Lipids in Archaeological Pottery: A Review on Their Sampling and Extraction Techniques
Abstract
:1. Introduction
2. Lipids and Archaeological Biomarkers
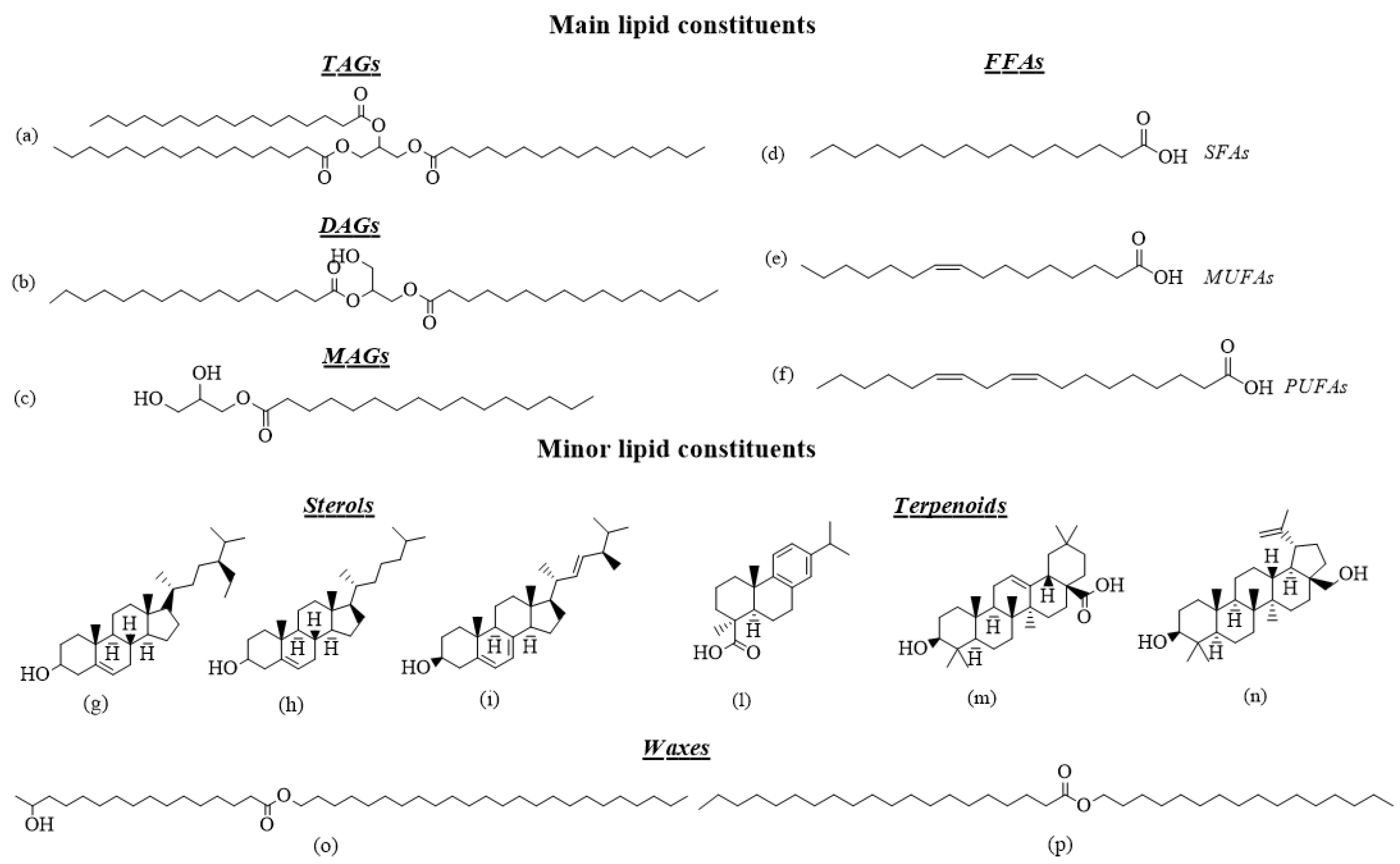
2.1. Triacylglycerols, Diacylglycerols and Monoacylglycerols in Pottery
2.2. Free Fatty Acids as Archaeological Biomarkers
2.3. Minor Lipid Constituents in Archaeological Samples
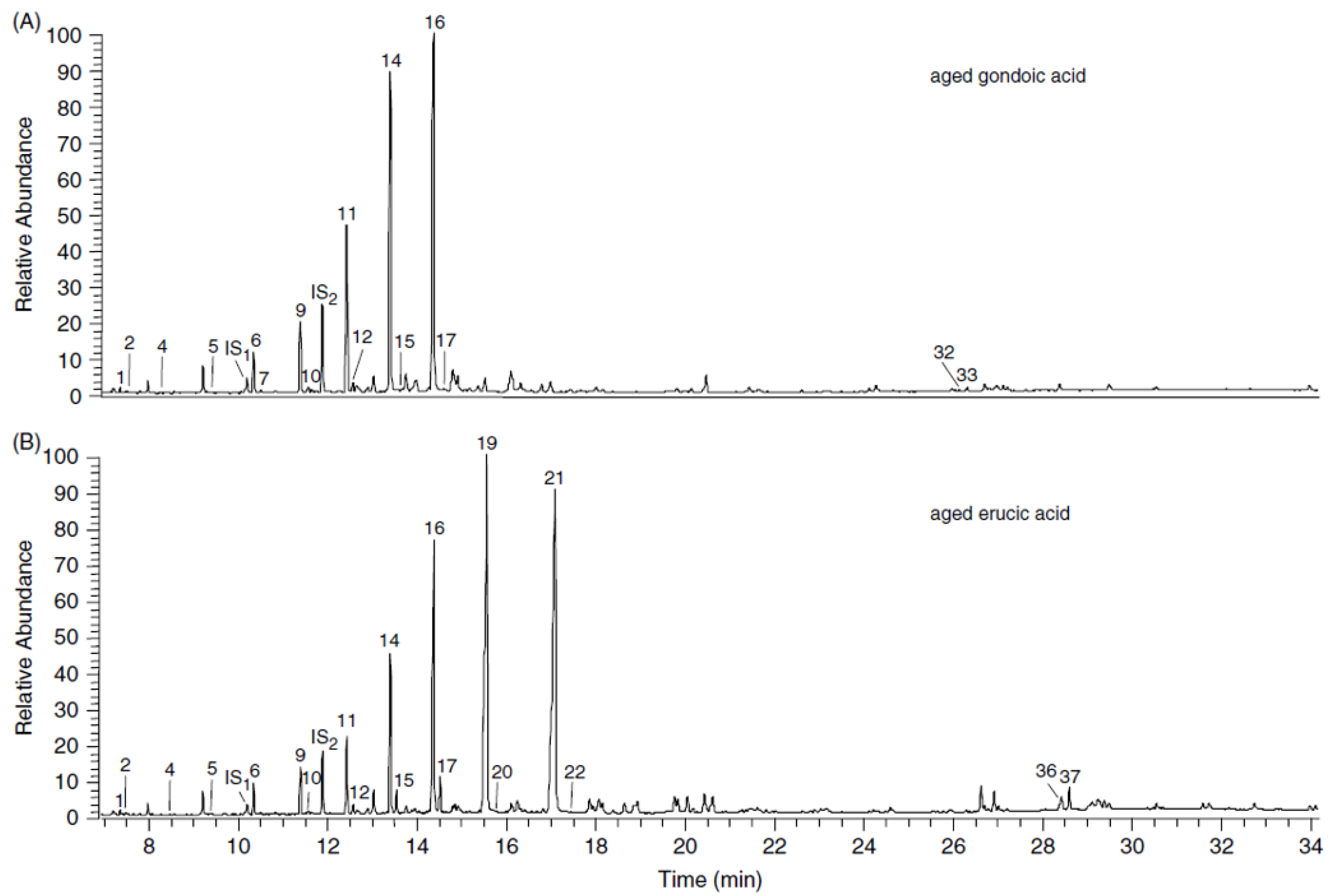
3. Artificial Ageing Studies
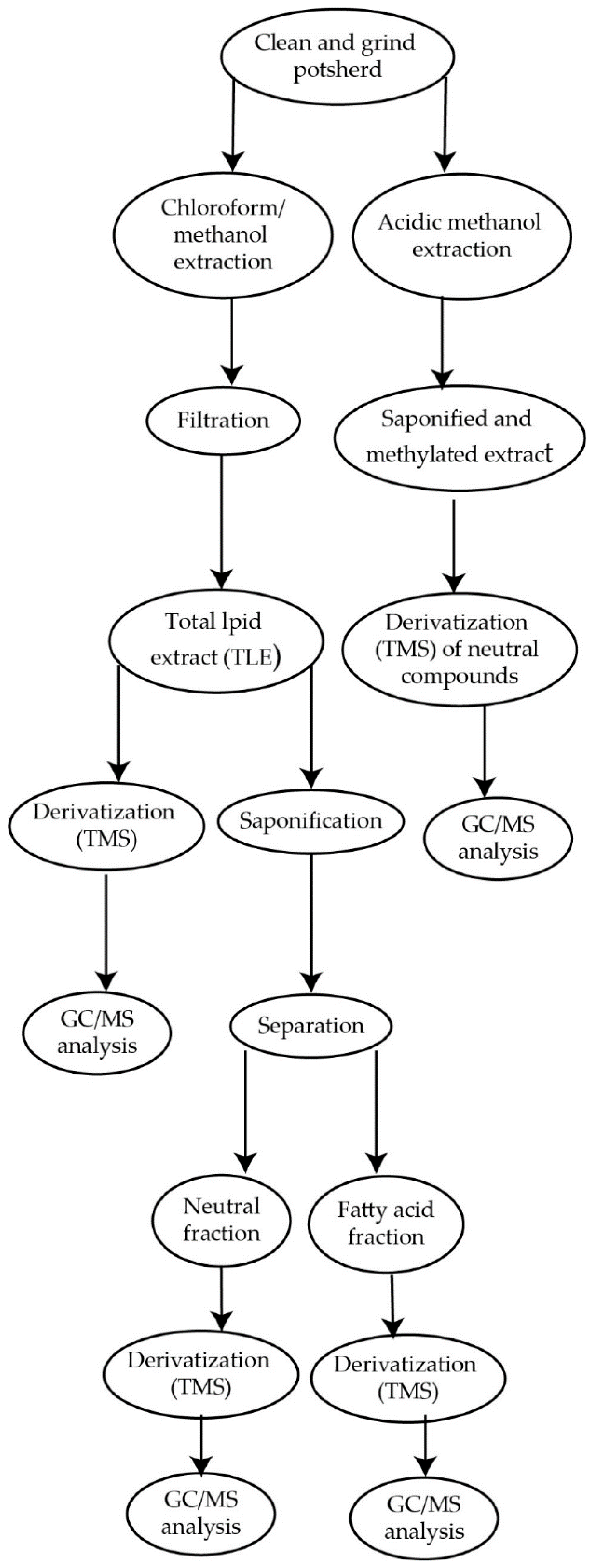
4. Sampling and Extraction Protocols of Lipids from Ancient Pottery
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Dunne, J.; Chapman, A.; Blinkhorn, P.; Evershed, R.P. Fit for purpose? Organic residue analysis and vessel specialisation: The perfectly utilitarian medieval pottery assemblage from West Cotton, Raunds. J. Archaeol. Sci. 2020, 120, 105178. [Google Scholar] [CrossRef]
- Lundy, J.; Drieu, L.; Meo, A.; Sacco, V.; Arcifa, L.; Pezzini, E.; Aniceti, V.; Fiorentino, G.; Alexander, M.; Orecchioni, P.; et al. New insights into early medieval Islamic cuisine: Organic residue analysis of pottery from rural and urban Sicily. PLoS ONE 2021, 16, e0252225. [Google Scholar] [CrossRef] [PubMed]
- Regert, M. Analytical strategies for discriminating archeological fatty substances from animal origin. Mass. Spectrom. Rev. 2011, 30, 177–220. [Google Scholar] [CrossRef] [PubMed]
- Suryanarayan, A.; Cubas, M.; Craig, O.E.; Heron, C.P.; Shinde, V.S.; Singh, R.N.; O’Connell, T.C.; Petrie, C.A. Lipid residues in pottery from the Indus Civilisation in northwest India. J. Archaeol. Sci. 2021, 125, 105291. [Google Scholar] [CrossRef] [PubMed]
- Tanasi, D.; Greco, E.; Noor, R.E.; Feola, S.; Kumar, V.; Crispino, A.; Gelis, I. 1H NMR, 1H–1H 2D TOCSY and GC-MS analyses for the identification of olive oil in Early Bronze Age pottery from Castelluccio (Noto, Italy). Anal. Methods 2018, 10, 2756–2763. [Google Scholar] [CrossRef]
- Craig, O.E.; Saul, H.; Lucquin, A.; Nishida, Y.; Taché, K.; Clarke, L.; Thompson, A.; Altoft, D.T.; Uchiyama, J.; Ajimoto, M.; et al. Earliest evidence for the use of pottery. Nature 2013, 496, 351–354. [Google Scholar] [CrossRef]
- Dunne, J.; Grillo, K.M.; Casanova, E.; Whelton, H.L.; Evershed, R.P. Pastoralist Foodways Recorded in Organic Residues from Pottery Vessels of Modern Communities in Samburu, Kenya. J. Archaeol. Method Theory 2019, 26, 619–642. [Google Scholar] [CrossRef] [Green Version]
- Heron, C.; Evershed, R.P. The Analysis of Organic Residues and the Study of Pottery Use. J. Archaeol. Method Theory 1993, 5, 247–284. [Google Scholar]
- Yoneda, M.; Kisida, K.; Gakuhari, T.; Omori, T.; Abe, Y. Interpretation of bulk nitrogen and carbon isotopes in archaeological foodcrusts on potsherds. Rapid Commun. Mass. Spectrom. 2019, 33, 1097–1106. [Google Scholar] [CrossRef]
- Stern, B.; Heron, C.; Tellefsen, T.; Serpico, M. New investigations into the Uluburun resin cargo. J. Archaeol. Sci. 2008, 35, 2188–2203. [Google Scholar] [CrossRef]
- Colombini, M.P.; Modugno, F.; Ribechini, E. Direct exposure electron ionization mass spectrometry and gas chromatography/mass spectrometry techniques to study organic coatings on archaeological amphorae. J. Mass. Spectrom. 2005, 40, 675–687. [Google Scholar] [CrossRef]
- Tanasi, D.; Cucina, A.; Cunsolo, V.; Saletti, R.; Di Francesco, A.; Greco, E.; Foti, S. Paleoproteomic profiling of organic residues on prehistoric pottery from Malta. Amino Acids 2021, 53, 295–312. [Google Scholar] [CrossRef] [PubMed]
- Evershed, R.P. Organic residue in archaeology: The archaeological biomarker revolution. Archaeometry 2008, 50, 895–924. [Google Scholar] [CrossRef]
- Colonese, A.C.; Lucquin, A.; Guedes, E.P.; Thomas, R.; Best, J.; Fothergill, B.T.; Sykes, N.; Foster, A.; Miller, H.; Poole, K.; et al. The identification of poultry processing in archaeological ceramic vessels using in-situ isotope references for organic residue analysis. J. Archaeol. Sci. 2017, 78, 179–192. [Google Scholar] [CrossRef] [Green Version]
- Evershed, R.P. Biomolecular archaeology and lipids. World Archaeol. 1993, 25, 74–93. [Google Scholar] [CrossRef] [PubMed]
- Mayyas, A.S. Organic residues in ancient pottery sherds from sites in Jordan. Mediterr. Archaeol. Archaeom. 2018, 18, 61–75. [Google Scholar]
