Serbia. Rom. Biotechnol. Lett. 2016, 21, 11115–11125. [Google Scholar]
- Gegiu, G.; Branza, A.D.; Bucur, L.; Grigorian, M.; Tache, T.; Badea, V. Contributions to the antimicrobial and antifungal study of the aqueous extract of Prunus spinosa L. Farmacia 2015, 63, 275–279. [Google Scholar]
- Pinacho, R.; Cavero, R.Y.; Astiasaran, I.; Ansorena, D.; Calvo, M.I. Phenolic compounds of blackthorn (Prunus spinosa L.) and influence of in vitro digestion on their antioxidant capacity. J. Funct. Foods 2015, 19, 49–62. [Google Scholar] [CrossRef]
- Rutkowska, M.; Kolodziejczyk-Czepas, J.; Owczarek, A.; Zakrzewska, A.; Magiera, A.; Olszewska, M.A. Novel insight into biological activity and phytochemical composition of Sorbus aucuparia L. fruits: Fractionated extracts as inhibitors of protein glycation and oxidative/nitrative damage of human plasma components. Food Res. Int. 2021, 147, 110526. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.; Xu, Z. Changes of anthocyanins, anthocyanidins, and antioxidant activity in bilberry extract during dry heating. J. Food Sci. 2008, 73, C494–C499. [Google Scholar] [CrossRef]
- Heras-Ramirez, M.E.; Quintero-Ramos, A.; Camacho-Davila, A.A.; Barnard, J.; Talamas-Abbud, R.; Torres-Munoz, J.V.; Salas-Munoz, E. Effect of blanching and drying temperature on polyphenolic compound stability and antioxidant capacity of apple pomace. Food Bioproc. Tech. 2012, 5, 2201–2210. [Google Scholar] [CrossRef]
- Kessy, H.N.; Hu, Z.; Zhao, L.; Zhou, M. Effect of steam blanching and drying on phenolic compounds of Litchi Pericarp. Molecules 2016, 21, 729. [Google Scholar] [CrossRef] [Green Version]
- Méndez-Lagunas, L.; Rodríguez-Ramírez, J.; Cruz-Gracida, M.; Sandoval-Torres, S.; Barriada-Bernal, G. Convective drying kinetics of strawberry (Fragaria ananassa): Effects on antioxidant activity, anthocyanins and total phenolic content. Food Chem. 2017, 230, 174–181. [Google Scholar] [CrossRef]
- ALjahdali, N.; Carbonero, F. Impact of Maillard reaction products on nutrition and health: Current knowledge and need to understand their fate in the human digestive system. Crit. Rev. Food Sci. Nutr. 2019, 59, 474–487. [Google Scholar] [CrossRef] [PubMed]
- Parisi, S.; Luo, W. Maillard reaction and processed foods—Main chemical products. In Chemistry of Maillard Reactions in Processed Foods; Parisi, S., Ed.; Springer Briefs in Molecular Science; Springer: Cham, Switzerland, 2018; p. 53. [Google Scholar]
- Shapla, U.M.; Solayman, M.; Alam, N.; Khalil, M.I.; Gan, S.H. 5-Hydroxymethylfurfural (HMF) levels in honey and other food products: Effects on bees and human health. Chem. Cent. J. 2018, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Adefegha, S.A. Functional foods and nutraceuticals as dietary intervention in chronic diseases; Novel perspectives for health promotion and disease prevention. J. Diet. Suppl. 2018, 15, 977–1009. [Google Scholar] [CrossRef] [PubMed]
