Materials Selection for Antifouling Systems in Marine Structures
Abstract
1. Introduction
2. Antifouling
2.1. Biocide Antifouling Systems
2.2. Biomimicry and Natural Components
3. Delivery Mechanisms and Systems
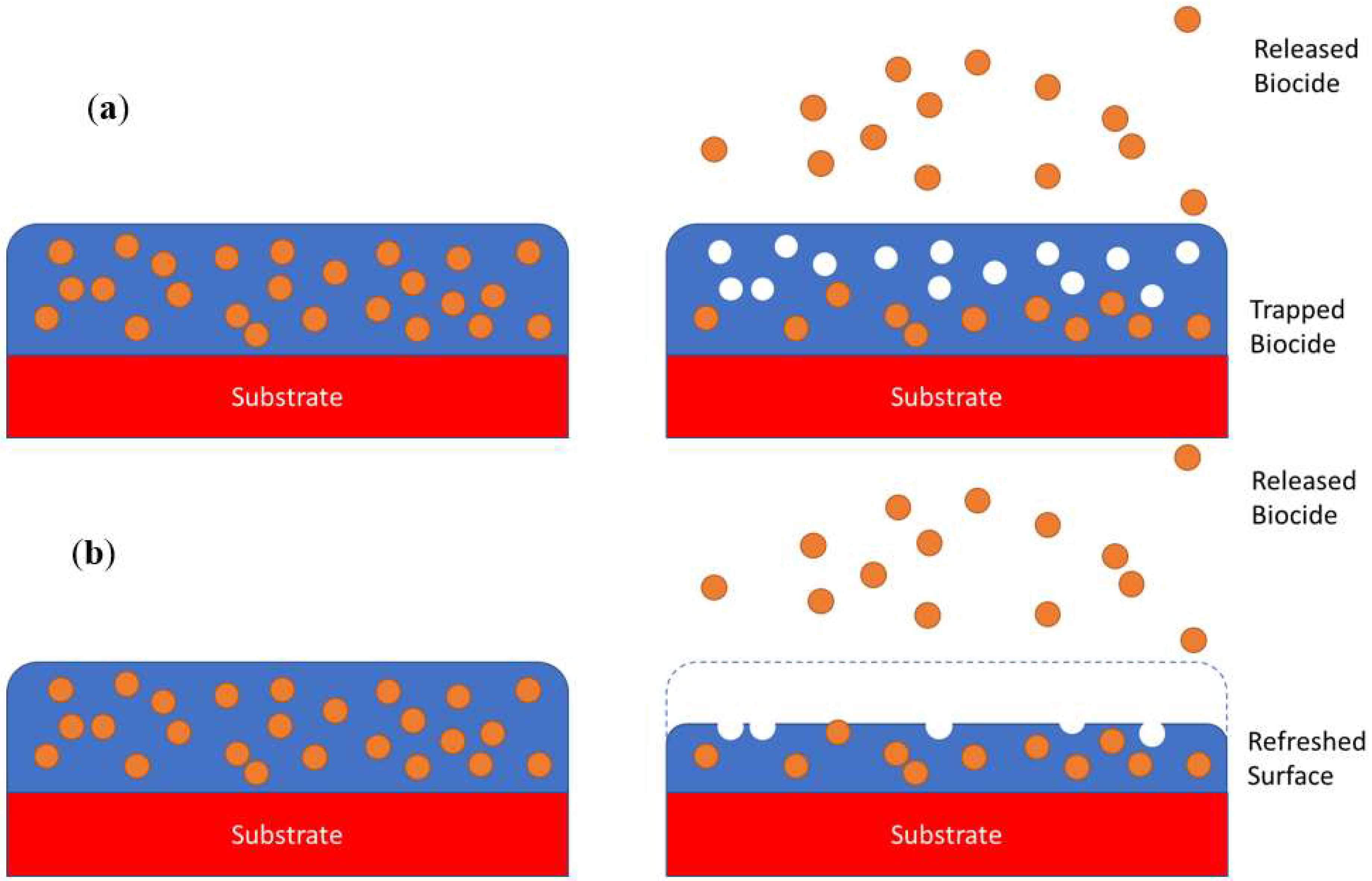
3.1. Degradation of Antifouling Matrix/Coating
3.2. Metal Organic Frameworks
3.3. Foul Release/Resistant Systems
3.4. Superhydrophobic
3.5. Hydrophilic
3.6. Amphiphilic
3.7. Micro-Texture
3.8. Hybrid Antifouling Systems
3.9. Active Cleaning Systems
4. Marine Sensors
5. Summary and Prospective Work
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fusetani, N. Biofouling and antifouling. Nat. Prod. Rep. 2004, 21, 94. [Google Scholar] [CrossRef] [PubMed]
- Schultz, M.P.; Bendick, J.A.; Holm, E.R.; Hertel, W.M. Economic impact of biofouling on a naval surface ship. Biofouling 2011, 27, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Hulme, P.E. Trade, transport and trouble: Managing invasive species pathways in an era of globalization. J. Appl. Ecol. 2009, 46, 10–18. [Google Scholar] [CrossRef]
- Murray, C.C.; Pakhomov, E.A.; Therriault, T.W. Recreational boating: A large unregulated vector transporting marine invasive species. Divers. Distrib. 2011, 17, 1161–1172. [Google Scholar] [CrossRef]
- Wahl, M. Marine epibiosis. I. Fouling and antifouling: Some basic aspects. Mar. Ecol. Prog. Ser. 1989, 58, 175–189. [Google Scholar] [CrossRef]
- Abarzua, S.; Jakubowski, S. Biotechnological investigation for the prevention of biofouling. 1. Biological and biochemical principles for the prevention of biofouling. Mar. Ecol. Prog. Ser. 1995, 123, 301–312. [Google Scholar] [CrossRef]
- Chambers, L.D.; Stokes, K.R.; Walsh, F.C.; Wood, R.J.K. Modern approaches to marine antifouling coatings. Surf. Coat. Technol. 2006, 201, 3642–3652. [Google Scholar] [CrossRef]
- Jin, H.; Tian, L.; Bing, W.; Zhao, J.; Ren, L. Bioinspired marine antifouling coatings: Status, prospects, and future. Prog. Mater. Sci. 2022, 124, 100889. [Google Scholar] [CrossRef]
- Müller, W.E.; Wang, X.; Proksch, P.; Perry, C.C.; Osinga, R.; Gardères, J.; Schröder, H.C. Principles of biofouling protection in marine sponges: A model for the design of novel biomimetic and bio-inspired coatings in the marine environment? Mar. Biotechnol. 2013, 15, 375–398. [Google Scholar] [CrossRef]
- Callow, J.A.; Callow, M.E. Trends in the development of environmentally friendly fouling-resistant marine coatings. Nat. Commun. 2011, 2, 244. [Google Scholar] [CrossRef]
- Callow, M.E.; Callow, J.A.; Pickett-Heaps, J.D.; Wetherbee, R. Primary adhesion of Enteromorpha (Chlorophyta, Ulvales) propagules: Quantitative settlement studies and video microscopy. Oceanogr. Lit. Rev. 1998, 45, 1195. [Google Scholar] [CrossRef]
- Roberts, D.; Rittschof, D.; Holm, E.; Schmidt, A.R. Factors influencing initial larval settlement: Temporal, spatial and surface molecular components. J. Exp. Mar. Biol. Ecol. 1991, 150, 203–221. [Google Scholar] [CrossRef]