- Mayyas, A.S.; Khrisat, B.R.; Hoffmann, T.; El Khalili, M.M. Fuel For Lamps: Organic Residues Preserved in Iron Age Lamps Excavated at the Site of Sahab in Jordan. Archaeometry 2017, 59, 934–948. [Google Scholar] [CrossRef]
- Whelton, H.L.; Hammann, S.; Cramp, L.J.E.; Dunne, J.; Roffet-Salque, M.; Evershed, R.P. A call for caution in the analysis of lipids and other small biomolecules from archaeological contexts. J. Archaeol. Sci. 2021, 132, 105397. [Google Scholar] [CrossRef]
- Copley, M.S.; Bland, H.A.; Rose, P.; Horton, M.; Evershed, R.P. Gas chromatographic, mass spectrometric and stable carbon isotopic investigations of organic residues of plant oils and animal fats employed as illuminants in archaeological lamps from Egypt. Analyst 2005, 130, 860–871. [Google Scholar] [CrossRef]
- Steele, V.J. Organic residues in archaeology—The highs and lows of recent research. In ACS Symposium Series; Armitage, R.A., Burton, J.H., Eds.; ACS: New Orleans, LA, USA, 2013; Volume 1147, pp. 89–108. [Google Scholar]
- Yang, Y.; Shevchenko, A.; Knaust, A.; Abuduresule, I.; Li, W.; Hu, X.; Wang, C.; Shevchenko, A. Proteomics evidence for kefir dairy in Early Bronze Age China. J. Archaeol. Sci. 2014, 45, 178–186. [Google Scholar] [CrossRef]
- Evershed, R.P.; Copley, M.S.; Dickson, L.; Hansel, F.A. Experimental evidence for the processing of marine animal products and other commodities containing polyunsaturated fatty acids in pottery vessels. Archaeometry 2008, 50, 101–113. [Google Scholar] [CrossRef]
- Evershed, R.P.; Vaughan, S.J.; Dudd, S.N.; Soles, J.S. Fuel for thought? Beeswax in lamps and conical cups from Late Minoan Crete. Antiquity 2015, 71, 979–985. [Google Scholar] [CrossRef]
- Blanco-Zubiaguirre, L.; Ribechini, E.; Degano, I.; La Nasa, J.; Carrero, J.A.; Iñañez, J.; Olivares, M.; Castro, K. GC–MS and HPLC-ESI-QToF characterization of organic lipid residues from ceramic vessels used by Basque whalers from 16th to 17th centuries. Microchem. J. 2018, 137, 190–203. [Google Scholar] [CrossRef]
- Evershed, R.P. Experimental approaches to the interpretation of absorbed organic residues in archaeological ceramics. World Archaeol. 2008, 40, 26–47. [Google Scholar] [CrossRef]
- Jones, M.K.; Briggs, D.E.G.; Eglington, G.; Hagelberg, E.; Evershed, R.P.; Dudd, S.N.; Charters, S.; Mottram, H.; Stott, A.W.; Raven, A.; et al. Lipids as carriers of anthropogenic signals from prehistory. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1999, 354, 19–31. [Google Scholar]
- Krueger, M.; Wicenciak, U.; Kowarska, Z.; Niedzielski, P.; Kozak, L.; Jakubowski, K.; Proch, J.; Mleczek, M.; Waśkiewicz, A. First results of organic residue analysis on ceramic vessels (Jiyeh and ChhÎm, Lebanon) by high perfomance liquid chromatography with tandem mass spectrometry. Mediterr. Archaeol. Archaeom. 2018, 18, 209–220. [Google Scholar]
- Oudemans, T.F.M.; Boon, J.J. Molecular archaeology: Analysis of charred (food) remains from prehistoric pottery by pyrolysis—Gas chromatography/mass spectrometry. J. Anal. Appl. Pyrolysis 1991, 20, 197–227. [Google Scholar] [CrossRef]
- Colombini, M.P.; Modugno, F.; Ribechini, E. Organic mass spectrometry in archaeology: Evidence for Brassicaceae seed oil in Egyptian ceramic lamps. J Mass. Spectrom. 2005, 40, 890–898. [Google Scholar] [CrossRef]
- Dunne, J.; Evershed, R.P.; Heron, C.; Brettell, R.; Barclay, A.; Smyth, J.; Cramp, L. Organic Residue Analysis and Archaeology Guidance for Good Practice; Historic England Publishing: Liverpool, UK, 2018. [Google Scholar]
- Hammann, S.; Scurr, D.J.; Alexander, M.R.; Cramp, L.J.E. Mechanisms of lipid preservation in archaeological clay ceramics revealed by mass spectrometry imaging. Proc. Natl. Acad. Sci. USA 2020, 117, 14688–14693. [Google Scholar] [CrossRef]
- Dudd, S.N.; Evershed, R.P. Direct demonstration of milk as an element of archaeological economies. Science 1998, 282, 1478–1481. [Google Scholar] [CrossRef]
- Hammann, S.; Cramp, L.J.E. Towards the detection of dietary cereal processing through absorbed lipid biomarkers in archaeological pottery. J. Archaeol. Sci. 2018, 93, 74–81. [Google Scholar] [CrossRef]
- Regert, M.; Bland, H.A.; Dudd, S.N.; Bergen, P.F.V.; Evershed, R.P. Free and bound fatty acid oxidation products in archaeological ceramic vessels. Proc. Royal Soc. B 1998, 265, 2027–2032. [Google Scholar] [CrossRef]