- Karwowska, K.; Przybył, J. Suszarnictwo i Przetwórstwo Ziół (Drying and Processing of Herbs), 1st ed.; SGGW: Warsaw, Poland, 2005; pp. 35–48. [Google Scholar]
- Castillo-Fraireac, C.M.; Poupard, P.; Guilois-Dubois, S.; Salas, E.; Guyota, S. Preparative fractionation of 5′-O-caffeoylquinic acid oxidation products using centrifugal partition chromatography and their investigation by mass spectrometry. J. Chrom. A. 2019, 1592, 19–30. [Google Scholar] [CrossRef]
- Aneklaphakij, C.; Saigo, T.; Watanabe, M.; Naake, T.; Fernie, A.R.; Bunsupa, S.; Satitpatipan, V.; Tohge, T. Diversity of chemical structures and biosynthesis of polyphenols in Nut-bearing species. Front. Plant Sci. 2021, 12, 642581. [Google Scholar] [CrossRef]
- Calín-Sánchez, Á.; Lipan, L.; Cano-Lamadrid, M.; Kharaghani, A.; Masztalerz, K.; Carbonell-Barrachina, Á.A.; Figiel, A. Comparison of traditional and novel drying techniques and its effect on quality of fruits, vegetables and aromatic herbs. Foods 2020, 9, 1261. [Google Scholar] [CrossRef]
- Piga, A.; Del Caro, A.; Corda, G. From plums to prunes: Influence of drying parameters on polyphenols and antioxidant activity. J. Agric. Food Chem. 2003, 51, 3675–3681. [Google Scholar] [CrossRef]
- Khanal, R.C.; Howard, L.R.; Prior, R.L. Effect of heating on the stability of grape and blueberry pomace procyanidins and total anthocyanins. Food Res. Int. 2010, 43, 1464–1469. [Google Scholar] [CrossRef]
- Madrau, M.A.; Sanguinetti, A.M.; Caro, A.D.; Fadda, C.; Piga, A. Contribution of melanoidins to the antioxidant activity of prunes. J. Food Qual. 2010, 33, 155–170. [Google Scholar] [CrossRef]
- Richards, S.A.; Hollerton, J.C. Essential Practical NMR for Organic Chemistry; John Wiley & Sons: Chichester, UK, 2011; pp. 57, 138. [Google Scholar] [CrossRef]
- Cottier, L.; Descotes, G.; Soro, Y. Synthesis of acetylated ranunculin diastereoisomers and δ–glucosyloxy–γ–oxo esters from α or β glucosylmethylfurfural. J. Carbohydr. Chem. 2005, 24, 55–71. [Google Scholar] [CrossRef]
- Boffo, E.F.; Tavares, L.A.; Tobias, A.C.T.; Ferreira, M.M.C.; Ferreira, A.G. Identification of components of Brazilian honey by 1H NMR and classification of its botanical origin by chemometric methods. LWT 2012, 49, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, A.; Kumar, V.; Kumar, S.; Majid, I.; Aggarwal, P.; Suri, S. 5-Hydroxymethylfurfural (HMF) formation, occurrence and potential health concerns: Recent developments. Toxin Rev. 2021, 40, 545–561. [Google Scholar] [CrossRef]
- Abraham, K.; Gürtler, R.; Berg, K.; Heinemeyer, G.; Lampen, A.; Appel, K.E. Toxicology and risk assessment of 5-hydroxymethylfurfural in food. Mol. Nutr. Food Res. 2011, 55, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Pastoriza de la Cueva, S.; Álvarez, J.; Végvári, Á.; Montilla-Gómez, J.; Cruz-López, O.; Delgado-Andrade, C.; Rufián-Henares, J.A. Relationship between HMF intake and SMF formation in vivo: An animal and human study. Mol. Nutr. Food Res. 2017, 61, 1600773. [Google Scholar] [CrossRef]
