A Mini-Review on Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Topical Delivery of Phytochemicals for the Treatment of Acne Vulgaris
Abstract
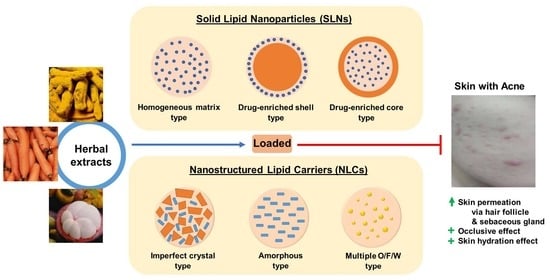
:1. Introduction
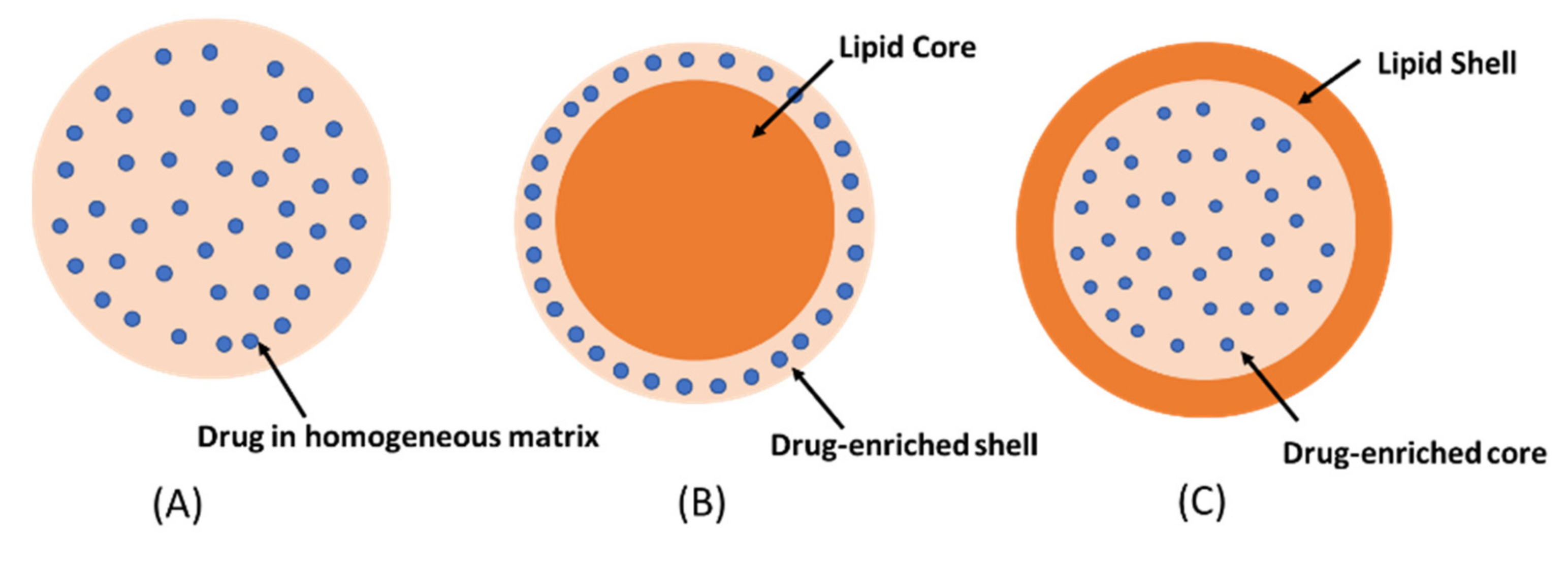
2. Solid Lipid Nanoparticles (SLNs)
- Homogeneous matrix model: the drug is dispersed in lipid matrix without the use of solubilizers or surfactants. This model is usually prepared by the cold homogenization technique [15].
- The drug-enriched shell model: a mixture of lipid and drug is heated at temperature above the melting point of the lipid. On rapid cooling, lipid precipitates at the core whereas the drug is concentrated at the outer melted lipid. The drug-enriched shell is completely formed when the melted mixture is cooled to room temperature [16].
- The drug-enriched core model: the concentration of drug in the melted lipid is close to its saturation solubility. The cooling process creates supersaturation of the drug in the melted lipid, resulting in drug precipitation at the core prior to lipid crystallization. Further cooling will lead to the crystallization of the lipid surrounding the drug core as a shell [17]. The drug-enriched shell and the drug-enriched core models are usually produced by hot homogenization technique.
- SLNs are biodegradable and biocompatible.
- They can modulate the drug release.
- SLNs can enhance the penetration of drugs through the stratum corneum.
- They have occlusive property which can increase skin hydration.
- SLNs can be sterilized using the autoclave. The high temperature (121 °C) during sterilization by autoclaving certainly causes a hot o/w microemulsion. On subsequent cooling, the SLNs reformed. However, it should be noted that the average particle size of SLNs is usually increased after sterilization by heating.
- Ease of large-scale production and low production cost.
- They are safe because of avoidance of organic solvents in the preparation.
- Incorporation of lipophilic and hydrophilic drugs is feasible.
- They can increase storage stability of loaded drug.
- Low loading capacity and drug expulsion during storage. Since the lipids crystallize in high energy modification (α form) during the preparation, they can transform to more organized, lower energy modification (β form) during storage. This modification resulted in fewer imperfections of lipid matrix for drug loading leading to drug expulsion.
- The irreversible transformation of a low viscous SLNs dispersion into a viscous gel, known as gelation phenomena, may occur rapidly and is unpredictable upon storage. In this circumstance, the surfactants can no longer stabilize the new surfaces, and hence the particle aggregation occurred. It has been reported that the addition of co-emulsifying surfactants with high mobility such as glycocholate can retard a gelation in SLNs.
