Synthesis, Redox and Spectroscopic Properties of Pterin of Molybdenum Cofactors
Abstract
:1. Introduction
2. Synthesis of Pterin
2.1. Gabriel-Isay Condensation
2.2. Viscontini Reaction
2.3. Timmis Reaction
2.4. Polonovski–Boon Cyclization
2.5. Taylor Method
2.6. Sonogashira Coupling
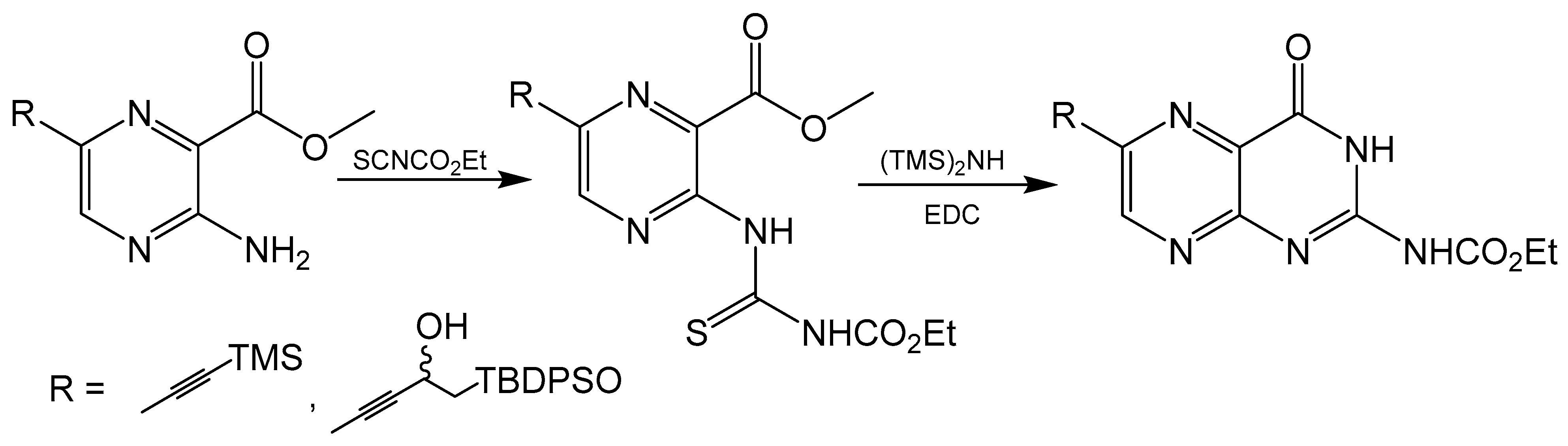
2.7. Aminopyrazine Pathway
3. Discerning between C6 and C7 Substituted Pterins
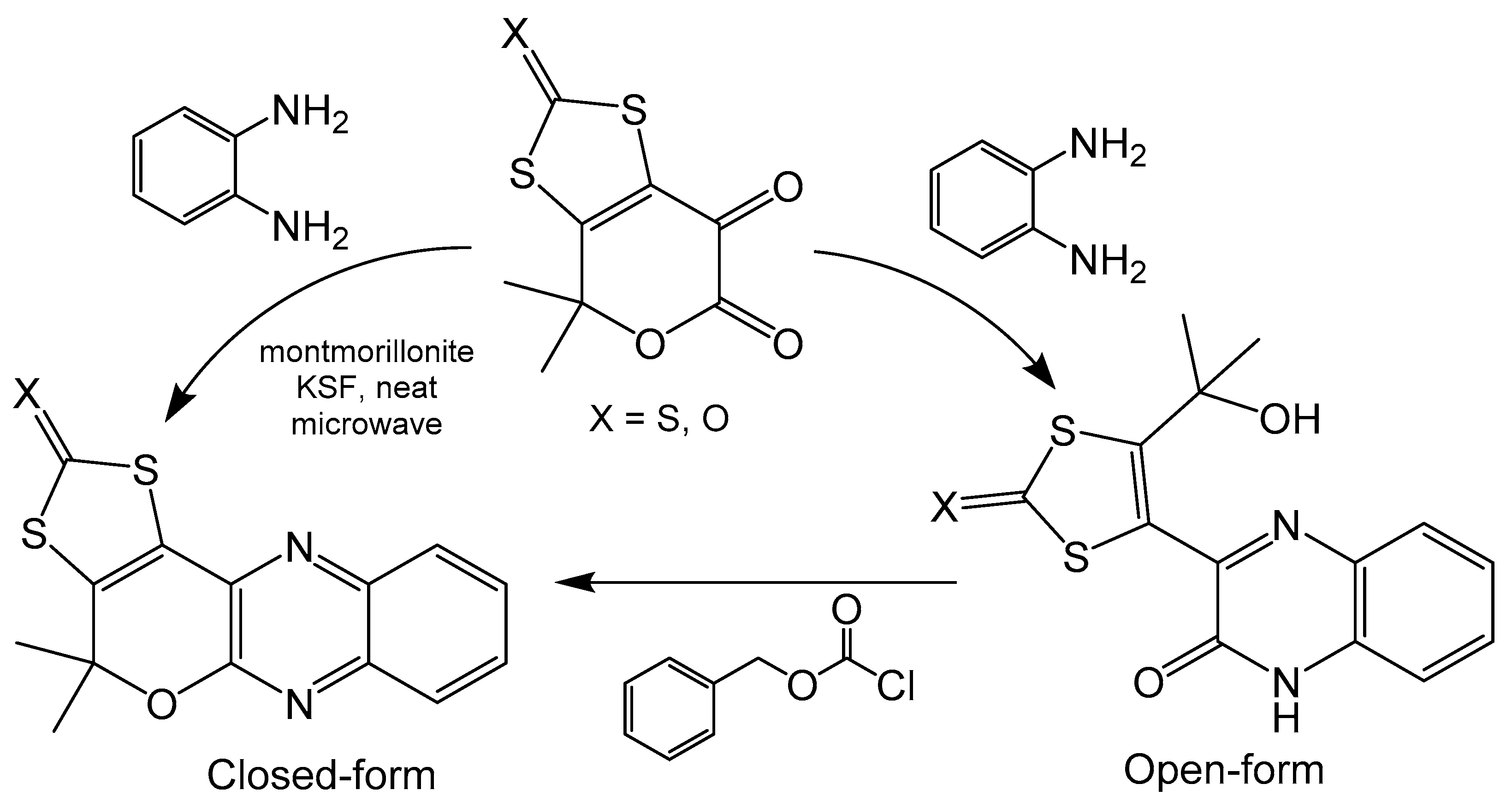
4. Impact of Pyran Cyclization on Redox Chemistry
5. Pterin as Fluorophores
6. Computational Studies
7. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Citation Diversity Statement
References
- Johnson, J.L.; Wuebbens, M.M.; Rajagopalan, K.V. The structure of a molybdopterin precursor. Characterization of a stable, oxidized derivative. J. Biol. Chem. 1989, 264, 13440. [Google Scholar] [CrossRef]
- Hine, F.J.; Taylor, A.J.; Garner, C.D. Dithiolene complexes and the nature of molybdopterin. Coord. Chem. Rev. 2010, 254, 1570–1579. [Google Scholar] [CrossRef]
- Moens, A.L.; Kass, D.A. Therapeutic Potential of Tetrahydrobiopterin for Treating Vascular and Cardiac Disease. J. Cardiovasc. Pharmacol. 2007, 50, 238–246. [Google Scholar] [CrossRef]
- Ishikawa, T. Product class 21: Pteridines and related structures. Sci. Synth. 2004, 16, 1291–1335. [Google Scholar] [CrossRef]
- Daubner, S.C.; Fitzpatrick, P.F. Pteridines. In Encyclopedia of Biological Chemistry; Elsevier: Amsterdam, The Netherlands, 2004; Volume 3, p. 556. [Google Scholar]
- Thony, B.; Auerbach, G.; Blau, N. Tetrahydrobiopterin biosynthesis, regeneration and functions. Biochem. J. 2000, 347, 1–16. [Google Scholar] [CrossRef]
- Kappock, T.J.; Caradonna, J.P. Pterin-Dependent Amino Acid Hydroxylases. Chem. Rev. 1996, 96, 2659–2756. [Google Scholar] [CrossRef] [PubMed]
- Kotsonis, P.; Matter, H. Pteridine-based inhibitors of nitric oxide synthases as therapeutic agents. Recent Res. Dev. Biochem. 2004, 5, 53–80. [Google Scholar]
- Wei, C.-C.; Crane, B.R.; Stuehr, D.J. Tetrahydrobiopterin Radical Enzymology. Chem. Rev. 2003, 103, 2365–2383. [Google Scholar] [CrossRef]
- Andrade, P.; Carneiro, M. Pterin-based pigmentation in animals. Biol. Lett. 2021, 17, 20210221. [Google Scholar] [CrossRef]
- Burgmayer, S.J.N.; Williams, B.R.; Basu, P. Pterin-inspired model compounds of molybdenum enzymes. RSC Metallobiol. 2017, 6, 8–67. [Google Scholar]
- Feirer, N.; Fuqua, C. Pterin function in bacteria. Pteridines 2017, 28, 23–36. [Google Scholar] [CrossRef] [Green Version]
