Pedicularis resupinata Extract Prevents Depressive-like Behavior in Repeated Corticosterone-Induced Depression in Mice: A Preliminary Study
Abstract
:1. Introduction
2. Results
2.1. Effects of PRE on OFT in CORT-Injected Mice
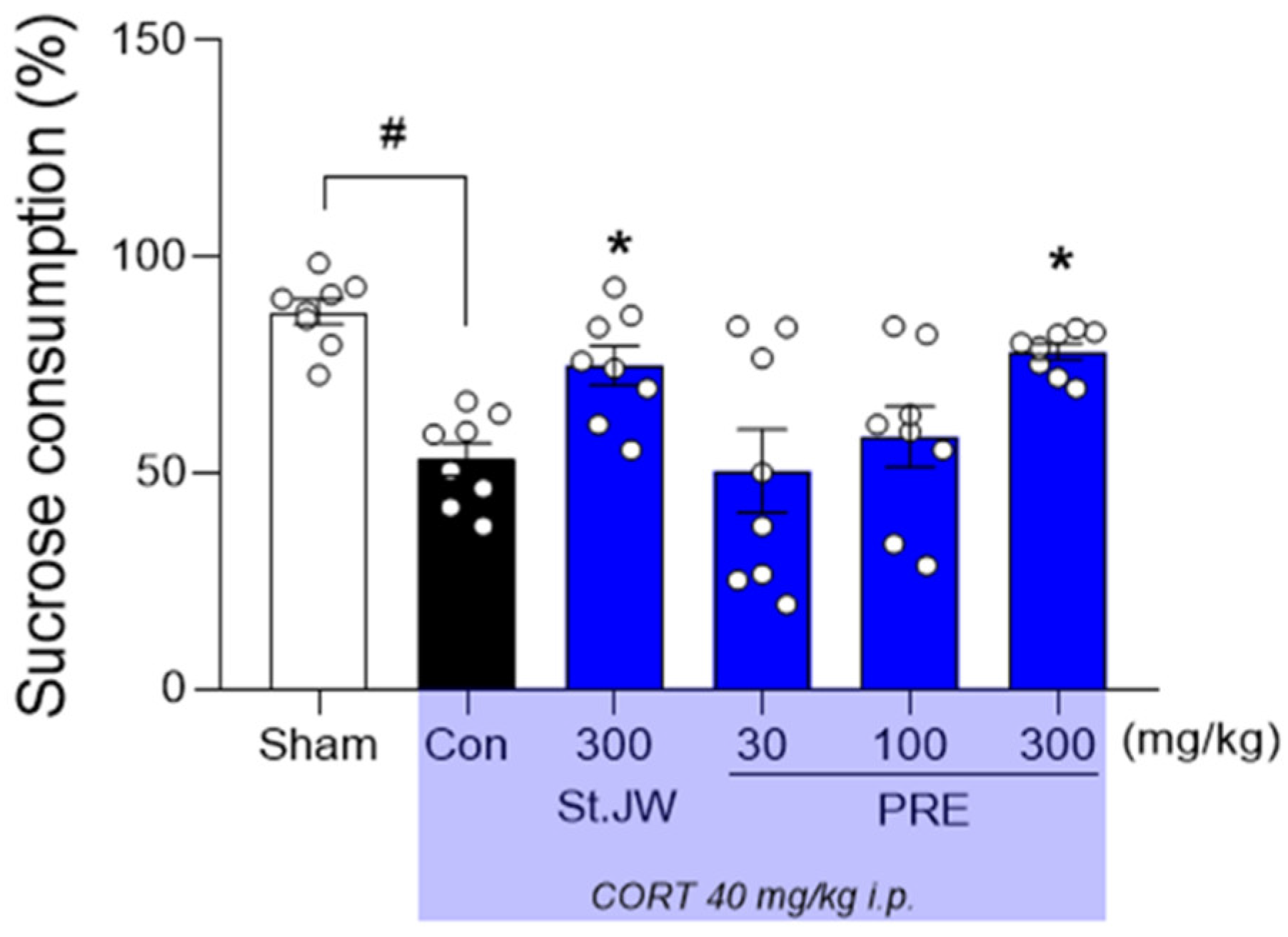
2.2. Effect of PRE on SPT in CORT-Injected Mice
2.3. Effect of PRE on PAT in CORT-Injected Mice
2.4. Effect of PRE on TST and FST in CORT-Injected Mice
2.5. Effect of PRE on GR and BDNF Expression
3. Discussion
4. Materials and Methods
4.1. Sample Preparation
4.2. Animals and Treatments
4.3. Open-Field Test (OFT)
4.4. Sucrose Preference Test (SPT)
4.5. Passive Avoidance Test (PAT)
4.6. Tail Suspension Test (TST) and Forced Swim Test (FST)
4.7. Western Blotting
4.8. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ali, A.M.; Alkhamees, A.A.; Hori, H.; Kim, Y.; Kunugi, H. The Depression Anxiety Stress Scale 21: Development and Validation of the Depression Anxiety Stress Scale 8-Item in Psychiatric Patients and the General Public for Easier Mental Health Measurement in a Post COVID-19 World. Int. J. Envior. Res. Public Health 2021, 18, 10142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.X.; Miller, S.O.; Xu, W.; Yin, A.; Chen, B.Z.; Delios, A.; Dong, R.K.; Chen, R.Z.; McIntyre, R.S.; Wan, X.; et al. Meta-analytic evidence of depression and anxiety in Eastern Europe during the COVID-19 pandemic. Eur. J. Psychotraumatol. 2022, 13, 2000132. [Google Scholar] [CrossRef] [PubMed]
- Mazza, M.G.; De Lorenzo, R.; Conte, C.; Poletti, S.; Vai, B.; Bollettini, I.; Melloni, E.M.T.; Furlan, R.; Ciceri, F.; Rovere-Querini, P.; et al. Anxiety and depression in COVID-19 survivors: Role of inflammatory and clinical predictors. Brain Behav. Immun. 2020, 89, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Racagni, G.; Popoli, M. The pharmacological properties of antidepressants. Int. Clin. Psychopharmacol. 2010, 25, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Baloch, Z.; Mao, F.B. Natural Products as a Source for New Leads in Depression Treatment. Evid.-Based Compl. Altern. 2022, 2022, 9791434. [Google Scholar] [CrossRef] [PubMed]
- Pirotta, M.; Willis, K.; Carter, M.; Forsdike, K.; Newton, D.; Gunn, J. ‘Less like a drug than a drug’: The use of St John’s wort among people who self-identify as having depression and/or anxiety symptoms. Complement Ther. Med. 2014, 22, 870–876. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.Q.; Yan, Y.; Li, F.; Zhang, D.F. Fruit and vegetable consumption and the risk of depression: A meta-analysis. Nutrition 2016, 32, 296–302. [Google Scholar] [CrossRef]
- Guideline Development Panel for the Treatment of Depressive Disorders. Summary of the clinical practice guideline for the treatment of depression across three age cohorts. Am. Psychol. 2021. [Google Scholar] [CrossRef]
- De Kloet, E.R.; Vreugdenhil, E.; Oitzl, M.S.; Joëls, M. Brain corticosteroid receptor balance in health and disease. Endocr. Rev. 1998, 19, 269–301. [Google Scholar]