- Irritation and sensitizing potential of some surfactants. Three surfactant types including cationic (such as cetylpyridinium chloride, cetyltrimethyl ammonium bromide), anionic (such as sodium dodecyl sulfate, sodium glycocholate), and non-ionic (such as poloxamer, Tween, phospholipid, Cremophor) are generally used in the preparation of both SLNs and NLCs. They are chosen based on their hydrophile-lipophile balance (HLB) values, effects on the particle size and lipid modification of the lipid carriers, as well as the route of administration of the lipid carriers [20]. It has been reported that the type and concentration of surfactants exert influence on the potential toxicity of these lipid carriers. According to the studies of Scholer et al. (2001), SLN formulation containing cetylpyridinium chloride exhibited strong cytotoxic effect on murine peritoneal macrophages, whereas other SLN formulations containing poloxamer, Tween, phospholipid, and sodium dodecyl sulfate at the same concentration, reduced cell viability slightly [21]. Furthermore, cationic and anionic surfactants are broadly regarded as potent irritants to human skin, with cationic surfactants being more cytotoxic than anionic surfactants. In contrast, non-ionic surfactants are considered to be safe (lowest irritation potential) [22]. Therefore, the safety test of SLNs and NLCs should be investigated prior to in vivo use of these lipid carriers.
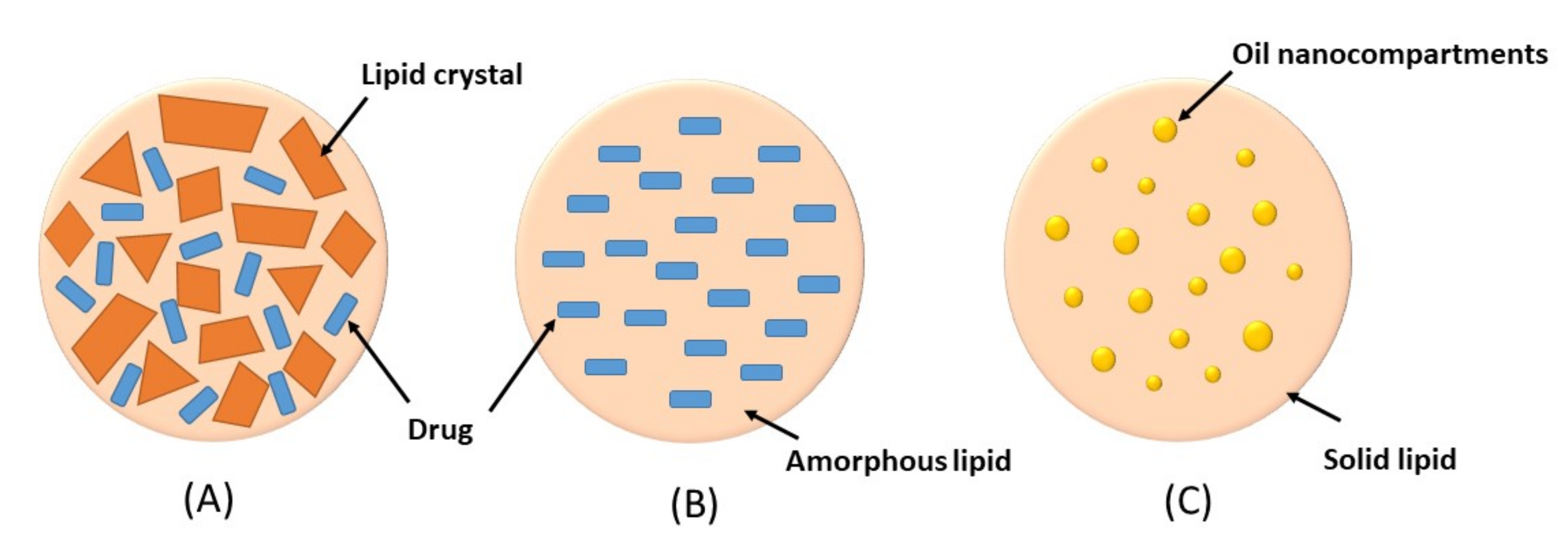
3. Nanostructured Lipid Carriers (NLCs)
- Imperfect crystal type: this type involves mixing of spatially different liquid lipids such as glycerides and solid lipids which introduce imperfections in the crystal order leading to more space for drug loading. The imperfection can be increased by using a mixture of various glycerides which vary in saturation and length of carbon chains.
- Amorphous type: this type is formed by incorporating special liquid oils such as isopropyl myristate or hydroxyoctacosanyl hydroxystearate in a lipid matrix. The matrix will solidify in an amorphous form that potentially reduces the expulsion of the loaded drug by delaying the crystallization of lipids during the preparation and storage of the NLCs
- Multiple O/F/W type: this type is formed by adding high amount of liquid lipid beyond its solubility in the lipid matrix. This will create oil nanocompartments distributed in the solid matrix. Drug solubility in oil nanocompartment is higher than in solid matrix which enables higher drug loading. Moreover, a solid lipid matrix around the oil nanocompartments acts as a barrier that prevents drug leakage and provides controlled drug release.
- Higher drug loading capacity by the formation of imperfect matrix and greater drug solubility in liquid lipid in the matrix.
- NLCs reduce drug expulsion during long-term storage. As known, drug expulsion has been occurred during the ongoing crystallization process of the lipid matrix. By mixing the special liquid oil in NLCs formulation, it forms amorphous structure which limits the crystallization of lipid matrix leading to a reduction of drug expulsion. More flexibility for modulation of drug release by modifying the types and amounts of liquid lipids or surfactants.
- Irritation and sensitizing potential of some surfactants. Hwang et al. (2014) revealed that NLC formulation with cationic surfactants induced cell death and the release of inflammatory mediators [29].