- Evershed, R.P.; Dudd, S.N.; Copley, M.S.; Berstan, R.; Stott, A.W.; Mottram, H.; Buckley, S.A.; Crossman, Z. Chemistry of archaeological animal fats. Acc. Chem. Res. 2002, 35, 660–668. [Google Scholar] [CrossRef]
- Mahesar, S.A.; Sherazi, S.T.H.; Khaskheli, A.R.; Kandhro, A.A.; Uddin, S. Analytical approaches for the assessment of free fatty acids in oils and fats. Anal. Methods 2014, 6, 4956–4963. [Google Scholar] [CrossRef]
- Casanova, E.; Knowles, T.; Bayliss, A.; Walton-Doyle, C.; Barclay, A.; Evershed, R. Compound-specific radiocarbon dating of lipid residues in pottery vessels: A new approach for detecting the exploitation of marine resources. J. Archaeol. Sci. 2022, 137, 105528. [Google Scholar] [CrossRef]
- Han, B.; Sun, Z.; Chong, J.; Lyu, N.; Rao, H.; Yang, Y. Lipid residue analysis of ceramic vessels from the Liujiawa site of the Rui State (early Iron Age, north China). J. Quat. Sci. 2022, 37, 114–122. [Google Scholar] [CrossRef]
- Skibo, J.M.; Malainey, M. Residue Analysis; Springer Science+Business Media: New York, NY, USA, 2013. [Google Scholar]
- Gunstone, F.D.; Harwood, J.L.; Dijkstra, A.J. The Lipid Handbook with CD-ROM, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Kałużna-Czaplińska, J.; Rosiak, A.; Kwapińska, M.; Kwapiński, W. Different Analytical Procedures for the Study of Organic Residues in Archeological Ceramic Samples with the Use of Gas Chromatography-mass Spectrometry. Crit. Rev. Anal. Chem. 2016, 46, 67–81. [Google Scholar] [CrossRef]
- Rosiak, A.; Kałużna-Czaplińska, J.; Gątarek, P. Analytical Interpretation of Organic Residues from Ceramics As a Source of Knowledge About Our Ancestors. Crit. Rev. Anal. Chem. 2020, 50, 189–195. [Google Scholar] [CrossRef]
- Keeney, M.; Katz, I.; Allison, M.J. On the probable origin of some milk fat acids in rumen microbial lipids. J. Am. Oil Chem. Soc. 1962, 39, 198–201. [Google Scholar] [CrossRef]
- Blanco-Zubiaguirre, L.; Olivares, M.; Castro, K.; Carrero, J.A.; García-Benito, C.; García-Serrano, J.; Pérez-Pérez, J.; Pérez-Arantegui, J. Wine markers in archeological potteries: Detection by GC-MS at ultratrace levels. Anal Bioanal Chem 2019, 411, 6711–6722. [Google Scholar] [CrossRef]
- Pecci, A.; Borgna, E.; Mileto, S.; Dalla Longa, E.; Bosi, G.; Florenzano, A.; Mercuri, A.M.; Corazza, S.; Marchesini, M.; Vidale, M. Wine consumption in Bronze Age Italy: Combining organic residue analysis, botanical data and ceramic variability. J. Archaeol. Sci. 2020, 123, 105256. [Google Scholar] [CrossRef]
- Pecci, A.; Reynolds, P.; Mileto, S.; Vargas Girón, J.M.; Bernal-Casasola, D. Production and Transport of Goods in the Roman Period: Residue Analysis and Wine Derivatives in Late Republican Baetican Ovoid Amphorae. Environ. Archaeol. 2021, 1–13. [Google Scholar] [CrossRef]
- Amir, A.; Finkelstein, I.; Shalev, Y.; Uziel, J.; Chalaf, O.; Freud, L.; Neumann, R.; Gadot, Y. Residue analysis evidence for wine enriched with vanilla consumed in Jerusalem on the eve of the Babylonian destruction in 586 BCE. PLoS ONE 2022, 17, e0266085. [Google Scholar] [CrossRef] [PubMed]
- Briggs, L.; Demesticha, S.; Katzev, S.; Wylde Swiny, H.; Craig, O.E.; Drieu, L. There’s more to a vessel than meets the eye: Organic residue analysis of ‘wine’ containers from shipwrecks and settlements of ancient Cyprus (4th–1st century bce). Archaeometry 2022, 64, 779–797. [Google Scholar] [CrossRef]
- Zhang, T.; Xu, S.; Li, Y.; Wen, R.; Yang, G. Orthogonal optimization of extraction and analysis for red wine residues in simulated and archaeological materials using LC/MS and HPLC methods. Microchem. J. 2018, 142, 175–180. [Google Scholar] [CrossRef]
- Francés-Negro, M.; Iriarte, E.; Galindo-Pellicena, M.A.; Gerbault, P.; Carrancho, A.; Pérez-Romero, A.; Arsuaga, J.L.; Carretero, J.M.; Roffet-Salque, M. Neolithic to Bronze Age economy and animal management revealed using analyses lipid residues of pottery vessels and faunal remains at El Portalón de Cueva Mayor (Sierra de Atapuerca, Spain). J. Archaeol. Sci. 2021, 131, 105380. [Google Scholar] [CrossRef]
- Correa-Ascencio, M.; Evershed, R.P. High throughput screening of organic residues in archaeological potsherds using direct acidified methanol extraction. Anal. Methods 2014, 6, 1330–1340. [Google Scholar] [CrossRef]