- Keller, J.; Gomez, R.; Williams, G.; Lembke, A.; Lazzeroni, L.; Murphy, G.M.; Schatzberg, A.F. HPA axis in major depression: Cortisol, clinical symptomatology and genetic variation predict cognition. Mol. Psychiatry 2017, 22, 527–536. [Google Scholar] [CrossRef]
- Gregus, A.; Wintink, A.J.; Davis, A.C.; Kalynchuk, L.E. Effect of repeated corticosterone injections and restraint stress on anxiety and depression-like behavior in male rats. Behav. Brain Res. 2005, 156, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Shim, I.; Lee, H.J.; Yang, Y.; Hahm, D.H. Effects of acupuncture on chronic corticosterone-induced depression-like behavior and expression of neuropeptide Y in the rats. Neurosci. Lett. 2009, 453, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Fujii, N. Chloroplast DNA phylogeography of Pedicularis ser. Gloriosae (Orobanchaceae) in Japan. J. Plant Res. 2007, 120, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Bae, J.H.; Jung, H.Y.; Kim, J.H.; Park, D.S. Antioxidant activity in water and methanol extracts from Korean edible wild plants. J. Korean Soc. Food Sci. Nutr. 2011, 40, 29–36. [Google Scholar] [CrossRef]
- Wang, P.; Kang, J.; Zheng, R.; Yang, Z.; Lu, J.; Gao, J.; Jia, Z. Scavenging effects of phenylpropanoid glycosides from Pedicularis on superoxide anion and hydroxyl radical by the spin trapping method(95)02255-4. Biochem. Pharmacol. 1996, 51, 687–691. [Google Scholar] [CrossRef]
- Lee, H.D.; Kim, J.H.; Pang, Q.Q.; Jung, P.M.; Cho, E.J.; Lee, S. Antioxidant Activity and Acteoside Analysis of Abeliophyllum distichum. Antioxidants 2020, 9, 1148. [Google Scholar] [CrossRef]
- Qiao, Z.G.; Tang, J.X.; Wu, W.; Tang, J.; Liu, M. Acteoside inhibits inflammatory response via JAK/STAT signaling pathway in osteoarthritic rats. BMC Complem. Altern. Med. 2019, 19, 264. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.Q.; Xu, Y.X.; Yan, J.; Zhao, X.Y.; Sun, X.B.; Zhang, Y.P.; Guo, J.C.; Zhu, C.Q. Acteoside protects human neuroblastoma SH-SY5Y cells against beta-amyloid-induced cell injury. Brain Res. 2009, 1283, 139–147. [Google Scholar] [CrossRef]
- Cui, Q.L.; Pan, Y.N.; Zhang, W.; Zhang, Y.A.; Ren, S.M.; Wang, D.M.; Wang, Z.Z.; Liu, X.Q.; Xiao, W. Metabolites of Dietary Acteoside: Profiles, Isolation, Identification, and Hepatoprotective Capacities. J. Agric. Food Chem. 2018, 66, 2660–2668. [Google Scholar] [CrossRef]
- Zhao, Y.; Ma, R.; Shen, J.; Su, H.; Xing, D.M.; Du, L.J. A mouse model of depression induced by repeated corticosterone injections. Eur. J. Pharmacol. 2008, 581, 113–120. [Google Scholar] [CrossRef]
- Kalueff, A.V.; Murphy, D.L. The importance of cognitive phenotypes in experimental modeling of animal anxiety and depression. Neural Plast 2007, 2007, 52087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badr, A.M.; Attia, H.A.; Al-Rasheed, N. Oleuropein Reverses Repeated Corticosterone-Induced Depressive-like Behavior in mice: Evidence of Modulating Effect on Biogenic Amines. Sci. Rep. 2020, 10, 3336. [Google Scholar] [CrossRef] [PubMed]