4. Preparation Methods of SLNs and NLCs
- Hot homogenization
- Cold homogenization
- Ultrasonication or high-shear homogenization
- Micro-emulsification
- Double-emulsion
- Solvent emulsification/evaporation
- Solvent diffusion and injection
- Membrane contractor technique
- Phase inversion technique
- Supercritical fluid extraction of emulsions (SFEE)
5. Characterization of SLNs and NLCs
- Drug loading and entrapment efficiency:
- Particle size and size distribution
- Zeta potential:
- Degree of crystallinity:
- Co-existence of different colloidal species:
- In vitro drug release:
6. SLNs and NLCs as Topical Carriers for Anti-Acne Phytochemicals
6.1. SLNs as a Promising Carrier System for the Topical Delivery of Anti-Acne Phytochemicals
6.2. NLCs as a Promising Carrier System for the Topical Delivery of Anti-Acne Phytochemicals
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xu, N.; Deng, W.; He, G.; Gan, X.; Gao, S.; Chen, Y.; Gao, Y.; Xu, K.; Qi, J.; Lin, H.; et al. Alpha- and gamma-mangostins exhibit anti-acne activities via multiple mechanisms. Immunopharmacol. Immunotoxicol. 2018, 40, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.; Sachan, A.K.; Jain, S.; Barik, R. Studies on inhibitory effect of Eucalyptus oil on sebaceous glands for the management of acne. Indian J. Nat. Prod. Resour. 2011, 2, 345–349. [Google Scholar]
- Fabbrocini, G.; Staibano, S.; De Rosa, G.; Battimiello, V.; Fardella, N.; Ilardi, G.; La Rotonda, M.M.; Longobardi, A.; Mazzella, M.; Siano, M.; et al. Resveratrol-containing gel for the treatment of acne vulgaris: A single-blind, vehicle-controlled, pilot study. Am. J. Clin. Dermatol. 2011, 12, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Garg, T. Current nanotechnological approaches for an effective delivery of bio-active drug molecules in the treatment of acne. Artif. Cells Nanomed. Biotechnol. 2016, 44, 98–105. [Google Scholar] [CrossRef]
- Battaglia, L.; Gallarate, M. Lipid nanoparticles: State of the art, new preparation methods and challenges in drug delivery. Expert Opin. Drug Deliv. 2012, 9, 497–508. [Google Scholar] [CrossRef]
- Beloqui, A.; Solinís, M.; Rodríguez-Gascón, A.; Almeida, A.J.; Préat, V. Nanostructured lipid carriers: Promising drug delivery systems for future clinics. Nanomedicine 2016, 12, 143–161. [Google Scholar] [CrossRef]
- Jain, S.; Patel, N.; Shah, M.K.; Khatri, P.; Vora, N. Recent advances in lipid-based vesicles and particulate carriers for topical and transdermal application. J. Pharm. Sci. 2017, 106, 423–445. [Google Scholar] [CrossRef]
- Verma, S.; Utreja, P.; Duvedi, D.R.; Prasad, D.; Kumar, L. Nanotechnological carriers for treatment of acne. Recent Pat. Antiinfect Drug Discov. 2018, 13, 105–126. [Google Scholar] [CrossRef]
- Müller, R.H.; Mehnert, W.; Lucks, J.S.; Schwarz, C.; Zur Mühlen, A.; Weyhers, H.; Freitas, C.; Rühl, D. Solid lipid nanoparticles (SLN)—An alternative colloidal carrier system for controlled drug delivery. Eur. J. Pharm. Biopharm. 1995, 41, 62–69. [Google Scholar]
- Müller, R.H.; Lippacher, A.; Gohla, S. Solid lipid nanoparticles (SLN) as a carrier system for the controlled release of drugs. In Handbook of Pharmaceutical Controlled Release Technology; Wise, D., Ed.; Marcel Dekker: New York, NY, USA, 2000; pp. 377–392. [Google Scholar]
- Mukherjee, S.; Ray, S.; Thakur, R.S. Solid lipid nanoparticles: A modern formulation approach in drug delivery system. Indian J. Pharm. Sci. 2009, 71, 349–358. [Google Scholar] [CrossRef] [Green Version]
- Mishra, V.; Bansal, K.K.; Verma, A.; Yadav, N.; Thakur, S.; Sudhakar, K.; Rosenholm, J.M. Solid lipid nanoparticles: Emerging colloidal nano drug delivery systems. Pharmaceutics 2018, 10, 191. [Google Scholar] [CrossRef] [Green Version]
- Smith, T.; Affram, K.; Nottingham, E.L.; Han, B.; Amissah, F.; Krishnan, S.; Trevino, J.; Agyare, E. Application of smart solid lipid nanoparticles to enhance the efficacy of 5-fluorouracil in the treatment of colorectal cancer. Sci. Rep. 2020, 10, 16989. [Google Scholar] [CrossRef]
- Mardhiah Adib, Z.; Ghanbarzadeh, S.; Kouhsoltani, M.; Khosroshahi, A.Y.; Hamishehkar, H. The effect of particle size on the deposition of solid lipid nanoparticles in different skin layers: A histological study. Adv. Pharm. Bull. 2016, 6, 31–36. [Google Scholar] [CrossRef] [Green Version]
- Zur Mühlen, A.; Mehnert, W. Drug release and release mechanisms of prednisolone loaded solid lipid nanoparticles. Pharmazie 1998, 53, 552–555. [Google Scholar]
- Müller, R.H.; Radtke, M.; Wissing, S.A. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv. Drug Deliv. Rev. 2002, 54 (Suppl. S1), S131–S155. [Google Scholar] [CrossRef]