- Yebra, D.M.; Kiil, S.; Dam-Johansen, K. Antifouling technology-Past, present and future steps towards efficient and environmentally friendly antifouling coatings. Prog. Org. Coat. 2004, 50, 75–104. [Google Scholar] [CrossRef]
- Readman, J.W. Development, occurrence and regulation of antifouling paint biocides: Historical review and future trends. In Handbook of Environmental Chemistry, Volume 5: Water Pollution; Springer: Berlin, Germany, 2006; pp. 1–15. [Google Scholar]
- Lunn, I. Antifouling: A Brief Introduction to the Origins and Developments of the Marine Antifouling Industry; BCA Publications: Thames, UK, 1974. [Google Scholar]
- Crisp, D.J. The role of the biologist in antifouling research. In Proceedings of the Third International Congress an Marine Corrosion and Fouling, Gaithersburg, MD, USA, 2–6 October 1972; pp. 88–93. [Google Scholar]
- Almeida, E.; Diamantino, T.C.; de Sousa, O. Marine paints: The particular case of antifouling paints. Prog. Org. Coat. 2007, 59, 2–20. [Google Scholar] [CrossRef]
- Ali, A.; Jamil, M.I.; Jiang, J.; Shoaib, M.; Amin, B.U.; Luo, S.; Zhan, X.; Chen, F.; Zhang, Q. An overview of controlled-biocide-release coating based on polymer resin for marine antifouling applications. J. Polym. Res. 2020, 27, 85. [Google Scholar] [CrossRef]
- Dafforn, K.A.; Lewis, J.A.; Johnston, E.L. Antifouling strategies: History and regulation, ecological impacts and mitigation. Mar. Pollut. Bull. 2011, 62, 453–465. [Google Scholar] [CrossRef]
- Finnie, A.A.; Williams, D.N. Paint and coatings technology for the control of marine fouling. In Biofouling; Drr, S., Thomason, J.C., Eds.; Wiley-Blackwell: London, UK, 2009; pp. 185–206. [Google Scholar]
- Kotrikla, A. Environmental management aspects for TBT antifouling wastes from the shipyards. J. Environ. Manag. 2009, 90, S77–S85. [Google Scholar] [CrossRef]
- Avelelas, F.; Martins, R.; Oliveira, T.; Maia, F.; Malheiro, E.; Soares, A.; Loureiro, S.; Tedim, J. Efficacy and ecotoxicity of novel anti-fouling nanomaterials in target and non-target marine species. Mar. Biotechnol. 2017, 19, 164–174. [Google Scholar] [CrossRef]
- Anyaogu, K.C.; Fedorov, A.V.; Neckers, D.C. Synthesis, characterization, and antifouling potential of functionalized copper nanoparticles. Langmuir 2008, 24, 4340–4346. [Google Scholar] [CrossRef]
- Ren, G.; Hu, D.; Cheng, E.W.C.; Vargas-Reus, M.A.; Reip, P.; Allaker, R.P. Characterisation of copper oxide nanoparticles for antimicrobial applications. Int. J. Antimicrob. Agents 2009, 33, 587–590. [Google Scholar] [CrossRef]
- Chang, Y.-N.; Zhang, M.; Xia, L.; Zhang, J.; Xing, G. The toxic effects and mechanisms of CuO and ZnO nanoparticles. Materials 2012, 5, 2850–2871. [Google Scholar] [CrossRef]
- Ingle, A.P.; Duran, N.; Rai, M. Bioactivity, mechanism of action, and cytotoxicity of copper-based nanoparticles: A review. Appl. Microbiol. Biotechnol. 2014, 98, 1001–1009. [Google Scholar] [CrossRef]