- Goossens, J.-F.; Thuru, X.; Bailly, C. Properties and reactivity of the folic acid and folate photoproduct 6-formylpterin. Free Radical Biol. Med. 2021, 171, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hanaya, T.; Yamamoto, H. Synthesis of biopterin and related pterin glycosides. IUBMB Life 2013, 65, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Leimkuehler, S. The biosynthesis of the molybdenum cofactors in Escherichia coli. Environ. Microbiol. 2020, 22, 2007–2026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendel, R.R. The Molybdenum Cofactor. J. Biol. Chem. 2013, 288, 13165–13172. [Google Scholar] [CrossRef] [Green Version]
- Basu, P.; Burgmayer, S.J.N. Recent developments in the study of molybdoenzyme models. JBIC J. Biol. Inorg. Chem. 2015, 20, 373–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hille, R.; Hall, J.; Basu, P. The Mononuclear Molybdenum Enzymes. Chem. Rev. 2014, 114, 3963–4038. [Google Scholar] [CrossRef] [Green Version]
- Kaufman, S.; Storm, C.B.; Shiman, R. Preparation of 6-substituted pterins via the Isay reaction. J. Org. Chem. 1971, 36, 3925. [Google Scholar] [CrossRef]
- Pimkov, I.V.; Serli-Mitasev, B.; Peterson, A.A.; Ratvasky, S.C.; Hammann, B.; Basu, P. Designing the Molybdopterin Core through Regioselective Coupling of Building Blocks. Chem. Eur. J. 2015, 21, 17057–17072. [Google Scholar] [CrossRef]
- Purrmann, R. Wing pigments of butterflies. X. Synthesis of xanthopterin. Justus Liebigs Ann. Chem. 1940, 546, 98–102. [Google Scholar] [CrossRef]
- Purrmann, R. Wing pigments of butterflies. XII. Constitution and synthesis of the so-called anhydroleucopterin. Justus Liebigs Ann. Chem. 1941, 548, 284. [Google Scholar] [CrossRef]
- Forrest, H.S.; Walker, J. Effect of hydrazine on the condensation of certain α-ketols and related substances with 2,4,5-triamino-6-hydroxypyrimidine. J. Chem. Soc. 1949, 2077–2082. [Google Scholar] [CrossRef]
- Ivery, M.T.G.; Gready, J.E. Isomer distributions and separation of 6- and 7- methyl-8-alkyl pterins. Pteridines 1992, 3, 105. [Google Scholar] [CrossRef]
- Pimkov, I.V.; Peterson, A.A.; Vaccarello, D.N.; Basu, P. A regioselective synthesis of the dephospho ditholene protected molybdopterin. RSC Adv. 2014, 4, 19072–19076. [Google Scholar] [CrossRef] [Green Version]
- Viscontini, M.; Provenzale, R.; Ohlgart, S.; Mallevialle, J. Pterin chemistry. XXXII. Synthesis of natural D-neopterin and L-monapterin. Helv. Chim. Acta 1970, 53, 1202. [Google Scholar] [CrossRef]
- Clinch, K.; Watt, D.K.; Dixon, R.A.; Baars, S.M.; Gainsford, G.J.; Tiwari, A.; Schwarz, G.; Saotome, Y.; Storek, M.; Belaidi, A.A.; et al. Synthesis of Cyclic Pyranopterin Monophosphate, a Biosynthetic Intermediate in the Molybdenum Cofactor Pathway. J. Med. Chem. 2013, 56, 1730–1738. [Google Scholar] [CrossRef]