- Renard, C.E.; Dailly, E.; David, D.J.; Hascoet, M.; Bourin, M. Monoamine metabolism changes following the mouse forced swimming test but not the tail suspension test. Fundam. Clin. Pharm. 2003, 17, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Crupi, R.; Abusamra, Y.A.K.; Spina, E.; Calapai, G. Preclinical Data Supporting/Refuting the Use of Hypericum perforatum in the Treatment of Depression. CNS Neurol. Disord.-Drug 2013, 12, 474–486. [Google Scholar] [CrossRef]
- Jovicic, M.; Maric, N.P.; Soldatovic, I.; Lukic, I.; Andric, S.; Mihaljevic, M.; Pavlovic, Z.; Mitic, M.; Adzic, M. The role of glucocorticoid receptor phosphorylation in the model of negative affective states. World J. Biol. Psychia. 2015, 16, 301–311. [Google Scholar] [CrossRef]
- Liu, B.J.; Zhang, H.Y.; Xu, C.Q.; Yang, G.A.; Tao, J.A.; Huang, J.H.; Wu, J.F.; Duan, X.H.; Cao, Y.X.; Dong, J.C. Neuroprotective effects of icariin on corticosterone-induced apoptosis in primary cultured rat hippocampal neurons. Brain Res. 2011, 1375, 59–67. [Google Scholar] [CrossRef]
- Bei, E.; Salpeas, V.; Pappa, D.; Anagnostara, C.; Alevizos, V.; Moutsatsou, P. Phosphorylation status of glucocorticoid receptor, heat shock protein 70, cytochrome c and Bax in lymphocytes of euthymic, depressed and manic bipolar patients. Psychoneuroendocrino 2009, 34, 1162–1175. [Google Scholar] [CrossRef]
- Numakawa, T.; Odaka, H.; Adachi, N. Actions of Brain-Derived Neurotrophic Factor and Glucocorticoid Stress in Neurogenesis. Int. J. Mol. Sci. 2017, 18, 2312. [Google Scholar] [CrossRef] [Green Version]
- Karstens, A.J.; Korzun, I.; Avery, E.T.; Kassel, M.T.; Keelan, R.; Kales, H.; Abercrombie, H.; Eisenlohr-Moul, T.; Langenecker, S.A.; Weisenbach, S. Examining HPA-axis functioning as a mediator of the relationship between depression and cognition across the adult lifespan. Neuropsychol. Dev. Cogn. B Aging Neuropsychol. Cogn. 2019, 26, 507–520. [Google Scholar] [CrossRef]
- Mizoguchi, K.; Ishige, A.; Aburada, M.; Tabira, T. Chronic stress attenuates glucocorticoid negative feedback: Involvement of the prefrontal cortex and hippocampus. Neuroscience 2003, 119, 887–897. [Google Scholar] [CrossRef]
- Jia, Y.; Liu, L.; Sheng, C.; Cheng, Z.; Cui, L.; Li, M.; Zhao, Y.; Shi, T.; Yau, T.O.; Li, F.; et al. Increased Serum Levels of Cortisol and Inflammatory Cytokines in People With Depression. J. Nerv. Ment. Dis. 2019, 207, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Mason, B.L.; Pariante, C.M. The effects of antidepressants on the hypothalamic-pituitary-adrenal axis. Drug News Perspect. 2006, 19, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Colle, R.; Cury, C.; Chupin, M.; Deflesselle, E.; Hardy, P.; Nasser, G.; Falissard, B.; Ducreux, D.; Colliot, O.; Corruble, E. Hippocampal volume predicts antidepressant efficacy in depressed patients without incomplete hippocampal inversion. Neuroimage Clin. 2016, 12, 949–955. [Google Scholar] [CrossRef]