- Chapman, J.; Le Nor, L.; Brown, R.; Kitteringham, E.; Russell, S.; Sullivan, T.; Regan, F. Antifouling performances of macro- to micro- to nano-copper materials for the inhibition of biofouling in its early stages. J. Mater. Chem. B 2013, 1, 6194–6200. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Gong, Y.; Suo, X.; Li, H. Liquid flame spray fabrication of polyimide-copper coatings for antifouling applications. Mater. Lett. 2017, 190, 217–220. [Google Scholar] [CrossRef]
- Jia, Z.; Liu, Y.; Wang, Y.; Gong, Y.; Jin, P.; Suo, X.; Li, H. Flame spray fabrication of polyethylene-Cu composite coatings with enwrapped structures: A new route for constructing antifouling layers. Surf. Coat. Technol. 2017, 309, 872–879. [Google Scholar] [CrossRef]
- Elmas, S.; Skipper, K.; Salehifar, N.; Jamieson, T.; Andersson, G.G.; Nydén, M.; Leterme, S.C.; Andersson, M.R. Cyclic copper uptake and release from natural seawater: A fully sustainable antifouling technique to prevent marine growth. Environ. Sci. Technol. 2021, 55, 757–766. [Google Scholar] [CrossRef]
- Soon, Z.Y.; Jung, J.H.; Jang, M.; Kang, J.H.; Jang, M.C.; Lee, J.S.; Kim, M. Zinc pyrithione (ZnPT) as an antifouling biocide in the marine environment-a literature review of its toxicity, environmental fates, and analytical methods. Water. Air. Soil Pollut. 2019, 230, 1–18. [Google Scholar] [CrossRef]
- Pasquet, J.; Chevalier, Y.; Pelletier, J.; Couval, E.; Bouvier, D.; Bolzinger, M.A. The contribution of zinc ions to the antimicrobial activity of zinc oxide. Colloids Surf. A Physicochem. Eng. Asp. 2014, 457, 263–274. [Google Scholar] [CrossRef]
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef]
- Hollinger, M.A. Toxicological aspects of topical silver pharmaceuticals. Crit. Rev. Toxicol. 1996, 26, 255–260. [Google Scholar] [CrossRef]
- Fusetani, N. Antifouling marine natural products. Nat. Prod. Rep. 2011, 28, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Qian, P.-Y.; Li, Z.; Xu, Y.; Li, Y.; Fusetani, N. Mini-review: Marine natural products and their synthetic analogs as antifouling compounds: 2009–2014. Biofouling 2015, 31, 101–122. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.L.; Wu, C.H.; Qian, P.Y. Marine natural products as antifouling molecules–a mini-review (2014–2020). Biofouling 2020, 36, 1210–1226. [Google Scholar] [CrossRef] [PubMed]
- Qian, P.Y.; Xu, Y.; Fusetani, N. Natural products as antifouling compounds: Recent progress and future perspectives. Biofouling 2010, 26, 223–234. [Google Scholar] [CrossRef]
- Wang, K.L.; Wu, Z.H.; Wang, Y.; Wang, C.Y.; Xu, Y. Mini-review: Antifouling natural products from marine microorganisms and their synthetic analogs. Mar. Drugs 2017, 15, 266. [Google Scholar] [CrossRef]
- Chapman, J.; Hellio, C.; Sullivan, T.; Brown, R.; Russell, S.; Kitteringham, E.; Le Nor, L.; Regan, F. Bioinspired synthetic macroalgae: Examples from nature for antifouling applications. Int. Biodeterior. Biodegrad. 2014, 86, 6–13. [Google Scholar] [CrossRef]
- Sullivan, T.; O’Callaghan, I. Recent developments in biomimetic antifouling materials: A review. Biomimetics 2020, 4, 58. [Google Scholar] [CrossRef]
- ABC 3. Available online: https://www.ppgpmc.com/products/ABC-3 (accessed on 31 March 2022).
