Phytochemical Profile and Biological Activity of the Ethanolic Extract from the Aerial Part of Crocus alatavicus Regel & Semen Growing Wildly in Southern Kazakhstan
Abstract
:1. Introduction
2. Results
2.1. Phytochemical Analysis
2.2. Antibacterial and Antifungal Activity
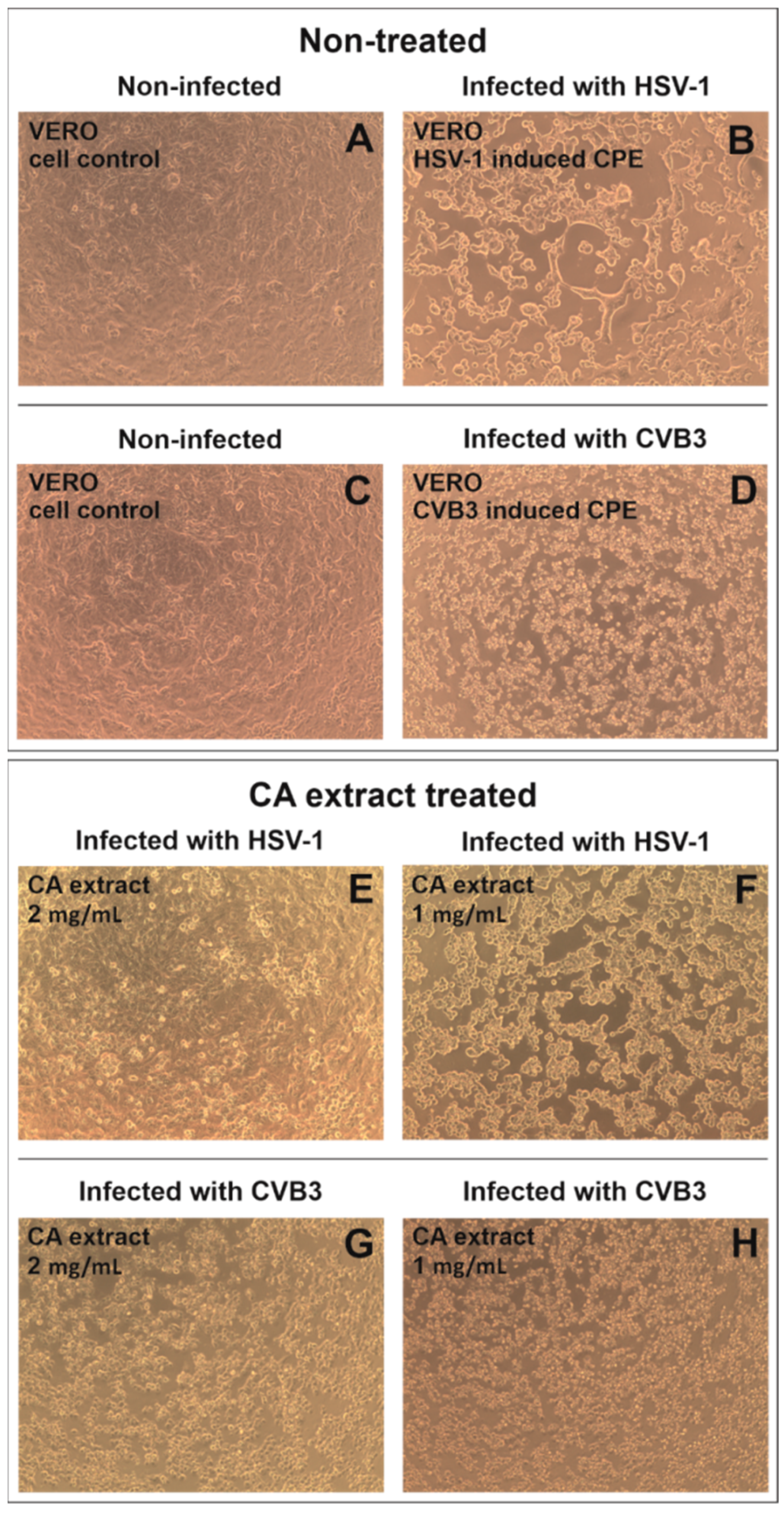
2.3. Antiviral Activity
2.4. Anticancer Activity
2.4.1. Cytotoxic Evaluation
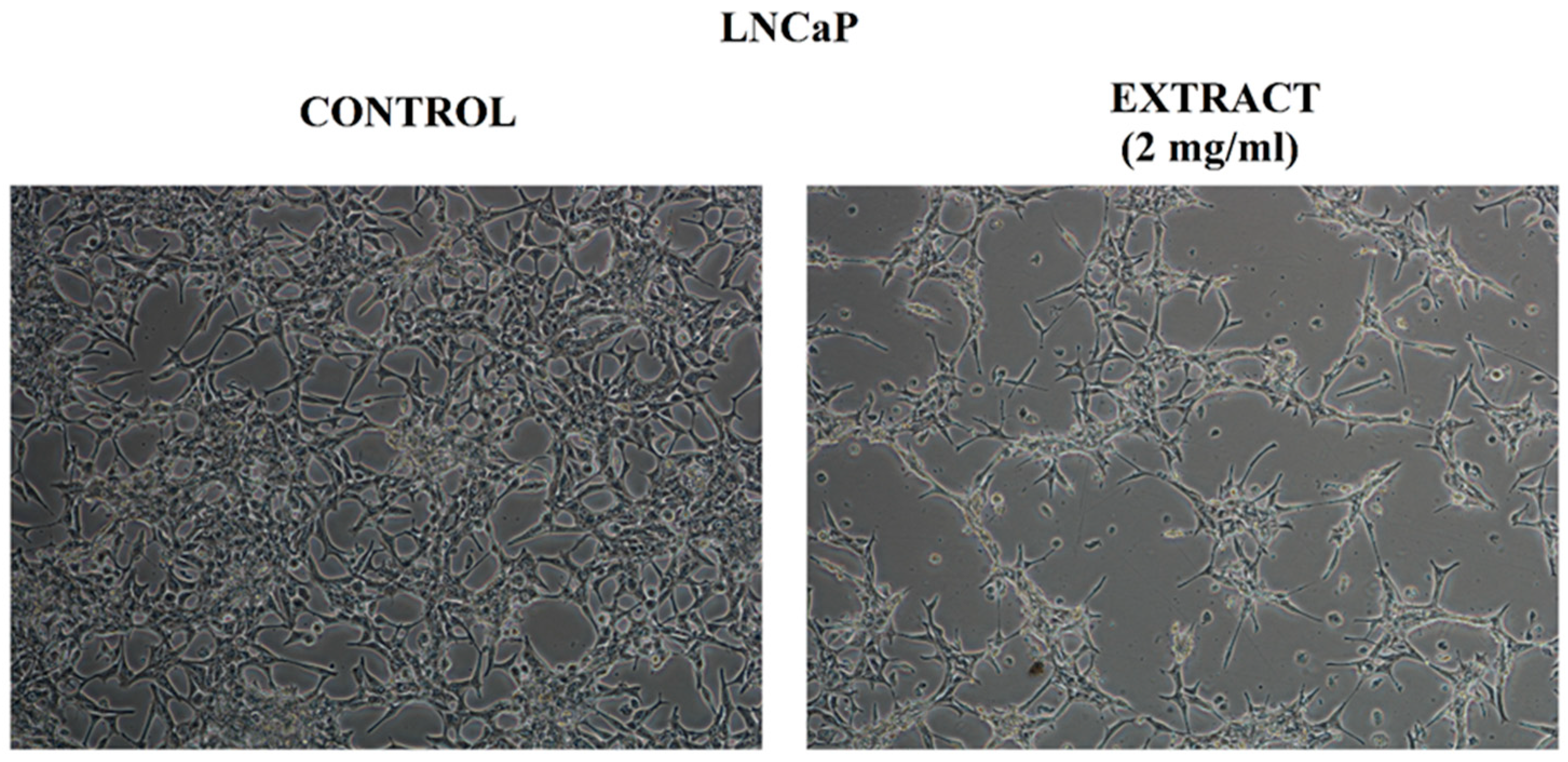
2.4.2. Assessment of Cell Morphology