- Iijima, M.; Ito, A.; Kurosu, S.; Chaki, S. Pharmacological characterization of repeated corticosterone injection-induced depression model in rats. Brain Res. 2010, 1359, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Naert, G.; Ixart, G.; Maurice, T.; Tapia-Arancibia, L.; Givalois, L. Brain-derived neurotrophic factor and hypothalamic-pituitary-adrenal axis adaptation processes in a depressive-like state induced by chronic restraint stress. Mol. Cell Neurosci. 2011, 46, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Castagne, V.; Moser, P.; Roux, S.; Porsolt, R.D. Rodent models of depression: Forced swim and tail suspension behavioral despair tests in rats and mice. Curr. Protoc. Neurosci. 2011, 49, 58. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.T.; Lei, H.; Wang, L.; Xue, L.; Wang, X.; Zhu, X.Z. Optimized animal model to mimic the reality of stress-induced depression in the clinic. BMC Psychiatry 2017, 17, 171. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.W.; Han, T.; Um, M.Y.; Yoon, M.; Kim, T.E.; Kim, Y.T.; Han, D.; Lee, J.; Lee, C.H. Administration of Asian Herb Bennet (Geum japonicum) Extract Reverses Depressive -Like Behaviors in Mouse Model of Depression Induced by Corticosterone. Nutrients 2019, 11, 2841. [Google Scholar] [CrossRef] [Green Version]
- Quax, R.A.; Manenschijn, L.; Koper, J.W.; Hazes, J.M.; Lamberts, S.W.J.; van Rossum, E.F.C.; Feelders, R.A. Glucocorticoid sensitivity in health and disease. Nat. Rev. Endocrinol. 2013, 9, 670–686. [Google Scholar] [CrossRef]
- Pariante, C.M. Glucocorticoid receptor function in vitro in patients with major depression. Stress 2004, 7, 209–219. [Google Scholar] [CrossRef]
- Wei, K.; Xu, Y.Z.; Zhao, Z.X.; Wu, X.; Du, Y.J.; Sun, J.; Yi, T.; Dong, J.C.; Liu, B.J. Icariin alters the expression of glucocorticoid receptor, FKBP5 and SGK1 in rat brains following exposure to chronic mild stress. Int. J. Mol. Med. 2016, 38, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Anacker, C.; Zunszain, P.A.; Cattaneo, A.; Carvalho, L.A.; Garabedian, M.J.; Thuret, S.; Price, J.; Pariante, C.M. Antidepressants increase human hippocampal neurogenesis by activating the glucocorticoid receptor. Mol. Psychiatr. 2011, 16, 738–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, K.; He, M.Y.; Wang, F.; Zhang, H.T.; Li, Y.T.; Yang, J.Y.; Wu, C.F. Revealing Antidepressant Mechanisms of Baicalin in Hypothalamus Through Systems Approaches in Corticosterone-Induced Depressed Mice. Front. Neurosci-Switz. 2019, 13, 834. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, A.L.; Barbosa, I.G.; Diniz, B.S.; Kummer, A. Circulating levels of brain-derived neurotrophic factor: Correlation with mood, cognition and motor function. Biomark Med. 2010, 4, 871–887. [Google Scholar] [CrossRef]
- Fernandes, B.S.; Gama, C.S.; Cereser, K.M.; Yatham, L.N.; Fries, G.R.; Colpo, G.; de Lucena, D.; Kunz, M.; Gomes, F.A.; Kapczinski, F. Brain-derived neurotrophic factor as a state-marker of mood episodes in bipolar disorders: A systematic review and meta-regression analysis. J. Psychiatr Res. 2011, 45, 995–1004. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, E.; Hashimoto, K.; Okamura, N.; Koike, K.; Komatsu, N.; Kumakiri, C.; Nakazato, M.; Watanabe, H.; Shinoda, N.; Okada, S.; et al. Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Biol. Psychiatry 2003, 54, 70–75. [Google Scholar] [CrossRef]