- Wissing, S.A.; Muller, R.H. The influence of the crystallinity of lipid nanoparticles on their occlusive properties. Int. J. Pharm. 2002, 242, 377–379. [Google Scholar] [CrossRef]
- Scalia, S.; Young, P.M.; Traini, D. Solid lipid microparticles as an approach to drug delivery. Expert Opin. Drug Deliv. 2015, 12, 583–599. [Google Scholar] [CrossRef]
- Borges, A.; Freitas, V.; Mateus, N.; Fernandes, I.; Oliveira, J. Solid lipid nanoparticles as carriers of natural phenolic compounds. Antioxidants 2020, 9, 998. [Google Scholar] [CrossRef]
- Chauhan, I.; Yasir, M.; Verma, M.; Singh, A.P. Nanostructured Lipid Carriers: A Groundbreaking Approach for Transdermal Drug Delivery. Adv. Pharm. Bull. 2020, 10, 150–165. [Google Scholar] [CrossRef]
- Scholer, N.; Olbrich, C.; Tabbatt, K.; Muller, R.H.; Hahn, H.; Liesenfeld, O. Surfactant, but not the size of solid lipid nanoparticles (SLN) influences viability and cytokine production of macrophages. Int. J. Pharm. 2001, 221, 57–67. [Google Scholar] [CrossRef]
- Effendy, I.; Maibach, H.I. Surfactants and experimental irritant contact dermatitis. Contact Dermat. 1995, 33, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.A.; Md, S.; Sahni, J.K.; Baboota, S.; Dang, S.; Ali, J. Nanostructured lipid carriers system: Recent advances in drug delivery. J. Drug Target. 2012, 20, 813–830. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.H.; Wu, C.T. Optimization of nanostructured lipid carriers for lutein delivery. Coll. Surf. A Physicochem. Eng. Asp. 2010, 353, 149–156. [Google Scholar] [CrossRef]
- Khosa, A.; Reddi, S.; Saha, R.N. Nanostructured lipid carriers for site-specific drug delivery. Biomed. Pharmacother. 2018, 103, 598–613. [Google Scholar] [CrossRef]
- Vyas, A.; Kumar Sonker, A.; Gidwani, B. Carrier-based drug delivery system for treatment of acne. Sci. World J. 2014, 2014, 276260. [Google Scholar] [CrossRef] [Green Version]
- Subramaniam, B.; Siddik, Z.H.; Nagoor, N.H. Optimization of nanostructured lipid carriers: Understanding the types, designs, and parameters in the process of formulations. J. Nanopart. Res. 2020, 22, 141. [Google Scholar] [CrossRef]
- Jaiswal, P.; Gidwani, B.; Vyas, A. Nanostructured lipid carriers and their current application in targeted drug delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 27–40. [Google Scholar] [CrossRef]
- Hwang, T.L.; Aljuffali, I.A.; Lin, C.F.; Chang, Y.T.; Fang, J.Y. Cationic additives in nanosystems activate cytotoxicity and inflammatory response of human neutrophils: Lipid nanoparticles versus polymeric nanoparticles. Int. J. Nanomed. 2015, 10, 371–385. [Google Scholar]
- Oláh, A.; Tóth, B.I.; Borbíró, I.; Sugawara, K.; Szöllõsi, A.G.; Czifra, G.; Pál, B.; Ambrus, L.; Kloepper, J.; Camera, E.; et al. Cannabidiol exerts sebostatic and antiinflammatory effects on human sebocytes. J. Clin. Investig. 2014, 124, 3713–3724. [Google Scholar] [CrossRef] [Green Version]
- Carlotti, M.E.; Sapino, S.; Trotta, M.; Battaglia, L.; Vione, D.; Pelizzetti, E. Photostability and stability over time of retinyl palmitate in an o/w emulsion and in SLN introduced in the emulsion. J. Disper. Sci. Technol. 2005, 26, 125–138. [Google Scholar] [CrossRef]
- Battaglia, L.; Gallarate, M.; Panciani, P.P.; Ugazio, E.; Sapino, S.; Peira, E.; Chirio, D. Techniques for the preparation of solid lipid nano and microparticles. In Application of Nanotechnology in Drug Delivery; Sezer, A.D., Ed.; IntechOpen: London, UK, 2014; pp. 51–75. [Google Scholar]
- Charcosse, C.; El-Harati, A.; Fessi, H. Preparation of solid lipid nanoparticles using a membrane contactor. J. Control. Release 2005, 108, 112–120. [Google Scholar] [CrossRef]
- Opatha, S.A.T.; Titapiwatanakun, V.; Chutoprapat, R. Transfersomes: A promising nanoencapsulation technique for transdermal drug delivery. Pharmaceutics 2020, 12, 855. [Google Scholar] [CrossRef]
- Söderman, O.; Stilbs, P.; Price, W.S. NMR studies of surfactants. Concept Magn. Reason. Part A 2004, 23, 121–135. [Google Scholar] [CrossRef]
- Furó, I. NMR spectroscopy of micelles and related systems. J. Mol. Liq. 2005, 117, 117–137. [Google Scholar] [CrossRef]
- Üner, M.; Karaman, E.F.; Aydoğmuş, Z. Solid lipid nanoparticles and nanostructured lipid carriers of loratadine for topical application: Physicochemical stability and drug penetration through rat skin. Trop. J. Pharm. Res. 2014, 13, 653–660. [Google Scholar] [CrossRef] [Green Version]
- Raza, K.; Singh, B.; Singal, P.; Wadhwa, S.; Katare, O.P. Systematically optimized biocompatible isotretinoin-loaded solid lipid nanoparticles (SLNs) for topical treatment of acne. Coll. Surf. B 2013, 105, 67–74. [Google Scholar] [CrossRef]
- Raza, K.; Singh, B.; Singla, S.; Wadhwa, S.; Garg, B.; Chhibber, S.; Katare, O.P. Nanocolloidal carriers of isotretinoin: Antimicrobial activity against Propionibacterium acnes and dermatokinetic modeling. Mol. Pharm. 2013, 10, 1958–1963. [Google Scholar] [CrossRef]