- Gainsford, G.J.; Clinch, K.; Dixon, R.; Tiwari, A. tert-Butyl (2R,4aR,5aR,11aS,12R,12aR)-8-[bis(tert-butoxycarbonyl)amino]-12-hydroxy-2-methoxy-2,10-dioxo-4,4a,5a,6,9,10,11,11a,12,12a-decahydro-2H-1,3,5-trioxa-6,7,9,11-tetraaza-2λ5-phosphatetracene-6-carboxylate methanol monosolvate monohydrate. Acta Crystallogr. Sect. E Struct. Rep. Online 2012, 68, 02250. [Google Scholar] [CrossRef] [Green Version]
- Timmis, G.M. A new synthesis of pteridines. Nature 1949, 164, 139. [Google Scholar] [CrossRef]
- Sato, N.; Ono, M. Studies on pyrazines. Part 36. A novel synthesis of 6-(2-hydroxyethyl)-1,3-dimethyllumazine. J. Heterocycl. Chem. 2000, 37, 419–420. [Google Scholar] [CrossRef]
- Xu, M.; Vasella, A. A new synthesis of pteridines. Helv. Chim. Acta 2006, 89, 1140–1146. [Google Scholar] [CrossRef]
- Boon, W.R.; Jones, W.G.M.; Ramage, G.R. Pteridines. I. An unambiguous synthesis of 7,8-dihydro-6-hydroxypteridines. J. Chem. Soc. 1951, 96–102. [Google Scholar] [CrossRef]
- Polonovski, M.; Jerome, H. A new method for the synthesis of pteridines. Compt. Rend. 1950, 230, 392. [Google Scholar]
- Archer, M.C.; Vonderschmitt, D.J.; Scrimgeour, K.G. Mechanism of oxidation of tetrahydropterins. Can. J. Biochem. 1972, 50, 1174. [Google Scholar] [CrossRef] [PubMed]
- Milstien, S.; Katusic, Z. Oxidation of Tetrahydrobiopterin by Peroxynitrite: Implications for Vascular Endothelial Function. Biochem. Biophys. Res. Commun. 1999, 263, 681–684. [Google Scholar] [CrossRef]
- Taylor, E.C.; Kobayashi, T. Pteridines. XXXIX. Synthesis of 2,4-diamino-7-alkenylpteridines and their 8-oxides. J. Org. Chem. 1976, 41, 1299. [Google Scholar] [CrossRef]
- Taylor, E.C.; LaMattina, J.L. Pteridines. 40. Some reactions of 2-amino-3-cyano-5-bromomethylpyrazine and 2-amino-3-cyano-5-methylpyrazine. J. Org. Chem. 1977, 42, 1523. [Google Scholar] [CrossRef]
- Taylor, E.C.; Ray, P.S. Pteridines. 51. A new and unequivocal route to C-6 carbon-substituted pterins and pteridines. J. Org. Chem. 1987, 52, 3997–4000. [Google Scholar] [CrossRef]
- Taylor, E.C.; Ray, P.S. Pteridines. 54. A novel synthetic approach to C-6 carbon substituted pterins via intermolecular 1,3-dipolar cycloaddition reactions. J. Org. Chem. 1991, 56, 1812. [Google Scholar] [CrossRef]
- Taylor, E.C.; Ray, P.S.; Darwish, I.S.; Johnson, J.L.; Rajagopalan, K.V. Studies on the molybdenum cofactor. Determination of the structure and absolute configuration of form A. J. Am. Chem. Soc. 1989, 111, 7664. [Google Scholar] [CrossRef]
- Taylor, E.C.; Sabb, A.L. Studies on the molybdenum cofactor: Model synthetic routes directed at Form B. J. Org. Chem. 1988, 53, 5839. [Google Scholar] [CrossRef]