- Antifouling SeaGuardian. Available online: https://www.jotun.com/ww-en/industries/products/antifouling-seaguardian/ (accessed on 31 March 2022).
- Hempel’s Antifouling Olympic+72900-72900-Hempel. Available online: https://www.hempel.com/products/hempels-antifouling-olympic-72900-72900 (accessed on 31 March 2022).
- Interspeed 6400. Available online: https://www.international-marine.com/product/interspeed-6400 (accessed on 31 March 2022).
- Hempel’s Antifouling Basic-71950-Hempel. Available online: https://www.hempel.com/products/hempels-antifouling-basic-71950 (accessed on 31 March 2022).
- Intersmooth 7460HS SPC. Available online: https://www.international-marine.com/product/intersmooth-7460hs-spc (accessed on 31 March 2022).
- Ashter, S.A. Mechanisms of polymer degradation. In Introduction to Bioplastics Engineering; Elsevier: Amsterdam, The Netherlands, 2016; pp. 31–59. [Google Scholar]
- “Hydrolysis”. Available online: https://polymerdatabase.com/polymer%20chemistry/Hydrolysis.html (accessed on 18 May 2020).
- Ma, C.; Xu, L.; Xu, W.; Zhang, G. Degradable polyurethane for marine anti-biofouling. J. Mater. Chem. B 2013, 1, 3099. [Google Scholar] [CrossRef]
- Yao, J.; Dai, Z.; Yi, J.; Yu, H.; Wu, B.; Dai, L. Degradable polyurethane based on triblock polyols composed of polypropylene glycol and ε-caprolactone for marine antifouling applications. J. Coat. Technol. Res. 2020, 17, 865–874. [Google Scholar] [CrossRef]
- Chen, R.; Li, Y.; Tang, L.; Yang, H.; Lu, Z.; Wang, J.; Liu, L.; Takahashi, K. Synthesis of zinc-based acrylate copolymers and their marine antifouling application. RSC Adv. 2017, 7, 40020–40027. [Google Scholar] [CrossRef]
- Sancet, M.P.A.; Hanke, M.; Wang, Z.; Bauer, S.; Azucena, C.; Arslan, H.K.; Heinle, M.; Gliemann, H.; Wöll, C.; Rosenhahn, A. Surface anchored metal-organic frameworks as stimulus responsive antifouling coatings. Biointerphases 2013, 8, 29. [Google Scholar] [CrossRef]
- Firouzjaei, M.D.; Shamsabadi, A.A.; Aktij, S.A.; Seyedpour, S.F.; Sharifian, G.M.; Rahimpour, A.; Esfahani, M.R.; Ulbricht, M.; Soroush, M. Exploiting synergetic effects of graphene oxide and a silver-based metal-organic framework to enhance antifouling and anti-biofouling properties of thin-film nanocomposite membranes. ACS Appl. Mater. Interfaces 2018, 10, 42967–42978. [Google Scholar] [CrossRef]
- Hunter, R.J. Zeta Potential in Colloid Science: Principles and Applications, 2nd ed.; Academic Press: London, UK, 1981. [Google Scholar]
- Clogston, J.D.; Patri, A.K. Zeta potential measurement. Methods Mol. Biol. 2011, 697, 63–70. [Google Scholar]
- Zhou, Z.; Calabrese, D.R.; Taylor, W.; Finlay, J.A.; Callow, M.E.; Callow, J.A.; Fischer, D.; Kramer, E.J.; Ober, C.K. Amphiphilic triblock copolymers with PEGylated hydrocarbon structures as environmentally friendly marine antifouling and fouling-release coatings. Biofouling 2014, 30, 589–604. [Google Scholar] [CrossRef]
- Maan, A.M.C.; Hofman, A.H.; de Vos, W.M.; Kamperman, M. Recent developments and practical feasibility of polymer-based antifouling coatings. Adv. Funct. Mater. 2020, 30, 2000936. [Google Scholar] [CrossRef]
- Baier, R.E. The role of surface energy in thrombogenesis. Bull. N. Y. Acad. Med. 1972, 48, 257–272. [Google Scholar]
- Vesco, S.; Aversa, C.; Puopolo, M.; Barletta, M. Advances in design and manufacturing of environmentally friendly and biocide-free antifouling/foul-release coatings: Replacement of fluorinate species. J. Coat. Technol. Res. 2019, 16, 3. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Ren, B.; Zhang, D.; Xie, S.; Chang, Y.; Yang, J.; Wu, J.; Xu, L.; Zheng, J. Fundamentals and applications of zwitterionic antifouling polymers. J. Phys. D Appl. Phys. 2019, 52, 403001. [Google Scholar] [CrossRef]
- Schlenoff, J.B. Zwitteration: Coating surfaces with zwitterionic functionality to reduce nonspecific adsorption. Langmuir 2014, 30, 9625–9636. [Google Scholar] [CrossRef]