- Liu, J.; Hu, W.; Chen, H.; Ni, Q.; Xu, H.; Yang, X. Isotretinoin-loaded solid lipid nanoparticles with skin targeting for topical delivery. Int. J. Pharm. 2007, 328, 191–195. [Google Scholar] [CrossRef]
- Pokharkar, V.B.; Mendiratta, C.; Kyadarkunte, A.Y.; Bhosale, S.H.; Barhate, G.A. Skin delivery aspects of benzoyl peroxide-loaded solid lipid nanoparticles for acne treatment. Ther. Deliv. 2014, 5, 635–652. [Google Scholar] [CrossRef]
- Pradhan, M.; Singh, D.; Singh, M.R. Influence of selected variables on fabrication of Triamcinolone acetonide loaded solid lipid nanoparticles for topical treatment of dermal disorders. Artif. Cells Nanomed. Biotechnol. 2016, 44, 392–400. [Google Scholar] [CrossRef]
- Madan, J.R.; Khude, P.A.; Dua, K. Development and evaluation of solid lipid nanoparticles of mometasone furoate for topical delivery. Int. J. Pharm. Res. 2014, 4, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pople, P.V.; Singh, K.K. Development and evaluation of topical formulation containing solid lipid nanoparticles of vitamin A. Aaps Pharmscitech 2006, 7, E63–E69. [Google Scholar] [CrossRef] [Green Version]
- Kelidari, H.R.; Saeedi, M.; Hajheydari, Z.; Akbari, J.; Morteza-Semnani, K.; Akhtari, J.; Valizadeh, H.; Asare-Addo, K.; Nokhodchi, A. Spironolactone loaded nanostructured lipid carrier gel for effective treatment of mild and moderate acne vulgaris: A randomized, double-blind, prospective trial. Colloids Surf. B 2016, 146, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Fatima, N.; Rehman, S.; Nabi, B.; Baboota, S.; Ali, J. Harnessing nanotechnology for enhanced topical delivery of clindamycin phosphate. J. Drug Deliv. Sci. Technol. 2019, 54, 101253. [Google Scholar] [CrossRef]
- Jain, A.; Garg, N.K.; Jain, A.; Kesharwani, P.; Jain, A.K.; Nirbhavane, P.; Tyagi, R.K. A synergistic approach of adapalene-loaded nanostructured lipid carriers, and vitamin C co-administration for treating acne. Drug Dev. Ind. Pharm. 2016, 42, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Malik, D.S.; Kaur, G. Exploring therapeutic potential of azelaic acid loaded NLCs for the treatment of acne vulgaris. J. Drug Deliv. Sci. Technol. 2020, 55, 101418. [Google Scholar] [CrossRef]
- Patwekar, S.L.; Pedewad, S.R.; Gattani, S. Development and evaluation of nanostructured lipid carriers-based gel of isotretinoin. Part. Sci. Technol. 2018, 36, 832–843. [Google Scholar] [CrossRef]
- Woo, J.O.; Misran, M.; Lee, P.F.; Tan, L.P. Development of a controlled release of salicylic acid loaded stearic acid-oleic acid nanoparticles in cream for topical delivery. Sci. World J. 2014, 2014, 205703. [Google Scholar] [CrossRef]
- AlZahabi, S.; Sakr, O.S.; Ramadan, A.A. Nanostructured lipid carriers incorporating prickly pear seed oil for the encapsulation of vitamin A. J. Cosmet. Dermatol. 2019, 18, 1875–1884. [Google Scholar] [CrossRef]
- Chen, J.; Wei, N.; Lopez-Garcia, M.; Ambrose, D.; Lee, J.; Annelin, C.; Peterson, T. Development and evaluation of resveratrol, Vitamin E, and epigallocatechin gallate loaded lipid nanoparticles for skin care applications. Eur. J. Pharm. Biopharm. 2017, 117, 286–291. [Google Scholar] [CrossRef]
- Yoon, J.Y.; Kwon, H.H.; Min, S.U.; Thiboutot, D.M.; Suh, D.H. Epigallocatechin-3-gallate improves acne in humans by modulating intracellular molecular targets and inhibiting P. acnes. J. Investig. Dermatol. 2013, 133, 429–440. [Google Scholar] [CrossRef] [Green Version]
- Szulc-Musioł, B.; Sarecka-Hujar, B. The use of micro- and nanocarriers for resveratrol delivery into and across the skin in different skin diseases—A Literature Review. Pharmaceutics 2021, 13, 451. [Google Scholar] [CrossRef]
- Shrotriya, S.; Ranpise, N.; Satpute, P.; Vidhate, B. Skin targeting of curcumin solid lipid nanoparticles-engrossed topical gel for the treatment of pigmentation and irritant contact dermatitis. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1471–1482. [Google Scholar] [CrossRef] [Green Version]
- Kakkar, V.; Kaur, I.P.; Kaur, A.P.; Saini, K.; Singh, K.K. Topical delivery of tetrahydrocurcumin lipid nanoparticles effectively inhibits skin inflammation: In vitro and in vivo study. Drug Dev. Ind. Pharm. 2018, 44, 1701–1712. [Google Scholar] [CrossRef]
- Hamishehkar, H.; Same, S.; Adibkia, K.; Zarza, K.; Shokri, J.; Taghaee, M.; Kouhsoltani, M. A comparative histological study on the skin occlusion performance of a cream made of solid lipid nanoparticles and Vaseline. Res. Pharm. Sci. 2015, 10, 378–387. [Google Scholar]