- Chinchilla, R.; Najera, C. The Sonogashira reaction: A booming methodology in synthetic organic chemistry. Chem. Rev. 2007, 107, 874–922. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.C.; Kobylecki, R. Pteridines. 43. A facile synthesis of 6-chloropterin and 2,4-diamino-6-chloropteridine. J. Org. Chem. 1978, 43, 680. [Google Scholar] [CrossRef]
- Burgmayer, S.J.N.; Kim, M.; Petit, R.; Rothkopf, A.; Kim, A.; BelHamdounia, S.; Hou, Y.; Somogyi, A.; Habel-Rodriguez, D.; Williams, A.; et al. Synthesis, characterization, and spectroscopy of model molybdopterin complexes. J. Inorg. Biochem. 2007, 101, 1601–1616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matz, K.G.; Mtei, R.P.; Rothstein, R.; Kirk, M.L.; Burgmayer, S.J.N. Study of Molybdenum(4+) Quinoxalyldithiolenes as Models for the Noninnocent Pyranopterin in the Molybdenum Cofactor. Inorg. Chem. 2011, 50, 9804–9815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, B.R.; Fu, Y.; Yap, G.P.A.; Burgmayer, S.J.N. Structure and Reversible Pyran Formation in Molybdenum Pyranopterin Dithiolene Models of the Molybdenum Cofactor. J. Am. Chem. Soc. 2012, 134, 19584–19587. [Google Scholar] [CrossRef] [Green Version]
- Williams, B.R.; Gisewhite, D.; Kalinsky, A.; Esmail, A.; Burgmayer, S.J.N. Solvent-Dependent Pyranopterin Cyclization in Molybdenum Cofactor Model Complexes. Inorg. Chem. 2015, 54, 8214–8222. [Google Scholar] [CrossRef] [Green Version]
- Pilato, R.S.; Eriksen, K.A.; Greaney, M.A.; Stiefel, E.I.; Goswami, S.; Kilpatrick, L.; Spiro, T.G.; Taylor, E.C.; Rheingold, A.L. Model complexes for molybdopterin-containing enzymes: Preparation and crystallographic characterization of a molybdenum ene-1-perthiolate-2-thiolate (trithiolate) complex. J. Am. Chem. Soc. 1991, 113, 9372. [Google Scholar] [CrossRef]
- O’Connor, B.R.; Jones, F.N. Reactions of ethylene di- and trithiocarbonates with acetylenes. Anomalous reaction with bromocyanoacetylene to give a thioacyl bromide. J. Org. Chem. 1970, 35, 2002. [Google Scholar] [CrossRef]
- Klewe, A.; Kruse, T.; Lindel, T. Aminopyrazine Pathway to the Moco Metabolite Dephospho Form A. Chem. Eur. J. 2017, 23, 11230–11233. [Google Scholar] [CrossRef]
- Koren, B.; Stanovnik, B.; Tisler, M. Transformations of 1,2,4-thiadiazolo[2,3-x]azines. Heterocycles 1987, 26, 689. [Google Scholar]
- Chowdhury, A.Z.M.S.; Shibata, Y.; Morita, M.; Kaya, K.; Hiratani, K. Synthesis of new heterocondensed pteridines. J. Heterocycl. Chem. 2001, 38, 1173–1177. [Google Scholar] [CrossRef]