- Frezza, C.; Venditti, A.; Toniolo, C.; De Vita, D.; Serafini, I.; Ciccola, A.; Franceschin, M.; Ventrone, A.; Tomassini, L.; Foddai, S.; et al. Genus: Systematics, Botany, Phytochemistry, Chemotaxonomy, Ethnopharmacology, and Other. Plants-Basel 2019, 8, 306. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.X.; Xie, W.D.; Jia, Z.H. Glycosides from two Pedicularis species. Biochem. Syst. Ecol. 2008, 36, 467–472. [Google Scholar] [CrossRef]
- Pieretti, S.; Saviano, A.; Mollica, A.; Stefanucci, A.; Aloisi, A.M.; Nicoletti, M. Calceolarioside A, a Phenylpropanoid Glycoside from Calceolaria spp., Displays Antinociceptive and Anti-Inflammatory Properties. Molecules 2022, 27, 2183. [Google Scholar] [CrossRef]
- Chae, S.; Kang, K.A.; Kim, J.S.; Hyun, J.W.; Kang, S.S. Trichotomoside: A new antioxidative phenylpropanoid glycoside from Clerodendron trichotomum. Chem. Biodivers. 2006, 3, 41–48. [Google Scholar] [CrossRef]
- Lopez-Munguia, A.; Hernandez-Romero, Y.; Pedraza-Chaverri, J.; Miranda-Molina, A.; Regla, I.; Martinez, A.; Castillo, E. Phenylpropanoid glycoside analogues: Enzymatic synthesis, antioxidant activity and theoretical study of their free radical scavenger mechanism. PLoS ONE 2011, 6, e20115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, D.W.; Park, J.; Jung, J.; Kim, S.H.; Um, M.Y.; Yoon, M.; Kim, Y.T.; Han, D.; Lee, C.; Lee, J. Dicaffeoylquinic acids alleviate memory loss via reduction of oxidative stress in stress-hormone-induced depressive mice. Pharmacol. Res 2020, 161, 105252. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Hu, X.P.; Zeng, Y.; Li, Y.; Wu, H.Q.; Qiu, R.Z.; Ma, W.J.; Li, T.; Li, C.Y.; He, Z.D. Advanced research on acteoside for chemistry and bioactivities. J. Asian Nat. Prod. Res. 2011, 13, 449–464. [Google Scholar] [CrossRef] [PubMed]
- Pu, X.; Song, Z.; Li, Y.; Tu, P.; Li, H. Acteoside from Cistanche salsa inhibits apoptosis by 1-methyl-4-phenylpyridinium ion in cerebellar granule neurons. Planta. Med. 2003, 69, 65–66. [Google Scholar] [CrossRef] [PubMed]
- Dell, R.B.; Holleran, S.; Ramakrishnan, R. Sample size determination. ILAR J. 2002, 43, 207–213. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, D.W.; Han, D.; Lee, C. Pedicularis resupinata Extract Prevents Depressive-like Behavior in Repeated Corticosterone-Induced Depression in Mice: A Preliminary Study. Molecules 2022, 27, 3434. https://doi.org/10.3390/molecules27113434
Lim DW, Han D, Lee C. Pedicularis resupinata Extract Prevents Depressive-like Behavior in Repeated Corticosterone-Induced Depression in Mice: A Preliminary Study. Molecules. 2022; 27(11):3434. https://doi.org/10.3390/molecules27113434
Chicago/Turabian StyleLim, Dong Wook, Daeseok Han, and Changho Lee. 2022. "Pedicularis resupinata Extract Prevents Depressive-like Behavior in Repeated Corticosterone-Induced Depression in Mice: A Preliminary Study" Molecules 27, no. 11: 3434. https://doi.org/10.3390/molecules27113434
APA StyleLim, D. W., Han, D., & Lee, C. (2022). Pedicularis resupinata Extract Prevents Depressive-like Behavior in Repeated Corticosterone-Induced Depression in Mice: A Preliminary Study. Molecules, 27(11), 3434. https://doi.org/10.3390/molecules27113434