- Shao, Q.; Jiang, S. Effect of carbon spacer length on zwitterionic carboxybetaines. J. Phys. Chem. B. 2013, 117, 1357–1366. [Google Scholar] [CrossRef]
- Chen, S.; Shi, J.; Zhao, Y.; Wang, W.; Liao, H.; Liu, G. Rapid fabrication of zwitterionic coating on 316L stainless steel surface for marine biofouling resistance. Prog. Org. Coat. 2021, 161, 106552. [Google Scholar] [CrossRef]
- Syuart, M.I.A.N. Method of Preparing a Zwitterionic Copolymer. European Patent EP 3 172 253 B1, 2017. [Google Scholar]
- Van Zoelen, W.; Buss, H.G.; Ellebracht, N.C.; Lynd, N.A.; Fischer, D.A.; Finlay, J.; Hill, S.; Callow, M.E.; Callow, J.A.; Kramer, E.J.; et al. Sequence of hydrophobic and hydrophilic residues in amphiphilic polymer coatings affects surface structure and marine antifouling/fouling release properties. ACS Macro Lett. 2014, 3, 364–368. [Google Scholar] [CrossRef]
- Guo, H.; Chen, P.; Tian, S.; Ma, Y.; Li, Q.; Wen, C.; Yang, J.; Zhang, L. Amphiphilic marine antifouling coatings based on a hydrophilic polyvinylpyrrolidone and hydrophobic fluorine-silicon-containing block copolymer. Langmuir 2020, 36, 14573–14581. [Google Scholar] [CrossRef]
- Koschitzki, F.; Wanka, R.; Sobota, L.; Gardner, H.; Hunsucker, K.Z.; Swain, G.W.; Rosenhahn, A. Amphiphilic zwitterionic acrylate/methacrylate copolymers for marine fouling-release coatings. Langmuir 2021, 37, 5591–5600. [Google Scholar] [CrossRef]
- Intersleek 1100SR. Available online: https://www.international-marine.com/product/intersleek-1100sr (accessed on 31 March 2022).
- Scardino, A.J.; Guenther, J.; de Nys, R. Attachment point theory revisited: The fouling response to a microtextured matrix. Biofouling 2008, 24, 45–53. [Google Scholar] [CrossRef]
- Scardino, A.J.; de Nys, R. Mini review: Biomimetic models and bioinspired surfaces for fouling control. Biofouling 2011, 27, 73–86. [Google Scholar] [CrossRef]
- Scardino, A.J.; Harvey, E.; de Nys, R. Testing attachment point theory: Diatom attachment on microtextured polyimide biomimics. Biofouling 2006, 22, 55–60. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhou, X.; Xing, Z.; Tu, T. Fabrication of a superhydrophobic surface with underwater air-retaining properties by electrostatic flocking. RSC Adv. 2018, 8, 10719–10726. [Google Scholar] [CrossRef]
- Greco, G.; Lanero, T.S.; Torrassa, S.; Young, R.; Vassalli, M.; Cavaliere, A.; Rolandi, R.; Pelucchi, E.; Faimali, M.; Davenport, J. Microtopography of the eye surface of the crab Carcinus maenas: An atomic force microscope study suggesting a possible antifouling potential. J. R. Soc. Interface 2013, 10, 84. [Google Scholar] [CrossRef][Green Version]
- Akuzov, D.; Brümmer, F.; Vladkova, T. Some possibilities to reduce the biofilm formation on transparent siloxane coatings. Colloids Surf. B Biointerfaces 2013, 104, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Guo, H.; Zhao, W.; Zhang, L. Environmentally friendly marine antifouling coating based on a synergistic strategy. Langmuir 2020, 36, 2396–2402. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.E.; Maillard, J.-Y.; Russell, A.D.; Catrenich, C.E.; Charbonneau, D.L.; Bartolo, R.G. Activity and mechanisms of action of selected biocidal agents on Gram-positive and -negative bacteria. J. Appl. Microbiol. 2003, 94, 240–247. [Google Scholar] [CrossRef]
- Sigma Sailadvance DX II. Available online: https://www.ppgpmc.com/products/SIGMA-SAILADVANCE-DX-II (accessed on 31 March 2022).
- SeaQuantum Pro U. Available online: https://www.jotun.com/ww-en/industries/products/seaquantum-pro-u/ (accessed on 31 March 2022).
- Hempel’s Antifouling Dynamic 8000 79450-79450-Hempel. Available online: https://www.hempel.com/products/hempels-antifouling-dynamic-8000-79450-79450 (accessed on 31 March 2022).