- Murkovic, M.; Pichler, N. Analysis of 5-hydroxymethylfurfual in coffee, dried fruits and urine. Mol. Nutr. Food Res. 2006, 50, 842–846. [Google Scholar] [CrossRef]
- Chung, H.Y.; Cesari, M.; Anton, S.; Marzetti, E.; Giovannini, S.; Seo, A.Y.; Carter, C.; Yu, B.P.; Leeuwenburgh, C. Molecular inflammation: Underpinnings of aging and age-related diseases. Ageing Res. Rev. 2009, 8, 18–30. [Google Scholar] [CrossRef] [Green Version]
- Jones, H.R.; Robb, C.T.; Perretti, M.; Rossi, A.G. The role of neutrophils in inflammation resolution. Semin. Immunol. 2016, 28, 137–145. [Google Scholar] [CrossRef]
- Iyer, S.S.; Cheng, G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit. Rev. Immunol. 2012, 32, 23–63. [Google Scholar] [CrossRef] [Green Version]
- Marchelak, A.; Owczarek, A.; Matczak, M.; Pawlak, A.; Kolodziejczyk-Czepas, J.; Nowak, P.; Olszewska, M.A. Bioactivity potential of Prunus spinosa L. flower extracts: Phytochemical profiling, cellular safety, pro-inflammatory enzymes inhibition and protective effects against oxidative stress in vitro. Front. Pharmacol. 2017, 8, 680. [Google Scholar] [CrossRef] [Green Version]
- Bourgonje, A.R.; Feelisch, M.; Faber, K.N.; Pasch, A.; Dijkstra, G.; van Goor, H. Oxidative stress and redox-modulating therapeutics in inflammatory bowel disease. Trends Mol. Med. 2020, 26, 1034–1046. [Google Scholar] [CrossRef]
- Tiwari, B.K.; Pandey, K.B.; Abidi, A.B.; Rizvi, S.I. Markers of oxidative stress during diabetes mellitus. J. Biomark. 2013, 2013, 378790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuno, Y.; Ina, K.; Nishiwaki, T.; Tsuzuki, T.; Shimada, M.; Imada, A.; Nishio, Y.; Nobata, K.; Suzuki, T.; Ando, T.; et al. Possible involvement of neutrophil elastase in impaired mucosal repair in patients with ulcerative colitis. J. Gastroenterol. 2002, 37, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Lessieur, E.M.; Saadane, A.; Lindstrom, S.I.; Taylor, P.R.; Kern, T.S. Neutrophil elastase contributes to the pathological vascular permeability characteristic of diabetic retinopathy. Diabetologia 2019, 62, 2365–2374. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.M.; An, J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalliolias, G.D.; Ivashkiv, L.B. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat. Rev. Rheumatol. 2016, 12, 49–62. [Google Scholar] [CrossRef]
- Saraiva, M.; Vieira, P.; O’Garra, A. Biology and therapeutic potential of interleukin-10. J. Exp. Med. 2020, 217, e20190418. [Google Scholar] [CrossRef] [Green Version]
- Husøy, T.; Haugen, M.; Murkovic, M.; Jöbstl, D.; Stølen, L.H.; Bjellaas, T.; Rønningborg, C.; Glatt, H.; Alexander, J. Dietary exposure to 5-hydroxymethylfurfural from Norwegian food and correlations with urine metabolites of short-term exposure. Food Chem. Toxicol. 2008, 46, 3697–3702. [Google Scholar] [CrossRef]