- Evershed, R.P.; Mottram, H.R.; Dudd, S.N.; Charters, S.; Stott, A.W.; Lawrence, G.J.; Gibson, A.M.; Conner, A.; Blinkhorn, P.W.; Reeves, V. New Criteria for the Identification of Animal Fats Preserved in Archaeological Pottery. Naturwissenschaften 1997, 84, 402–406. [Google Scholar] [CrossRef]
- Gregg, M.W.; Slater, G.F. A new method for extraction, isolation and transesterification of free fatty acids from archaeological pottery. Archaeometry 2010, 52, 833–854. [Google Scholar] [CrossRef]
- Kimpe, K.; Jacobs, P.A.; Waelkens, M. Mass spectrometric methods prove the use of beeswax and ruminant fat in late Roman cooking pots. J. Chromatogr. A 2002, 968, 151–160. [Google Scholar] [CrossRef]
- Marchbanks, M.L. Lipid Analysis in Archaeology: An Initial Study of Ceramics and Subsistence at the George C. Davis Site; University of Texas: Austin, TX, USA, 1989. [Google Scholar]
- McClure, S.B.; Magill, C.; Podrug, E.; Moore, A.M.T.; Harper, T.K.; Culleton, B.J.; Kennett, D.J.; Freeman, K.H. Fatty acid specific δ13C values reveal earliest Mediterranean cheese production 7200 years ago. PLoS ONE 2018, 13, e0202807. [Google Scholar] [CrossRef] [PubMed]
- Olsson, M.; Isaksson, S. Molecular and isotopic traces of cooking and consumption of fish at an Early Medieval manor site in eastern middle Sweden. J. Archaeol. Sci. 2008, 35, 773–780. [Google Scholar] [CrossRef]
- Spangenberg, J.E.; Jacomet, S.; Schibler, J. Chemical analyses of organic residues in archaeological pottery from Arbon Bleiche 3, Switzerland—Evidence for dairying in the late Neolithic. J. Archaeol. Sci. 2006, 33, 1–13. [Google Scholar] [CrossRef]
- Keute, J.; Isaksson, S.; Deviese, T.; Hein, A. Insights into ceramic use in prehistoric Northwest China obtained from residue analysis: A pilot study on the Andersson Collection at the Museum of Far Eastern Antiquities, Stockholm. Bull. Mus. Far East. Antiq. 2021, 82, 1–24. [Google Scholar]
- Copley, M.S.; Berstan, R.; Harden, S.; Docherty, G.; Mukherjee, A.J.; Straker, V.; Payne, S.; Evershed, R. Direct Chemical Evidence for Widespread Dairying in Prehistoric Britain. Proc. Natl. Acad. Sci. USA 2003, 100, 1524–1529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shoda, S.; Lucquin, A.; Yanshina, O.; Kuzmin, Y.; Shevkomud, I.; Medvedev, V.; Derevianko, E.; Lapshina, Z.; Craig, O.E.; Jordan, P. Late Glacial hunter-gatherer pottery in the Russian Far East: Indications of diversity in origins and use. Quat. Sci. Rev. 2020, 229, 106124. [Google Scholar] [CrossRef]
- Fernandes, R.; Millard, A.R.; Brabec, M.; Nadeau, M.-J.; Grootes, P. Food Reconstruction Using Isotopic Transferred Signals (FRUITS): A Bayesian Model for Diet Reconstruction. PLoS ONE 2014, 9, e87436. [Google Scholar] [CrossRef] [Green Version]
- Lucquin, A.; Colonese, A.C.; Farrell, T.F.G.; Craig, O.E. Utilising phytanic acid diastereomers for the characterisation of archaeological lipid residues in pottery samples. Tetrahedron Lett. 2016, 57, 703–707. [Google Scholar] [CrossRef] [Green Version]
- Dunne, J.; Rebay-Salisbury, K.; Salisbury, R.B.; Frisch, A.; Walton-Doyle, C.; Evershed, R.P. Milk of ruminants in ceramic baby bottles from prehistoric child graves. Nature 2019, 574, 246–248. [Google Scholar] [CrossRef] [Green Version]
- Dunne, J.; di Lernia, S.; Chłodnicki, M.; Kherbouche, F.; Evershed, R.P. Timing and pace of dairying inception and animal husbandry practices across Holocene North Africa. Quat. Int. 2018, 471, 147–159. [Google Scholar] [CrossRef]
- Notarstefano, F. Ceramica E Alimentazione: L’analisi Chimica Dei Residui Organici Nelle Ceramiche Applicata Ai Contesti Archeologici; Edipuglia: Bari, Italy, 2012. [Google Scholar]
- Sikorski, Z.E.; Staroszczyk, H. Chemia Żywności, 1st ed.; PWN: Warsaw, Poland, 2017; Volume 2. [Google Scholar]
- Eerkens, J.W. GC–MS analysis and fatty acid ratios of archaeological potsherds from the western great basin of north america. Archaeometry 2005, 47, 83–102. [Google Scholar] [CrossRef]
- Malainey, M.E. The Reconstruction and Testing of Subsistence and Settlement Strategies for the Plains, Parkland, and Southern Boreal Forest. Ph.D. Thesis, University of Manitoba, Winnipeg, MB, Canada, 1997. [Google Scholar]