- Intersmooth 7465Si SPC. Available online: https://www.international-marine.com/product/intersmooth-7465si-spc (accessed on 31 March 2022).
- Hempaguard X8 89940-89940-Hempel. Available online: https://www.hempel.com/products/hempaguard-x8-89940-89940 (accessed on 31 March 2022).
- Intercept 8500 LPP. Available online: https://www.international-marine.com/product/intercept-8500-lpp (accessed on 31 March 2022).
- Song, C.; Cui, W. Review of underwater ship hull cleaning technologies. J. Mar. Sci. Appl. 2020, 19, 415–429. [Google Scholar] [CrossRef]
- Pivetta, R.; Lammas, A.; Sammut, K. 3D adaptive coverage planning for confined space inspection robots. In Technology and Science for the Ships of the Future; IOS Press: Amsterdam, The Netherlands, 2018; pp. 496–503. [Google Scholar]
- McQuillan, J.; Hopper, D.; Magiopoulos, I.; Arundell, M.; Brown, R.; Shorter, S.; Mowlem, M.; Pascal, R.; Connelly, D. Buzz off! An evaluation of ultrasonic acoustic vibration for the disruption of marine micro-organisms on sensor-housing materials. Lett. Appl. Microbiol. 2016, 63, 393–399. [Google Scholar] [CrossRef]
- Guo, S.; Lee, H.P.; Khoo, B.C. Inhibitory effect of ultrasound on barnacle (Amphibalanus amphitrite) cyprid settlement. J. Exp. Mar. Biol. Ecol. 2011, 409, 253–258. [Google Scholar] [CrossRef]
- Guo, S.F.; Lee, H.P.; Chaw, K.C.; Miklas, J.; Teo, S.L.M.; Dickinson, G.H.; Birch, W.R.; Khoo, B.C. Effect of ultrasound on cyprids and juvenile barnacles. Biofouling. 2011, 27, 185–192. [Google Scholar] [CrossRef]
- Dular, M.; Bachert, B.; Stoffel, B.; Širok, B. Relationship between cavitation structures and cavitation damage. Wear 2004, 257, 1176–1184. [Google Scholar] [CrossRef]
- Park, J.-S.; Lee, J.-H. Sea-trial verification of ultrasonic antifouling control. Biofouling 2018, 34, 98–110. [Google Scholar] [CrossRef]
- Naval Sea Systems Command, Waterborne underwater hull cleaning of navy ships. In Naval Ships’ Technical Manual, Rev. 5; Department of the Navy, Naval Sea Systems Command: Washington, DC, USA, 2006; pp. 1–18.
- Legg, M.; Yücel, M.K.; de Carellan, I.G.; Kappatos, V.; Selcuk, C.; Gan, T.H. Acoustic methods for biofouling control: A review. Ocean Eng. 2015, 103, 237–247. [Google Scholar] [CrossRef]
- Hunsucker, K.Z.; Braga, C.; Gardner, H.; Jongerius, M.; Hietbrink, R.; Salters, B.; Swain, G. Using ultraviolet light for improved antifouling performance on ship hull coatings. Biofouling 2019, 35, 658–668. [Google Scholar] [CrossRef]
- Ryan, E.; Turkmen, S.; Benson, S. An investigation into the application and practical use of (UV) ultraviolet light technology for marine antifouling. Ocean Eng. 2020, 216, 107690. [Google Scholar] [CrossRef]
- Delgado, A.; Briciu-Burghina, C.; Regan, F. Antifouling strategies for sensors used in water monitoring: Review and future perspectives. Sensors 2021, 21, 389. [Google Scholar] [CrossRef]
- Alliance for Coastal Technologies. Biofouling Prevention Technologies for Coastal Sensors/Sensor Platforms; University of Maryland Center of Environmental Science: Cambridge, MD, USA, 2003. [Google Scholar]
- Fitzgerald, J.W.; Davis, M.E.; Hurdle, B.G. Some acoustic properties of marine fouling. J. Acoust. Soc. Am. 1947, 19, 332–337. [Google Scholar] [CrossRef]
- Heupel, M.R.; Reiss, K.L.; Yeiser, B.G.; Simpfendorfer, C.A. Effects of biofouling on performance of moored data logging acoustic receivers. Limnol. Oceanogr. Methods 2008, 6, 327–335. [Google Scholar] [CrossRef]