- Qiu, Y.; Lin, X.; Chen, Z.; Li, B.; Zhang, Y. 5-Hydroxymethylfurfural exerts negative effects on gastric mucosal epithelial cells by inducing oxidative stress, apoptosis, and tight junction disruption. J. Agric. Food Chem. 2022, 70, 3852–3861. [Google Scholar] [CrossRef]
- Kong, F.; Lee, B.H.; Wei, K. 5-Hydroxymethylfurfural mitigates lipopolysaccharide-stimulated inflammation via suppression of MAPK, NF-κB and mTOR activation in RAW 264.7 Cells. Molecules 2019, 24, 275. [Google Scholar] [CrossRef] [Green Version]
- Uchida, R.; Kato, M.; Hattori, Y.; Kikuchi, H.; Watanabe, E.; Kobayashi, K.; Nishida, K. Identification of 5-Hydroxymethylfurfural (5-HMF) as an active component Citrus Jabara that suppresses FcεRI-mediated mast Cell Activation. Int. J. Mol. Sci. 2020, 21, 2472. [Google Scholar] [CrossRef] [Green Version]
- Marchelak, A.; Olszewska, M.A.; Owczarek, A. Simultaneous quantification of thirty polyphenols in blackthorn flowers and dry extracts prepared thereof: HPLC-PDA method development and validation for quality control. J. Pharm. Biomed. Anal. 2020, 184, 113121. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wu, C.-J.; Tang, X.-Y.; Yu, S.-J. Determination of four bitter compounds in caramel colors and beverages using modified QuEChERS coupled with liquid chromatography-diode array detector-mass spectrometry. Food Anal. Methods 2019, 12, 1674–1683. [Google Scholar] [CrossRef]
- Gökmen, V.; Senyuva, H.Z. Improved method for the determination of hydroxymethylfurfural in baby foods using liquid chromatography−mass spectrometry. J. Agric. Food Chem. 2006, 54, 2845–2849. [Google Scholar] [CrossRef] [PubMed]
- Teixidó, E.; Moyano, E.; Santos, F.J.; Galceran, M.T. Liquid chromatography multi-stage mass spectrometry for the analysis of 5-hydroxymethylfurfural in foods. J. Chromatogr. A. 2008, 1185, 102–108. [Google Scholar] [CrossRef]
- Catarino, M.D.; Silva, A.M.S.; Saraiva, S.C.; Sobral, A.B.J.F.N.; Cardoso, S.M. Characterization of phenolic constituents and evaluation of antioxidant properties of leaves and stems of Eriocephalus africanus. Arab. J. Chem. 2018, 11, 62–69. [Google Scholar] [CrossRef] [Green Version]
- Jaiswal, R.; Sovdat, T.; Vivan, F.; Kuhnert, N. Profiling and characterization by LC-MSn of the chlorogenic acids and hy-droxycinnamoylshikimate esters in maté (Ilex paraguariensis). J. Agric. Food Chem. 2010, 58, 5471–5481. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, R.; Müller, H.; Müller, A.; Karar, M.G.; Kuhnert, N. Identification and characterization of chlorogenic acids, chlorogenic acid glycosides and flavonoids from Lonicera henryi L. (Caprifoliaceae) leaves by LC-MSn. Phytochemistry 2014, 108, 252–263. [Google Scholar] [CrossRef]
- Clifford, M.N.; Johnston, K.L.; Knight, S.; Kuhnert, N. Hierarchical scheme for LC-MSn identification of chlorogenic acids. J. Agric. Food Chem. 2003, 51, 2900–2911. [Google Scholar] [CrossRef]