- Isaksson, S. Food and Rank in Early Medieval Time. Ph.D. Thesis, Comprehensive Summary, Archaeological Research Laboratory, Stockholm University, Stockholm, Sweden, 2000. [Google Scholar]
- Isaksson, S.; Karlsson, C.; Eriksson, T. Ergosterol (5, 7, 22-ergostatrien-3β-ol) as a potential biomarker for alcohol fermentation in lipid residues from prehistoric pottery. J. Archaeol. Sci. 2010, 37, 3263–3268. [Google Scholar] [CrossRef]
- Dudd, S.N.; Evershed, R.P.; Gibson, A.M. Evidence for Varying Patterns of Exploitation of Animal Products in Different Prehistoric Pottery Traditions Based on Lipids Preserved in Surface and Absorbed Residues. J. Archaeol. Sci. 1999, 26, 1473–1482. [Google Scholar] [CrossRef]
- Hjulström, B.; Isaksson, S.; Karlsson, C. Prominent migration period building. Acta Archaeol. 2008, 79, 62–78. [Google Scholar] [CrossRef]
- Dunne, J. Organic Residue Analysis and Archaeology: Supporting Information; Historic England Publishing: London, UK, 2017. [Google Scholar]
- Rigano, F.; Oteri, M.; Micalizzi, G.; Mangraviti, D.; Dugo, P.; Mondello, L. Lipid profile of fish species by liquid chromatography coupled to mass spectrometry and a novel linear retention index database. J. Sep. Sci. 2020, 43, 1773–1780. [Google Scholar] [CrossRef]
- Aillaud, S. Field and Laboratory Studies of Diagenetic Reactions Affecting Lipid Residues Absorbed in Unglazed Archaeological Pottery Vessels. Ph.D. Thesis, University of Bristol, Bristol, UK, 2002. [Google Scholar]
- Rottlander, R.C.; Schlichtherle, H. Chemical analysis of fat residues in prehistoricvessels. Naturwissenschaften 1983, 70, 33–38. [Google Scholar]
- Hansel, F.A.; Evershed, R.P. Formation of dihydroxy acids from Z-monounsaturated alkenoic acids and their use as biomarkers for the processing of marine commodities in archaeological pottery vessels. Tetrahedron Lett. 2009, 50, 5562–5564. [Google Scholar] [CrossRef]
- Copley, M.S.; Hansel, F.A.; Sadr, K.; Evershed, R.P. Organic residue evidence for the processing of marine animal products in pottery vessels from the pre-colonial archaeological site of Kasteelberg D east, South Africa: Research article. S. Afr. J. Sci 2004, 100, 279–283. [Google Scholar]
- Hansel, F.A.; Copley, M.S.; Madureira, L.A.S.; Evershed, R.P. Thermally produced ω-(o-alkylphenyl)alkanoic acids provide evidence for the processing of marine products in archaeological pottery vessels. Tetrahedron Lett. 2004, 45, 2999–3002. [Google Scholar] [CrossRef]
- Budja, M. Neolithic pottery and the biomolecular archaeology of lipids. Doc. Praehist. 2014, 41, 196–224. [Google Scholar] [CrossRef] [Green Version]
- Pollard, A.; Batt, C.M.; Stern, B.; Young, S.M.M. Analytical Chemistry in Archaeology; Cambridge University Press: Cambridge, UK, 2007; pp. 1–404. [Google Scholar]
- Dudd, S.N. Molecular and Isotopic Characterisation of Animal Fats in Archaelogical Pottery. Ph.D. Thesis, University of Bristol, Bristol, UK, 1999. [Google Scholar]
- Hammann, S.; Cramp, L.J.E.; Whittle, M.; Evershed, R.P. Cholesterol degradation in archaeological pottery mediated by fired clay and fatty acid pro-oxidants. Tetrahedron Lett. 2018, 59, 4401–4404. [Google Scholar] [CrossRef] [Green Version]
- Papakosta, V.; Oras, E.; Isaksson, S. Early pottery use across the Baltic—A comparative lipid residue study on Ertebølle and Narva ceramics from coastal hunter-gatherer sites in southern Scandinavia, northern Germany and Estonia. J. Archaeol. Sci. Rep. 2019, 24, 142–151. [Google Scholar] [CrossRef]
- DeMan, J.M. Principles of Food Chemistry; Aspen Publishers, Inc.: Gaithersburg, MD, USA, 1999. [Google Scholar]
- Zak, I.; Balcerzyk, A. Chemia Medyczna; Sląska Akademia Medyczna: Katowice, Poland, 2001. [Google Scholar]
- Mukherjee, A.J.; Gibson, A.M.; Evershed, R.P. Trends in pig product processing at British Neolithic Grooved Ware sites traced through organic residues in potsherds. J. Archaeol. Sci. 2008, 35, 2059–2073. [Google Scholar] [CrossRef]
- Smyth, J.; Evershed, R.P. The molecules of meals: New insight into Neolithic foodways. Proc. R. Ir. Acad. C 2015, 115C, 27–46. [Google Scholar] [CrossRef]
- Eglinton, G.; Logan, G.A.; Ambler, R.P.; Boon, J.J.; Perizonius, W.R.K.; Eglinton, G.; Curry, G.B. Molecular preservation. Philos. Trans. R. Soc. B Biol. Sci. 1991, 333, 315–328. [Google Scholar]