- Talarico, L.; Consumi, M.; Leone, G.; Tamasi, G.; Magnani, A. Solid lipid nanoparticles produced via a coacervation method as promising carriers for controlled release of Quercetin. Molecules 2021, 26, 2694. [Google Scholar] [CrossRef]
- Tsai, T.H.; Huang, W.C.; Lien, T.J.; Huang, Y.H.; Chang, H.; Yu, C.H.; Tsai, P.J. Clove extract and eugenol suppress inflammatory responses elicited by Propionibacterium acnes in vitro and in vivo. Food Agric. Immunol. 2017, 28, 916–931. [Google Scholar] [CrossRef] [Green Version]
- Garg, A.; Singh, S. Targeting of eugenol-loaded solid lipid nanoparticles to the epidermal layer of human skin. Nanomedicine 2014, 9, 1223–1238. [Google Scholar] [CrossRef]
- Taylor, E.J.M.; Yu, Y.; Champer, J.; Kim, J. Resveratrol demonstrates antimicrobial effects against Propionibacterium acnes in vitro. Dermatol. Ther. 2014, 4, 249–257. [Google Scholar] [CrossRef] [Green Version]
- Rigon, R.B.; Fachinetti, N.; Severino, P.; Santana, M.H.; Chorilli, M. Skin delivery and in vitro biological evaluation of trans-resveratrol-loaded solid lipid nanoparticles for skin disorder therapies. Molecules 2016, 21, 116. [Google Scholar] [CrossRef] [Green Version]
- Rapalli, V.K.; Kaul, V.; Waghule, T.; Gorantla, S.; Sharma, S.; Roy, A.; Dubey, S.K.; Singhvi, G. Curcumin loaded nanostructured lipid carriers for enhanced skin retained topical delivery: Optimization, scale-up, in-vitro characterization and assessment of ex-vivo skin deposition. Eur. J. Pharm. Sci. 2020, 152, 105438. [Google Scholar] [CrossRef] [PubMed]
- Lacatusu, I.; Badea, G.; Popescu, M.; Bordei, N.; Istrati, D.; Moldovan, L.; Seciu, A.M.; Panteli, M.I.; Rasit, I.; Badea, N. Marigold extract, azelaic acid and black caraway oil into lipid nanocarriers provides a strong anti-inflammatory effect in vivo. Ind. Crops Prod. 2017, 109, 141–150. [Google Scholar] [CrossRef]
- Lacatusu, I.; Istrati, D.; Bordei, N.; Popescu, M.; Seciu, A.M.; Panteli, L.M.; Badea, N. Synergism of plant extract and vegetable oils-based lipid nanocarriers: Emerging trends in development of advanced cosmetic prototype products. Mater. Sci. Eng. C 2020, 108, 110412. [Google Scholar] [CrossRef]
- Istrati, D.; Lacatusu, I.; Bordei, N.; Badea, G.; Oprea, O.; Stefan, L.M.; Stan, R.; Badea, N.; Meghea, A. Phyto-mediated nanostructured carriers based on dual vegetable actives involved in the prevention of cellular damage. Mater. Sci. Eng. C 2016, 64, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Arsenie, L.V.; Lacatusu, I.; Oprea, O.; Bordei, N.; Bacalum, M.; Badea, N. Azelaic acid-willow bark extract-panthenol—Loaded lipid nanocarriers improve the hydration effect and antioxidant action of cosmetic formulations. Ind. Crops Prod. 2020, 154, 112658. [Google Scholar] [CrossRef]
- Sun, B.; Wu, L.; Wu, Y.; Zhang, C.; Qin, L.; Hayashi, M.; Kudo, M.; Gao, M.; Liu, T. Therapeutic potential of Centella asiatica and its triterpenes: A review. Front. Pharmacol. 2020, 11, 568032. [Google Scholar] [CrossRef] [PubMed]
- Da Rocha, P.B.R.; Souza, B.S.; Andrade, L.M.; Dos Anjos, J.L.V.; Mendanha, S.A.; Alonso, A.; Marreto, R.N.; Taveira, S.F. Enhanced asiaticoside skin permeation by Centella asiatica-loaded lipid nanoparticles: Effects of extract type and study of stratum corneum lipid dynamics. J. Drug Deliv. Sci. Technol. 2019, 50, 305–312. [Google Scholar] [CrossRef]
- Fontao, F.; Von Engelbrechten, M.; Seilaz, C.; Sorg, O.; Saurat, J.H. Microcomedones in non-lesional acne prone skin. New orientations on comedogenesis and its prevention. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 357–364. [Google Scholar] [CrossRef]
- Nocera, T.; Josse, G.; Garidou, L.; Rizz, N.C.; Bessou-Touya, S.; Saurat, J.H. 15410 Efficacy and tolerability of a new formulation containing Silybum marianum fruit extract in young adults with acne-prone skin: A comparative controlled study. J. Am. Acad. Dermatol. 2020, 83, AB150. [Google Scholar] [CrossRef]
- Singh, P.; Arya, M.; Kanoujia, J.; Singh, M.; Gupta, K.P.; Saraf, S.A. Design of topical nanostructured lipid carrier of silymarin and its effect on 7,12-dimethylbenz[a]anthracene (DMBA) induced cellular differentiation in mouse skin. RSC Adv. 2016, 6, 84965–84977. [Google Scholar] [CrossRef]
- Rosli, N.A.; Hasham, R.; Aziz, A.A.; Aziz, R. Formulation and characterization of nanostructured lipid carrier encapsulated Zingiber zerumbet oil using ultrasonication technique. J. Adv. Res. Appl. Mech. 2015, 11, 16–23. [Google Scholar]
- Yob, N.J.; Jofrry SMohd Meor Mohd Affandi, M.M.R.; Teh, L.K.; Salleh, M.Z.; Zakaria, Z.A. Zingiber zerumbet (L.) Smith: A Review of Its Ethnomedicinal, Chemical, and Pharmacological Uses. Evid. Based Complement. Altern. Med. 2011, 2011, 543216. [Google Scholar] [CrossRef]