- Bystrom, L.M.; Lewis, B.A.; Brown, D.L.; Rodriguez, E.; Obendorf, R.L. Characterization of phenolics by LC-UV/vis, LC-MS/MS and sugars by GC in Melicoccus bijugatus Jacq. ‘Montgomery’ fruits. Food Chem. 2008, 111, 1017–1024. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.; Xu, X.; Yuan, S.; Liu, H.; Liu, M.; Zhang, Y.; Zhang, H.; Gao, Y.; Lin, R.; Li, X. Identification and analysis of amygdalin, neoamygdalin and amygdalin amide in different processed bitter almonds by HPLC-ESI-MS/MS and HPLC-DAD. Molecules 2017, 22, 1425. [Google Scholar] [CrossRef]
- Ben Said, R.; Hamed, A.I.; Mahalel, U.A.; Al-Ayed, A.S.; Kowalczyk, M.; Moldoch, J.; Oleszek, W.; Stochmal, A. Tentative characterization of polyphenolic compounds in the male flowers of Phoenix dactylifera by liquid chromatography coupled with mass spectrometry and DFT. Int. J. Mol. Sci. 2017, 18, 512. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Li, X.; Saleri, F.; Guo, M. Analysis of flavonoids in Rhamnus davurica and its antiproliferative activities. Molecules 2016, 21, 1275. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; Knight, S.; Kuhnert, N. Discriminating between the six isomers of dicaffeoylquinic acid by LC-MSn. J. Agric. Food Chem. 2005, 53, 3821–3832. [Google Scholar] [CrossRef] [PubMed]





| MED | DEFD | EAFD | BFD | WRD | |
|---|---|---|---|---|---|
| Total contents: | |||||
| TPC (GAE) | 26.77 ± 0.47 d | 124.01 ± 0.70 a | 107.43 ± 4.08 b | 46.58 ± 2.28 c | 22.59 ± 0.05 d |
| TPH | 9.61 ± 0.60 d | 80.24 ± 1.16 b | 109.91 ± 1.26 a | 28.49 ± 0.96 c | 0.78 ± 0.01 e |
| TPA | 8.19 ± 0.51 d | 47.51 ± 1.50 b | 102.53 ± 0.85 a | 25.16 ± 0.80 c | 0.78 ± 0.01 e |
| TAC | n.d. | n.d. | n.d. | 0.22 ± 0.01 a | n.d. |
| TFL | 1.43 ± 0.09 d | 25.99 ± 0.77 a | 6.69 ± 0.46 b | 3.12 ± 0.24 c | n.d. |
| TTC (PB2) | 8.17 ± 0.24 a | n.d. | n.d. | n.d. | 5.37 ± 0.11 b |
| MRPs | 0.92 ± 0.07 c | 37.75 ± 2.39 a | 13.53 ± 0.78 b | 1.37 ± 0.03 c | 0.26 ± 0.003 c |
| Individual compounds: | |||||
| Avicularin (46) | 0.64 ± 0.09 b | 4.44 ± 0.22 a | 0.83 ± 0.14 b | n.d. | n.d. |
| Guaiaverin (45) | n.d. | 0.91 ± 0.05 a | 0.48 ± 0.03 b | n.d. | n.d. |
| Hyperoside (40) | n.d. | n.d. | 0.84 ± 0.04 a | n.d. | n.d. |
| Isoquercitrin (42) | n.d. | 0.13 ± 0.01 b | 1.10 ± 0.14 a | n.d. | n.d. |
| Reinutrin (44) | n.d. | 0.41 ± 0.003 b | 0.71 ± 0.07 a | n.d. | n.d. |
| Rutin (41) | 0.41 ± 0.03 c | n.d. | 0.74 ± 0.03 b | 2.13 ± 0.20 a | n.d. |
| Quercitrin (48) | 0.11 ± 0.01 c | 1.40 ± 0.07 a | 0.67 ± 0.03 b | n.d. | n.d. |
| Quercetin (53, QU) | 0.06 ± 0.01 c | 16.41 ± 0.40 a | 0.63 ± 0.04 b | n.d. | n.d. |
| Cyanidin 3-O-glucoside (24) | n.d. | n.d. | n.d. | 0.22 ± 0.01 a | n.d. |
| Protocatechuic acid (6) | n.d. | 11.04 ± 0.96 a | 0.57 ± 0.02 b | n.d. | n.d. |
| p-Hydroxybenzoic acid (11) | n.d. | 1.47 ± 0.12 a | 0.57 ± 0.04 b | n.d. | n.d. |
| Vanillic acid (19) | n.d. | 7.85 ± 0.31 a | n.d. | n.d. | n.d. |
| p-Coumaric acid (31) | n.d. | 1.89 ± 0.18 a | n.d. | n.d. | n.d. |