- Basu, P.; Burgmayer, S.J.N. Pterin chemistry and its relationship to the molybdenum cofactor. Coord. Chem. Rev. 2011, 255, 1016–1038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gisewhite, D.R.; Yang, J.; Williams, B.R.; Esmail, A.; Stein, B.; Kirk, M.L.; Burgmayer, S.J.N. Implications of Pyran Cyclization and Pterin Conformation on Oxidized Forms of the Molybdenum Cofactor. J. Am. Chem. Soc. 2018, 140, 12808–12818. [Google Scholar] [CrossRef]
- Gisewhite, D.R.; Nagelski, A.L.; Cummins, D.C.; Yap, G.P.A.; Burgmayer, S.J.N. Modeling Pyran Formation in the Molybdenum Cofactor: Protonation of Quinoxalyl-Dithiolene Promoting Pyran Cyclization. Inorg. Chem. 2019, 58, 5134–5144. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hammoudeh, D.; Lin, W.-W.; Das, S.; Yun, M.-K.; Li, Z.-M.; Griffith, E.; Chen, T.-S.; White, S.W.; Lee, R.E. Development of a Pterin-Based Fluorescent Probe for Screening Dihydropteroate Synthase. Bioconjugate Chem. 2011, 22, 2110–2117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goswami, S.; Das, M.K.; Manna, A. Pterin-based highly selective, ratiometric, and sensitive ‘naked-eye’ sensor for acetate. Tetrahedron Lett. 2014, 55, 2707–2710. [Google Scholar] [CrossRef]
- Grochocki, W.; Buszewska-Forajta, M.; Macioszek, S.; Markuszewski, M.J. Determination of urinary pterins by capillary electrophoresis coupled with LED-induced fluorescence detector. Molecules 2019, 24, 1166. [Google Scholar] [CrossRef] [Green Version]
- Miyako, K.; Yasuno, Y.; Shinada, T.; Fujita, M.J.; Sakai, R. Diverse Aromatic Metabolites in the Solitary Tunicate Cnemidocarpa irene. J. Nat. Prod. 2020, 83, 3156–3165. [Google Scholar] [CrossRef]
- Goswami, S.; Das, M.K.; Maity, A.C.; Quah, C.K.; Fun, H.-K. Synthesis of 7-methyl-6-indolopterin and 7-methyl-6-indoloquinoxaline. Synth. Commun. 2016, 46, 1529–1536. [Google Scholar] [CrossRef]
- Reibnegger, G. An ab initio and density functional theory study on neutral pterin radicals. Pteridines 2015, 26, 135–142. [Google Scholar] [CrossRef]
- Hoffbrand, A.V.; Weir, D.G. The history of folic acid. Br. J. Haematol. 2001, 113, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Koren, D.; Orban, C.; Gallo, N.; Kun, S.; Vecseri-Hegyes, B.; Kun-Farkas, G. Folic acid content and antioxidant activity of different types of beers available in Hungarian retail. J. Food Sci. Technol. 2017, 54, 1158–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, R.; Adhikari, S.; Patro, B.S.; Chattopadhyay, S.; Mukherjee, T. Free radical scavenging behavior of folic acid: Evidence for possible antioxidant activity. Free Radical Biol. Med. 2001, 30, 1390–1399. [Google Scholar] [CrossRef]