- Deshpande, P.; Venugopalan, P.; Teo, S.L.M. Effect of biofouling on acoustic signals. In Proceedings of the Institute of Marine Engineering, Science and Technology. Part B, Journal of Marine Design and Operations; Institute of Marine Engineering, Science and Technology: London, UK, 2007; Volume 12, pp. 17–23. [Google Scholar]
- Piola, R.; Leary, M.; Santander, R.; Shimeta, J. Antifouling performance of copper-containing fused filament fabrication (FFF) 3-D printing polymer filaments for marine applications. Biofouling 2021, 37, 206–221. [Google Scholar] [CrossRef]
- Nabi, G.; McLaughlin, R.W.; Hao, Y.; Wang, K.; Zeng, X.; Khan, S.; Wang, D. The possible effects of anthropogenic acoustic pollution on marine mammals’ reproduction: An emerging threat to animal extinction. Environ. Sci. Pollut. Res. 2018, 25, 19338–19345. [Google Scholar] [CrossRef]
- King, P.C.; Poole, A.J.; Horne, S.; de Nys, R.; Gulizia, S.; Jahedi, M.Z. Embedment of copper particles into polymers by cold spray. Surf. Coat. Technol. 2013, 216, 60–67. [Google Scholar] [CrossRef]
- Vucko, M.J.; King, P.C.; Poole, A.J.; Jahedi, M.Z.; de Nys, R. Polyurethane seismic streamer skins: An application of cold spray metal embedment. Biofouling 2013, 29, 1–9. [Google Scholar] [CrossRef]
- Casalino, G.; Caccia, M.; Caiti, A.; Antonelli, G.; Indiveri, G.; Melchiorri, C.; Caselli, S. MARIS: A national project on marine robotics for interventions. In Proceedings of the 22nd Mediterranean Conference on Control and Automation, Palermo, Italy, 16–19 June 2014; pp. 864–869. [Google Scholar]
- Murawski, L.; Ostachowicz, W.; Opoka, S.; Mieloszyk, M.; Majewska, K. Practical application of monitoring system based on optical sensors for marine constructions. Key Eng. Mater. 2012, 518, 261–270. [Google Scholar] [CrossRef]
- Booth, C.; Wheeler, P.; Hancock, J.; Ximenes, R.; Patterson, D.E. Optical behavior of antibiofouling additives in environment-friendly coverglass materials for bio-sensors and solar panels. Polym. Adv. Technol. 2009, 20, 626–630. [Google Scholar] [CrossRef]
- Ali, H.R.; Arifin, M.M.; Sheikh, M.A.; Shazili, N.A.M.; Bachok, Z. Occurrence and distribution of antifouling biocide Irgarol-1051 in coastal waters of Peninsular Malaysia. Mar. Pollut. Bull. 2013, 70, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, S.; Takeda, K.; Sakugawa, H. Occurrence of diuron and irgarol in seawater, sediments and planktons of Seto Inland Sea, Japan. Geochem. J. 2012, 46, 169–177. [Google Scholar] [CrossRef]
- Murphy, K.; Sullivan, T.; Heery, B.; Regan, F. Data analysis from a low-cost optical sensor for continuous marine monitoring. Sens. Actuators B Chem. 2015, 214, 211–217. [Google Scholar] [CrossRef]
- Joslin, J.; Polagye, B. Demonstration of biofouling mitigation methods for long-term deployments of optical cameras. Mar. Technol. Soc. J. 2016, 49, 88–96. [Google Scholar] [CrossRef]
- FoulfreeTM Transducer Coating|AIRMAR. Available online: https://www.airmar.com/productdescription.html?id=223 (accessed on 31 March 2022).
- Foulfree by Propspeed|For Transducers|Product Information. Available online: https://propspeed.com/products/foulfree/product-information (accessed on 31 March 2022).
- Osborne, M.; Aryasomayajula, A.; Shakeri, A.; Selvaganapathy, P.R.; Didar, T.F. Suppression of biofouling on a permeable membrane for dissolved oxygen sensing using a lubricant-infused coating. ACS Sens. 2019, 4, 687–693. [Google Scholar] [CrossRef]