- Carbone, C.; Martins-Gomes, C.; Caddeo, C.; Silva, A.M.; Musumeci, T.; Pignatello, R.; Puglisi, G.; Souto, E.B. Mediterranean essential oils as precious matrix components and active ingredients of lipid nanoparticles. Int. J. Pharm. 2018, 548, 217–226. [Google Scholar] [CrossRef]
- Bose, K.S.; Sharma, K.; Chhibber, S.; Harjai, K. Therapeutic potential of nanolipoidal α-terpineol in combating keratitis induced by Pseudomonas aeruginosa in the murine model. Int. J. Pharm. 2021, 594, 120175. [Google Scholar] [CrossRef]
- Esposito, E.; Drechsler, M.; Cortesi, R.; Nastruzzi, C. Encapsulation of cannabinoid drugs in nanostructured lipid carriers. Eur. J. Pharm. Biopharm. 2016, 102, 87–91. [Google Scholar] [CrossRef]
- Wen, M.M.; El-Zahaby, S.A.; Abdelwahab, I. Nanostructured lipid carriers: A promising topical system for delivering cinnamon oil in wound care treatment. In Proceedings of the Fifth Euro-Mediterranean conference of Natural Products, Pharma and Complementary Medicine, Limassol, Cyprus, 1 March 2018. [Google Scholar]
- Sun, P.; Zhao, L.; Zhang, N.; Wang, C.; Wu, W.; Mehmood, A.; Zhang, L.; Ji, B.; Zhou, F. Essential oil and juice from bergamot and sweet orange improve acne vulgaris caused by excessive androgen secretion. Mediat. Inflamm. 2020, 2020, 8868107. [Google Scholar] [CrossRef]
- Shaaban, M.; Nasr, M.; Tawfik, A.A.; Fadel, M.; Sammour, O. Bergamot oil as an integral component of nanostructured lipid carriers and a photosensitizer for photodynamic treatment of vitiligo: Characterization and clinical experimentation. Expert Opin. Drug Deliv. 2021, 18, 139–150. [Google Scholar] [CrossRef]
- Scalia, S.; Franceschinis, E.; Bertelli, D.; Iannuccelli, V. Comparative evaluation of the effect of permeation enhancers, lipid nanoparticles and colloidal silica on in vivo human skin penetration of quercetin. Skin Pharmacol. Physiol. 2013, 26, 57–67. [Google Scholar] [CrossRef]
- Zamarioli, C.M.; Martins, R.M.; Carvalho, E.C.; Freitas, L.A.P. Nanoparticles containing curcuminoids (Curcuma longa): Development of topical delivery formulation. Rev. Bras. Farmacogn. 2015, 25, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Vijayan, V.; Aafreen, S.; Sakthivel, S.; Reddy, K.R. Formulation and characterization of solid lipid nanoparticles loaded Neem oil for topical treatment of acne. J. Acute Dis. 2013, 2, 282–286. [Google Scholar] [CrossRef] [Green Version]
- Gokce, E.H.; Korkmaz, E.; Dellera, E.; Sandri, G.; Bonferoni, M.C.; Ozer, O. Resveratrol-loaded solid lipid nanoparticles versus nanostructured lipid carriers: Evaluation of antioxidant potential for dermal applications. Int. J. Nanomed. 2012, 7, 1841–1850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afra, B.; Mohammadi, M.; Soleimani, M.; Mahjub, R. Preparation, statistical optimization, in vitro characterization, and in vivo pharmacological evaluation of solid lipid nanoparticles encapsulating propolis flavonoids: A novel treatment for skin edema. Drug Dev. Ind. Pharm. 2020, 46, 1163–1176. [Google Scholar] [CrossRef] [PubMed]
- Yostawonkul, J.; Surassmo, S.; Namdee, K.; Khongkow, M.; Boonthum, C.; Pagseesing, S.; Saengkrit, N.; Ruktanonchai, U.R.; Chatdarong, K.; Ponglowhapan, S.; et al. Nanocarrier-mediated delivery of α-mangostin for non-surgical castration of male animals. Sci. Rep. 2017, 7, 16234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saporito, F.; Sandri, G.; Bonferoni, M.C.; Rossi, S.; Boselli, C.; Cornaglia, A.I.; Mannucci, B.; Grisoli, P.; Vigani, B.; Ferrari, F. Essential oil-loaded lipid nanoparticles for wound healing. Int. J. Nanomed. 2018, 13, 175–186. [Google Scholar] [CrossRef] [Green Version]
- Okonogi, S.; Riangjanapatee, P. Physicochemical characterization of lycopene-loaded nanostructured lipid carrier formulations for topical administration. Int. J. Pharm. 2015, 478, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Muller, R.H.; Mader, K.; Gohla, S. Solid lipid nanoparticles (SLN) for controlled drug delivery—A review of the state of the art. Eur. J. Pharm. Biopharm. 2000, 50, 161–177. [Google Scholar] [CrossRef]
- Schäfer-Korting, M.; Mehnert, W.; Korting, H.C. Lipid nanoparticles for improved topical application of drugs for skin diseases. Adv. Drug Deliv. Rev. 2007, 59, 427–443. [Google Scholar] [CrossRef] [PubMed]
- Pardeike, J.; Hommoss, A.; Muller, R.H. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int. J. Pharm. 2009, 366, 170–184. [Google Scholar] [CrossRef]
- Muller, R.H.; Shegokar, R.; Keck, C.M. 20 years of lipid nanoparticles (SLN and NLC): Present state of development and industrial applications. Curr. Drug Discov. Technol. 2011, 8, 207–227. [Google Scholar] [CrossRef]
- Naseri, N.; Valizadeh, H.; Milani, P.Z. Solid lipid nanoparticles and nanostructured lipid carriers: Structure, preparation and application. Adv. Pharm. Bull. 2015, 5, 305–313. [Google Scholar] [CrossRef] [Green Version]