| Neochlorogenic acid (10) | 4.52 ± 0.34 d | 11.10 ± 0.40 c | 29.28 ± 0.86 a | 16.19 ± 0.50 b | 0.78 ± 0.01 e |
| Chlorogenic acid (20, CHA) | 0.14 ± 0.01 b | 0.28 ± 0.01 b | 3.56 ± 0.21 a | 0.20 ± 0.01 b | n.d. |
| Cryptochlorogenic acid (23) | 2.11 ± 0.03 c | 6.40 ± 0.40 b | 50.85 ± 1.29 a | 7.65 ± 0.49 b | n.d. |
| Vanillin (27) | n.d. | 6.74 ± 0.47 a | 0.69 ± 0.05 b | n.d. | n.d. |
| 5-Hydroxymethylfurfural (2, HMF) | 0.78 ± 0.05 c | 32.08 ± 2.03 a | 11.50 ± 0.66 b | 1.16 ± 0.03 c | 0.22 ± 0.002 c |
| Position/ Group b | Compound 2 | Compound 3 | ||
|---|---|---|---|---|
| δC | δH | δC | δH | |
| C-2 | 163.8 | – | 154.8 | – |
| C-3 | 112.9 | 6.62 (1H, d, J = 3.1) | 107.6 | 6.39 (1H, d, J = 3.0) |
| C-4 | 129.3 | 7.42 (1H, d, J = 3.1) | 108.9 | 6.31 (1H, d, J = 3.0) |
| C-5 | 154.9 | - | 155.2 | - |
| -OCHO- | - | - | 98.2 | 5.41 (1H, s) |
| -CH2O- | 58.2 | 4.62 (1H, s) | 67.0 | 4.52 (2H, s) |
| -OCH3 | - | 55.9 | 3.37 (3H, s) | |
| -CHO | 182.6 | 9.56 (1H, s) | - | - |
| Analyte | λ (nm) | Regression (Linear Model) | r | Linear Range (μg/mL) | F-Test | LOD (μg/mL) | |
|---|---|---|---|---|---|---|---|
| Equation | n | ||||||
| HMF | 285 | y = 33981.70x − 15618.30 | 8 | 0.9999 | 1.08−108.0 | 167282.90 | 0.485 |
| Neutrophils | PBMCs | ||||
|---|---|---|---|---|---|
| TNF-α | IL-8 | TNF-α | IL-10 | IL-6 | |
| stimulated control | 0.5−1.8 | 43.9−88.8 | 1.5−5.8 | 1.4−2.8 | 2.3−2.7 |
| unstimulated control | 0.03−0.20 | 10.4−20.2 | 0.06−0.23 | 0.08−0.13 | 0.13−0.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magiera, A.; Czerwińska, M.E.; Owczarek, A.; Marchelak, A.; Granica, S.; Olszewska, M.A. Polyphenols and Maillard Reaction Products in Dried Prunus spinosa Fruits: Quality Aspects and Contribution to Anti-Inflammatory and Antioxidant Activity in Human Immune Cells Ex Vivo. Molecules 2022, 27, 3302. https://doi.org/10.3390/molecules27103302
Magiera A, Czerwińska ME, Owczarek A, Marchelak A, Granica S, Olszewska MA. Polyphenols and Maillard Reaction Products in Dried Prunus spinosa Fruits: Quality Aspects and Contribution to Anti-Inflammatory and Antioxidant Activity in Human Immune Cells Ex Vivo. Molecules. 2022; 27(10):3302. https://doi.org/10.3390/molecules27103302
Chicago/Turabian StyleMagiera, Anna, Monika Ewa Czerwińska, Aleksandra Owczarek, Anna Marchelak, Sebastian Granica, and Monika Anna Olszewska. 2022. "Polyphenols and Maillard Reaction Products in Dried Prunus spinosa Fruits: Quality Aspects and Contribution to Anti-Inflammatory and Antioxidant Activity in Human Immune Cells Ex Vivo" Molecules 27, no. 10: 3302. https://doi.org/10.3390/molecules27103302
APA StyleMagiera, A., Czerwińska, M. E., Owczarek, A., Marchelak, A., Granica, S., & Olszewska, M. A. (2022). Polyphenols and Maillard Reaction Products in Dried Prunus spinosa Fruits: Quality Aspects and Contribution to Anti-Inflammatory and Antioxidant Activity in Human Immune Cells Ex Vivo. Molecules, 27(10), 3302. https://doi.org/10.3390/molecules27103302