- Zhuiykov, S.; Kalantar-zadeh, K. Development of antifouling of electrochemical solid-state dissolved oxygen sensors based on nanostructured Cu0.4Ru3.4O7 + RuO2 sensing electrodes. Electrochim. Acta 2012, 73, 105–111. [Google Scholar] [CrossRef]
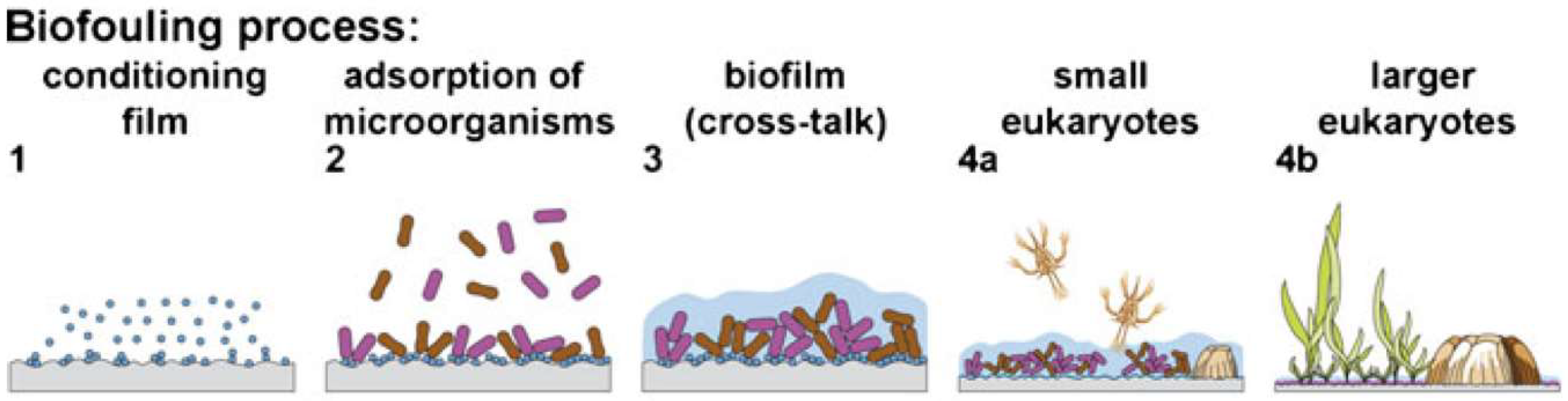


| Fouling Phase | Common Foulants | Settling Rate | Dominant Factors | Stage (Refer to Figure 1) |
|---|---|---|---|---|
| Conditioning Layer | Glycoprotein; Humic, amino and nucleic acids; Polysachides; Lipids | Minutes—hours | Hydrodynamic forces, surface chemical and electrical properties. | 1 |
| Biofilm | Phaeobacter sp.; Pseudoalteromonas sp.; Nitzschia sp.; Amphora sp. | Hours—days | Hydrodynamic forces, physical surface properties | 3 |
| Small Macrofoulers | Ulva sp.; Polysiphonia sp.; Ectocarpus sp.; Bugula sp.; | Hours—days | Hydrodynamic forces, physical surface properties, chemical cues | 4 a |
| Large Macrofoulers | Balanus sp.; Mytilus sp.; Spirorbis sp. | Days—weeks | Hydrodynamic forces, physical surface properties, chemical cues | 4 b |
| Mode of Degradation | Factors |
|---|---|
| Thermal degradation | Exposure to heat |
| Thermo-oxidative degradation | Exposure to heat and oxygen |
| Photo-degradation | Exposure to visible light and ultraviolet (UV) light |
| Irradiation degradation | Exposure of high-energy radiation such as X-rays and gamma irradiation |
| Mechanochemical degradation | Exposure to mechanical stress |
| Chemical degradation | Exposure to chemical attack such as solvolysis/hydrolysis, ozonolysis, catalytic degradation |
| Biodegradation | Exposure to aerobic and anaerobic environment |
| Class | Action | Maturity | Scale | Efficacy |
|---|---|---|---|---|
| SPC | Copper | High | High | High |
| Zinc | High | High | High | |
| CDP | Copper | High | Med | Med |
| Zinc | High | Med | Med | |
| Organic biocides | Med | Med | Med | |
| Nanoparticles/composite | Silver | Med | Low | Med |
| Copper | Med | Low | High | |
| Zinc | Low | Low | Med | |
| TiO2 | Med | Low | Med | |
| Graphene | High | Med | Med | |
| MOFs | Low | Low | High | |
| Low surface energy | Fluorinated polymers (PTFE) | High | Low | High |
| Silicone based polymers (PDMS) | High | Med | High | |
| Surface-topography | Marine organism inspired (Sharklet) | High | Low | Med |
| Lotus leaf inspired (Finsulate) | High | Med | Med | |
| Hydrophilic | PEG | High | Low | High |
| PVP | Med | Low | High | |
| Zwitterionic | Polybetaines | Med | Low | High |
| Phosphorylcholine | Med | Low | High | |
| Active cleaning | In-water cleaning | High | High | Med |
| Ultra-sonic cleaning | High | High | Med | |
| Ultraviolet cleaning | Med | Low | Med |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donnelly, B.; Sammut, K.; Tang, Y. Materials Selection for Antifouling Systems in Marine Structures. Molecules 2022, 27, 3408. https://doi.org/10.3390/molecules27113408
Donnelly B, Sammut K, Tang Y. Materials Selection for Antifouling Systems in Marine Structures. Molecules. 2022; 27(11):3408. https://doi.org/10.3390/molecules27113408
Chicago/Turabian StyleDonnelly, Bradley, Karl Sammut, and Youhong Tang. 2022. "Materials Selection for Antifouling Systems in Marine Structures" Molecules 27, no. 11: 3408. https://doi.org/10.3390/molecules27113408
APA StyleDonnelly, B., Sammut, K., & Tang, Y. (2022). Materials Selection for Antifouling Systems in Marine Structures. Molecules, 27(11), 3408. https://doi.org/10.3390/molecules27113408