- Lauterbach, A.; Müller-Goymann, C.C. Applications and limitations of lipid nanoparticles in dermal and transdermal drug delivery via the follicular route. Eur. J. Pharm. Biopharm. 2015, 97, 152–163. [Google Scholar] [CrossRef]
- Garcês, A.; Amaral, M.H.; Sousa Lobo, J.M.; Silva, A.C. Formulations based on solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for cutaneous use: A review. Eur. J. Pharm. Sci. 2018, 112, 159–167. [Google Scholar] [CrossRef]
- Montoto, S.S.; Muraca, G.; Ruiz, M.E. Solid lipid nanoparticles for drug delivery: Pharmacological and biopharmaceutical aspects. Front. Mol. Biosci. 2020, 7, 587997. [Google Scholar] [CrossRef]
- Souto, E.B.; Baldim, I.; Oliveira, W.P.; Rao, R.; Yadav, N.; Gama, F.M.; Mahant, S. SLN and NLC for topical, dermal, and transdermal drug delivery. Rev. Expert Opin. Drug Deliv. 2020, 17, 357–377. [Google Scholar] [CrossRef]
- Dhiman, N.; Awasthi, R.; Sharma, B.; Kharkwal, H.; Kulkarni, G.T. Lipid nanoparticles as carriers for bioactive delivery. Front. Chem. 2021, 9, 580118. [Google Scholar] [CrossRef]
| Lipid Carriers | Phytochemicals | Compositions | Findings | References |
|---|---|---|---|---|
| SLNs | Quercetin | Tristearin, phosphatidylcholine | The penetration through the stratum corneum of quercetin via quercetin-loaded SLNs in topical emulsion (21.2 ± 2.9%) was greater than that of control emulsion (18.1 ± 2.3%). | [81] |
| SLNs | Curcuminoids | Beeswax, Tween 80, lecithin | The formulation showed a sustained release with first order kinetics. | [82] |
| SLNs | Neem oil | Soya lecithin, cholesterol, Tween80 | Entrapment efficiency (EE) of neem oil-loaded SLNs was in the range of 67.23–82.10%. EE increased when the concentrations of lecithin and tween 80 increased. SLNs showed burst release (3.56–30.05%) within first 30 min. | [83] |
| SLNs | Resveratrol | Glyceryl behenate (Compritol 888), poloxamer 188 (Pluronic F68), Tween 80, Miglyol® 812 | Mean particle size of SLNs and NLCs were 287.2 nm ± 5.1 and 110.5 nm ± 1.3, respectively. The drug entrapment efficiency was 18% higher in NLCs. NLCs penetrated deeper into the skin than SLNs. | [84] |
| SLNs | Propolis flavonoids (PFs) | Glyceryl monostearate, soy lecithin, PEG400, Tween80 | The PFs-loaded SLNs exhibited prolonged drug release for 24 h and prolonged anti-inflammatory properties. No cytotoxicity observed. | [85] |
| NLCs | Resveratrol | Cetyl palmitate, sesame oil, tween 80 Glyceryl behenate (Compritol 888), sesame oil, tween 80 Phospholipid (Phospholipon80), sesame oil, Tween 80 | All NLC formulations showed a slow degradation of resveratrol over 24 h while resveratrol solution showed rapid degradation in the first 8 h. Phospholipon-based NLCs showed sustained release of resveratrol over 24 h and improved the penetration of resveratrol through stratum corneum. | [52] |
| NLCs | α-Mangostin | Cetyl palmitate, lavender oil, Montanov 82, Polyoxyethylene (20) sorbitanmonolaurate, poloxamer and glycerol | α -Mangostin-loaded NLC reduced the levels of inflammatory mediators including nitric oxide and TNF-α in macrophages induced by lipopolysaccharide. | [86] |
| NLCs | Eucalyptus essential oils or Rosemaryessential oils | Cocoa butter, olive oil or sesame oil, lecithin | All NLC formulations showed good bioadhesive properties. Eucalyptus oil-loaded NLCs prepared with olive oil showed antimicrobial and wound healing properties. | [87] |
| NLCs | Lycopene | Eumulgin SG, orange wax, rice oil | Lycopene-loaded NLCs gave a biphasic release profile (a relatively fast release during the first 6 h, followed by a sustained release during the next 18 h). The occlusive properties of NLC increased with increasing lycopene loading. The utilization of NLC increased the stability of lycopene. | [88] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chutoprapat, R.; Kopongpanich, P.; Chan, L.W. A Mini-Review on Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Topical Delivery of Phytochemicals for the Treatment of Acne Vulgaris. Molecules 2022, 27, 3460. https://doi.org/10.3390/molecules27113460
Chutoprapat R, Kopongpanich P, Chan LW. A Mini-Review on Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Topical Delivery of Phytochemicals for the Treatment of Acne Vulgaris. Molecules. 2022; 27(11):3460. https://doi.org/10.3390/molecules27113460
Chicago/Turabian StyleChutoprapat, Romchat, Peerawas Kopongpanich, and Lai Wah Chan. 2022. "A Mini-Review on Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Topical Delivery of Phytochemicals for the Treatment of Acne Vulgaris" Molecules 27, no. 11: 3460. https://doi.org/10.3390/molecules27113460