- Yamabe, S.; Tsuchida, N.; Yamazaki, S. How Is the Oxidation Related to the Tautomerization in Vitamin B9? J. Phys. Chem. A 2021, 125, 9346–9354. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.H.; Lorente, C.; Capparelli, A.L.; Pokhrel, M.R.; Braun, A.M.; Oliveros, E. Fluorescence of pterin, 6-formylpterin, 6-carboxypterin and folic acid in aqueous solution: pH effects. Photochem. Photobiol. Sci. 2002, 1, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Ledbeter, J.W., Jr.; Pfleiderer, W.; Freisheim, J.H. Photosensitized reduction of L-biopterin in the active ternary complex of dihydrofolate reductase. Photochem. Photobiol. 1995, 62, 71–81. [Google Scholar] [CrossRef]
- Oliveros, E.; Dantola, M.L.; Vignoni, M.; Thomas, A.H.; Lorente, C. Production and quenching of reactive oxygen species by pterin derivatives, an intriguing class of biomolecules. Pure Appl. Chem. 2011, 83, 801–811. [Google Scholar] [CrossRef] [Green Version]
- Thomas, A.H.; Lorente, C.; Capparelli, A.L.; Martinez, C.G.; Braun, A.M.; Oliveros, E. Singlet oxygen (1Δg) production by pterin derivatives in aqueous solutions. Photochem. Photobiol. Sci. 2003, 2, 245–250. [Google Scholar] [CrossRef]
- Buglak, A.A.; Telegina, T.A.; Vorotelyak, E.A.; Kononov, A.I. Theoretical study of photoreactions between oxidized pterins and molecular oxygen. J. Photochem. Photobiol. A 2019, 372, 254–259. [Google Scholar] [CrossRef]
- Lorente, C.; Capparelli, A.L.; Thomas, A.H.; Braun, A.M.; Oliveros, E. Quenching of the fluorescence of pterin derivatives by anions. Photochem. Photobiol. Sci. 2004, 3, 167–173. [Google Scholar] [CrossRef]
- Liu, L.; Yang, D.; Li, P. pH-Related and Site-Specific Excited-State Proton Transfer from Pterin to Acetate. J. Phys. Chem. B 2014, 118, 11707–11714. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, J.; Zurn, P.; Bassett, D.S. (In)citing Action to Realize an Equitable Future. Neuron 2020, 106, 890–894. [Google Scholar] [CrossRef] [PubMed]



















Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colston, K.J.; Basu, P. Synthesis, Redox and Spectroscopic Properties of Pterin of Molybdenum Cofactors. Molecules 2022, 27, 3324. https://doi.org/10.3390/molecules27103324
Colston KJ, Basu P. Synthesis, Redox and Spectroscopic Properties of Pterin of Molybdenum Cofactors. Molecules. 2022; 27(10):3324. https://doi.org/10.3390/molecules27103324
Chicago/Turabian StyleColston, Kyle J., and Partha Basu. 2022. "Synthesis, Redox and Spectroscopic Properties of Pterin of Molybdenum Cofactors" Molecules 27, no. 10: 3324. https://doi.org/10.3390/molecules27103324
APA StyleColston, K. J., & Basu, P. (2022). Synthesis, Redox and Spectroscopic Properties of Pterin of Molybdenum Cofactors. Molecules, 27(10), 3324. https://doi.org/10.3390/molecules27103324