- Colombini, M.P.; Giachi, G.; Modugno, F.; Ribechini, E. Characterisation of organic residues in pottery vessels of the Roman age from Antinoe (Egypt). Microchem. J. 2005, 79, 83–90. [Google Scholar] [CrossRef]
- Ribechini, E.; Modugno, F.; Colombini, M.P.; Evershed, R.P. Gas chromatographic and mass spectrometric investigations of organic residues from Roman glass unguentaria. J. Chromatogr. A 2008, 1183, 158–169. [Google Scholar] [CrossRef]
- Modugno, F.; Ribechini, E.; Colombini, M.P. Aromatic resin characterisation by gas chromatography-mass spectrometry. Raw and archaeological materials. J. Chromatogr. A 2006, 1134, 298–304. [Google Scholar] [CrossRef]
- Modugno, F.; Ribechini, E.; Colombini, M.P. Chemical study of triterpenoid resinous materials in archaeological findings by means of direct exposure electron ionisation mass spectrometry and gas chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 2006, 20, 1787–1800. [Google Scholar] [CrossRef]
- Heron, C.; Shoda, S.; Breu Barcons, A.; Czebreszuk, J.; Eley, Y.; Gorton, M.; Kirleis, W.; Kneisel, J.; Lucquin, A.; Müller, J.; et al. First molecular and isotopic evidence of millet processing in prehistoric pottery vessels. Sci. Rep. 2016, 6, 38767. [Google Scholar] [CrossRef] [Green Version]
- Bondetti, M.; Scott, E.; Courel, B.; Lucquin, A.; Shoda, S.; Lundy, J.; Labra-Odde, C.; Drieu, L.; Craig, O.E. Investigating the formation and diagnostic value of ω-(o-alkylphenyl)alkanoic acids in ancient pottery. Archaeometry 2020, 63, 594–608. [Google Scholar] [CrossRef] [PubMed]
- Cramp, L.J.E.; Evershed, R.P. Reconstructing Aquatic Resource Exploitation in Human Prehistory Using Lipid Biomarkers and Stable Isotopes; Elsevier: Oxford, UK, 2014. [Google Scholar]
- Dudd, S.N.; Regert, M.; Evershed, R.P. Assessing microbial lipid contributions during laboratory degradations of fats and oils and pure triacylglycerols absorbed in ceramic potsherds. Org. Geochem. 1998, 29, 1345–1354. [Google Scholar] [CrossRef]
- Christie, W.W. The composition, structure and function of lipids in the tissues of ruminant animals. Prog. Lipid Res. 1978, 17, 111–205. [Google Scholar] [CrossRef]
- Heron, C.; Evershed, R.P.; Goad, L.J. Effects of migration of soil lipids on organic residues associated with buried potsherds. J. Archaeol. Sci. 1991, 18, 641–659. [Google Scholar] [CrossRef]
- Hua, P.-Y.; Manikandan, M.; Abdelhamid, H.N.; Wu, H.-F. Graphene nanoflakes as an efficient ionizing matrix for MALDI-MS based lipidomics of cancer cells and cancer stem cells. J. Mater. Chem. B 2014, 2, 7334–7343. [Google Scholar] [CrossRef]
- Harper, C.S.; Macdonald, F.V.; Braun, K.L. Lipid Residue Analysis of Archaeological Pottery: An Introductory Laboratory Experiment in Archaeological Chemistry. J. Chem. Educ. 2017, 94, 1309–1313. [Google Scholar] [CrossRef]
- Evershed, R.P.; Heron, C.; Goad, L.J. Analysis of organic residues of archaeological origin by high-temperature gas chromatography and gas chromatography-mass spectrometry. Analyst 1990, 115, 1339–1342. [Google Scholar] [CrossRef]
- Tanasi, D.; Greco, E.; Pisciotta, F.; Hassam, S. Chemical characterization of organic residues on Late Roman amphorae from shipwrecks off the coast of Marsala (Trapani, Italy). J. Archaeol. Sci. Rep. 2021, 40, 103241. [Google Scholar] [CrossRef]
- Christie, W.W.; Han, X. Lipid Analysis. Isolation, Separation, Identification and Lipidomic Analysis, 4th ed.; Oily Press: Middlesex, UK, 2010. [Google Scholar]
- Eskilsson, C.S.; Björklund, E. Analytical-scale microwave-assisted extraction. J. Chromatogr. A 2000, 902, 227–250. [Google Scholar] [CrossRef]
- Andreotti, A.; Bonaduce, I.; Colombini, M.P.; Gautier, G.; Modugno, F.; Ribechini, E. Combined GC/MS Analytical Procedure for the Characterization of Glycerolipid, Waxy, Resinous, and Proteinaceous Materials in a Unique Paint Microsample. Anal. Chem. 2006, 78, 4490–4500. [Google Scholar] [CrossRef]
- Devièse, T.; Van Ham-Meert, A.; Hare, V.J.; Lundy, J.; Hommel, P.; Ivanovich Bazaliiskii, V.; Orton, J. Supercritical Fluids for Higher Extraction Yields of Lipids from Archeological Ceramics. Anal. Chem. 2018, 90, 2420–2424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Micalizzi, G.; Ragosta, E.; Farnetti, S.; Dugo, P.; Tranchida, P.Q.; Mondello, L.; Rigano, F. Rapid and miniaturized qualitative and quantitative gas chromatography profiling of human blood total fatty acids. Anal. Bioanal. Chem. 2020, 412, 2327–2337. [Google Scholar] [CrossRef] [PubMed]
- Schummer, C.; Delhomme, O.; Appenzeller, B.M.; Wennig, R.; Millet, M. Comparison of MTBSTFA and BSTFA in derivatization reactions of polar compounds prior to GC/MS analysis. Talanta 2009, 77, 1473–1482. [Google Scholar] [CrossRef] [PubMed]
- Ichihara, K.I.; Fukubayashi, Y. Preparation of fatty acid methyl esters for gas-liquid chromatography. J. Lipid Res. 2010, 51, 635–640. [Google Scholar] [CrossRef] [Green Version]
- Romanus, K.; Poblome, J.; Verbeke, K.; Luypaerts, A.; Jacobs, P.; De Vos, D.; Waelkens, M. An evaluation of analytical and interpretative methodologies for the extraction and identification of lipids associated with pottery sherds from the site of Sagalassos, Turkey. Archaeometry 2007, 49, 729–747. [Google Scholar] [CrossRef]
- Craig, O.E.; Steele, V.J.; Fischer, A.; Hartz, S.; Andersen, S.H.; Donohoe, P.; Glykou, A.; Saul, H.; Jones, D.M.; Koch, E.; et al. Ancient lipids reveal continuity in culinary practices across the transition to agriculture in Northern Europe. Proc. Natl. Acad. Sci. USA 2011, 108, 17910–17915. [Google Scholar] [CrossRef] [Green Version]
- Demirci, Ö.; Lucquin, A.; Craig, O.E.; Raemaekers, D.C.M. First lipid residue analysis of Early Neolithic pottery from Swifterbant (The Netherlands, ca. 4300–4000 BC). Archaeol. Anthropol. Sci. 2020, 12, 105. [Google Scholar] [CrossRef] [Green Version]
- Reber, E.A. Comparison of Neutral Compound Extraction from Archaeological Residues in Pottery Using Two Methodologies: A Preliminary Study. Separations 2021, 8, 6. [Google Scholar] [CrossRef]
- Papakosta, V.; Smittenberg, R.H.; Gibbs, K.; Jordan, P.; Isaksson, S. Extraction and derivatization of absorbed lipid residues from very small and very old samples of ceramic potsherds for molecular analysis by gas chromatography–mass spectrometry (GC–MS) and single compound stable carbon isotope analysis by gas chromatography–combustion–isotope ratio mass spectrometry (GC–C–IRMS). Microchem. J. 2015, 123, 196–200. [Google Scholar]
- Oliveira, C.; Araújo, A.; Ribeiro, A.; Delerue-Matos, C. Chromatographic analysis of honey ceramic artefacts. Archaeol. Anthropol. Sci. 2019, 11, 959–971. [Google Scholar] [CrossRef]
- Manhita, A.; Martins, S.; Gomes da Silva, M.; Lopes, M.d.C.; Barrocas Dias, C. Transporting Olive Oil in Roman Times: Chromatographic Analysis of Dressel 20 Amphorae from Pax Julia Civitas, Lusitania. Chromatographia 2020, 83, 1055–1064. [Google Scholar] [CrossRef]
- Lucejko, J.J.; La Nasa, J.; Porta, F.; Vanzetti, A.; Tanda, G.; Mangiaracina, C.F.; Corretti, A.; Colombini, M.P.; Ribechini, E. Long-lasting ergot lipids as new biomarkers for assessing the presence of cereals and cereal products in archaeological vessels. Sci. Rep. 2018, 8, 3935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beeston, R.F.; Palatinus, J.; Beck, C.; Stout, E.C.; Appendix, M. Organic Residue Analysis of Pottery Sherds from Chrysokamino. In The Chrysokamino Workshop and Its Territory (Hesperia Suppl. 36); American School: Princeton, NJ, USA, 2006; pp. 413–428. [Google Scholar]
- Pecci, A.; Degl’Innocenti, E.; Giorgi, G.; Cau Ontiveros, M.Á.; Cantini, F.; Solanes Potrony, E.; Alós, C.; Miriello, D. Organic residue analysis of experimental, medieval, and post-medieval glazed ceramics. Archaeol. Anthropol. Sci. 2016, 8, 879–890. [Google Scholar] [CrossRef]
- Colonese, A.C.; Hendy, J.; Lucquin, A.; Speller, C.F.; Collins, M.J.; Carrer, F.; Gubler, R.; Kühn, M.; Fischer, R.; Craig, O.E. New criteria for the molecular identification of cereal grains associated with archaeological artefacts. Sci. Rep. 2017, 7, 6633. [Google Scholar] [CrossRef] [Green Version]

| Pottery Samples and Archaeological Site | Lipid Biomarkers | Extraction | Derivatization | Analysis Method | Probable Origin | Ref. |
|---|---|---|---|---|---|---|
| n.35 from Zamostjen n.2 from Joton n.20 from Tianluoshan | APAAs | Solvent Extraction: MeOH/H2SO4 (4 h at 70 °C) | Direct extraction- derivatization | GC-MS GC-MSD | Aquatic | [96] |
| n.6 from Western Iberian Peninsula | ω-Hydroxy acids and cholesterol | Solvent Extraction: CH2Cl2/MeOH (2:1) | BSTFA + 1% TMCS * | GC-MS | Beeswax | [117] |
| n.14 from George Reeves, Mississippi Valley | Sterols, alkanols, alkanes and terpenoids | Solvent Extractions: CHCl3/MeOH (2:1) and MeOH/H2SO4 | BSTFA + 1% TMCS (70 °C for 1 h) NaOH in methanol (75 °C for 1 h) | GC-MS | Fish/shellfish and plants | [115] |
| n.20 from Pax Julia Civitas, Lusitania | FAs, acylglycerols and sterols | Solvent Extraction: CHCl3/MeOH (2:1) | BSTFA + 1% TMCS (microwaveoven 700 W for 30 s) | GC-MS | Plant oil | [118] |
| n.172 from Northwest India | FAs | Solvent Extraction: MeOH/H2SO4 (4 h at 70 °C) | Direct extraction- derivatization | GC-MS GC-C-IRMS | Animal fat | [4] |
| n.12 from three sites: Jneneh, Sahab and Tell Abu al-Kharaz. | FAs, alkanols, MAGs, DAGs, sterols | Solvent Extraction: CHCl3/MeOH (2:1) | BSTFA + 1% TMCS * | GC-MS | Plant oil and animal fat | [16] |
| 958 potsherds from 14 different sites in Britain | C16:0 and C18:0 | Solvent Extractions: CHCl3/MeOH (2:1) | BSTFA + 1% TMCS (70 °C for 1 h) BF3-methanol (14% w/v) (70 °C for 1 h) | GC-MS GC-C-IRMS | Ruminant adipose and dairy fats | [60] |
| n.63 from Samburu, Kenya | FAs | Solvent Extraction: MeOH/H2SO4 (1 h at 70 °C) | Direct extraction- derivatization | GC-MS GC-C-IRMS | Ruminant fats | [7] |
| n.15 from sites in Sardinia and Calabrian n.17 from Sicily | FAs, DAGs, TAGs and estolides | MAE extraction: KOH in ETOH (10% w:v) 200 W for 60 min | BSTFA + 1% TMCS (60 °C for 30 min) | GC-MS HPLC/ESI-Q-ToF | Cereal | [119] |
| n.101 from 13 different sites in Japan | FAs and isoprenoid FAs | Solvent Extraction: MeOH/H2SO4 (4 h at 70 °C) | Direct extraction-derivatization BF3-methanol (14% w/v) (70 °C for 1 h) | GC-MS GC-C-IRMS | Aquatic oils and marine foods | [6] |
| n. 5 from Sahab, Jordan | FAs | Solvent Extraction: CHCl3/MeOH (2:1) | BSTFA + 1% TMCS * | GC-MS | Animal and ruminant fat | [17] |
| n. 12 from Chrysokamino | FAs | Solvent Extraction: CH2Cl2/Et2O (1:1) | Diazomethane and KOH (25 °C for 24 h) | GC-MS | Plant oil | [120] |
| n. 10 from Qasr Ibrim, Egypt | TAGs, DAGs, MAGs, FAs, hydroxy FAs and (α, ω)-dicarboxylic acids | Solvent Extraction: CHCl3/MeOH (2:1) | BSTFA + 1% TMCS (70 °C for 1 h) BF3-methanol (14% w/v) (75 °C for 1 h) | GC-FID GC-MS GC-C-IRMS | Plant oil | [19] |
| n.6 from Florencen n.1 from the Pla d’Almatà site (Balaguer, Lleida, Spain) | FAs, MAGs and sterols | Solvent Extraction: CHCl3/MeOH (2:1) | BSTFA + 1% TMCS (70 °C for 1 h) | GC-MS | Animal fats, ruminants and vegetable oil | [121] |
| n. 15 from two sites, one in East Asia and one in Europe (Poland) | FAs and APAAs | Solvent Extraction: MeOH/H2SO4 (4 h at 70 °C) | BSTFA + 1% TMCS (70 °C for 1 h) | GC-MS GC-C-IRMS | Plant oil | [95] |
| n.2 from Switzerland | FAs, hydroxy FAs, alkylresorcinols and (α,ω)-dicarboxylic | Solvent Extractions: CH2Cl2/MeOH (2:1) and MeOH/H2SO4 | BSTFA + 1% TMCS (70 °C for 1 h) | GC-MS | Cereal grains | [122] |
| n.2 from old quarter of Lekeitio (Basque Country, northern Spain). | FAs, TAGs, (α,ω)-dicarboxylic acid and dihydroxy FAs | MAE extraction: (1) CHCl3:Hex (3:2) 600 W for 25 min (2) KOH in ETOH (10%) 200 W for 60 min | BSTFA + 1% TMCS (60 °C for 30 min) | GC-MS HPLC-ESI-Q-ToF | Fish oil | [24] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Irto, A.; Micalizzi, G.; Bretti, C.; Chiaia, V.; Mondello, L.; Cardiano, P. Lipids in Archaeological Pottery: A Review on Their Sampling and Extraction Techniques. Molecules 2022, 27, 3451. https://doi.org/10.3390/molecules27113451
Irto A, Micalizzi G, Bretti C, Chiaia V, Mondello L, Cardiano P. Lipids in Archaeological Pottery: A Review on Their Sampling and Extraction Techniques. Molecules. 2022; 27(11):3451. https://doi.org/10.3390/molecules27113451
Chicago/Turabian StyleIrto, Anna, Giuseppe Micalizzi, Clemente Bretti, Valentina Chiaia, Luigi Mondello, and Paola Cardiano. 2022. "Lipids in Archaeological Pottery: A Review on Their Sampling and Extraction Techniques" Molecules 27, no. 11: 3451. https://doi.org/10.3390/molecules27113451
APA StyleIrto, A., Micalizzi, G., Bretti, C., Chiaia, V., Mondello, L., & Cardiano, P. (2022). Lipids in Archaeological Pottery: A Review on Their Sampling and Extraction Techniques. Molecules, 27(11), 3451. https://doi.org/10.3390/molecules27113451