2.4.3. Cell Cycle Analysis
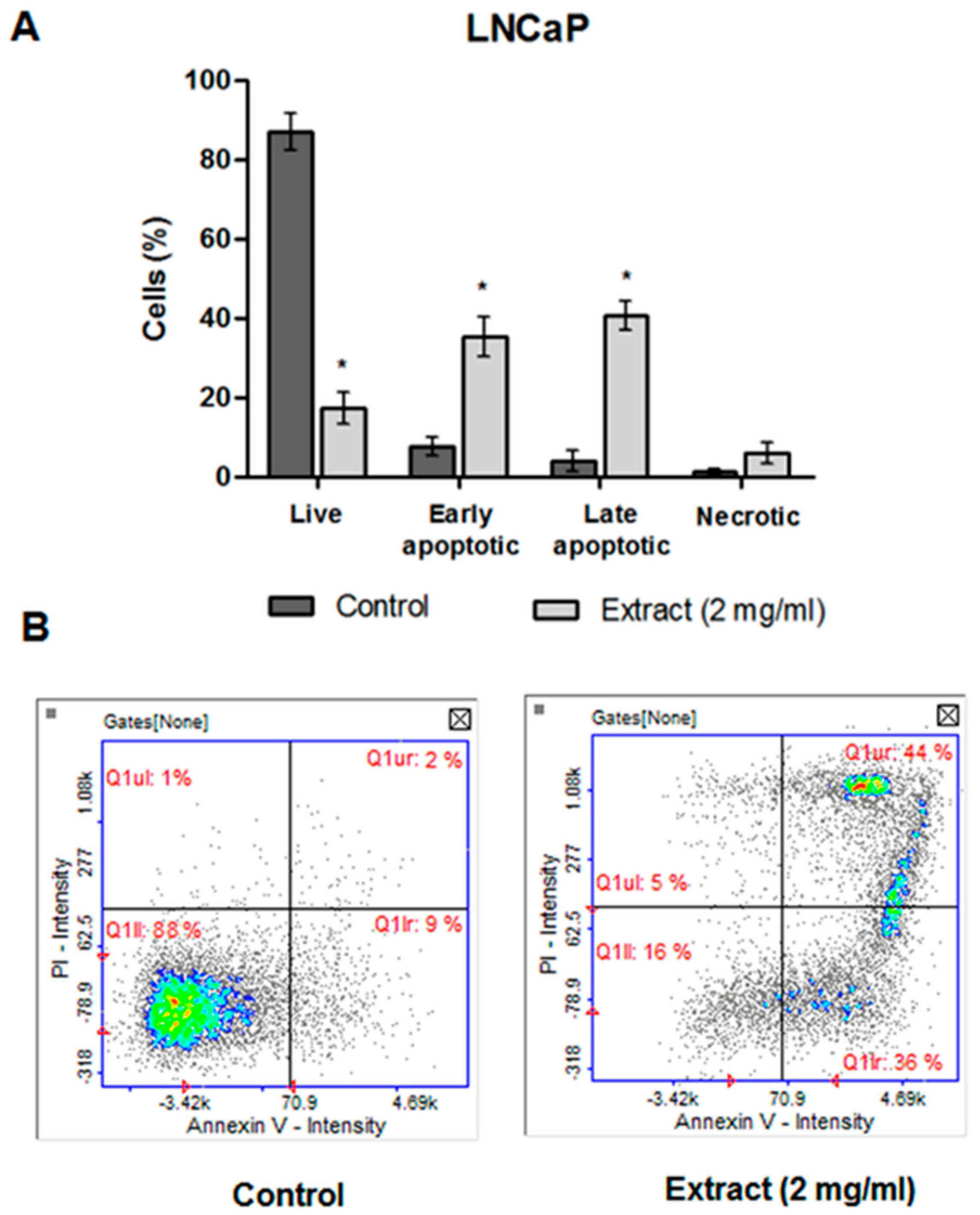
2.4.4. Apoptosis Detection
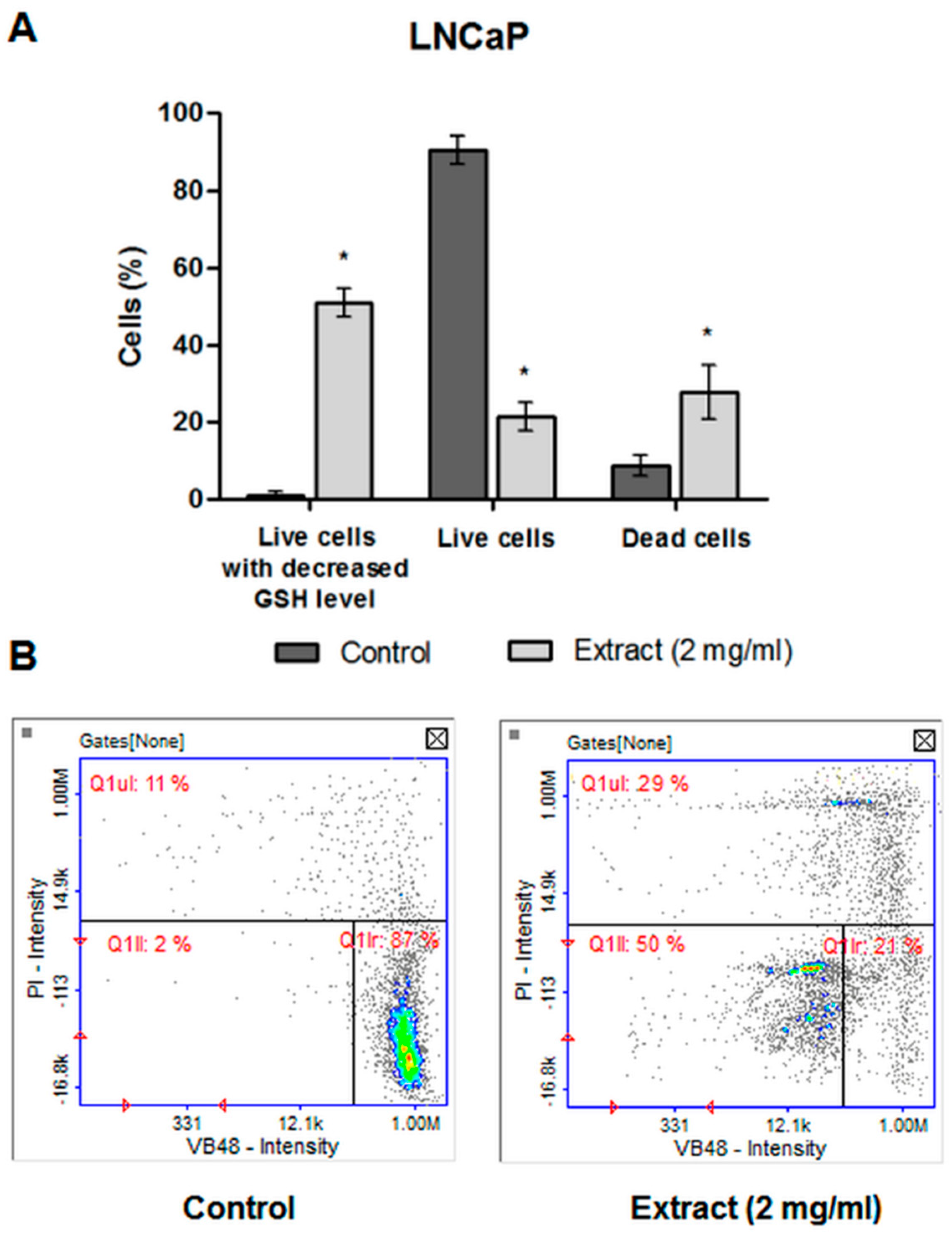
2.4.5. The Level of Cellular Thiols
3. Discussion
4. Materials and Methods
4.1. Plant Material Collection and Preparation
4.2. Extraction Procedure
4.3. Analysis of the CA Extract by RP-HPLC/PDA
4.4. Analysis of the CA Extract by HPLC/ESI-QTOF-MS
4.5. Determination of Antibacterial and Antifungal Activity
4.6. Determination of Antiviral Activity
4.6.1. Cytotoxicity Assessment
4.6.2. Antiviral Assay
4.6.3. End-Point Dilution Assay for HSV-1 Infectious Titre
4.6.4. Real-Time PCR for HSV-1 Viral Load
4.7. Anticancer Activity
4.7.1. Cell Culturing and Treatment
4.7.2. Assessment of Cell Morphology and Cell Cycle Analysis
4.7.3. Detection of Apoptosis
4.7.4. The Research of Oxidative Stress by the Level of Cellular Thiols
4.7.5. Statistical Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Conflicts of Interest
Sample Availability
References
- Mykhailenko, O.; Kovalyov, V.; Goryacha, O.; Ivanauskas, L.; Georgiyants, V. Biologically Active Compounds and Pharmacological Activities of Species of the Genus Crocus: A Review. Phytochemistry 2019, 162, 56–89. [Google Scholar] [CrossRef] [PubMed]
- Nørbæk, R.; Brandt, K.; Nielsen, J.K.; Ørgaard, M.; Jacobsen, N. Flower Pigment Composition of Crocus Species and Cultivars Used for a Chemotaxonomic Investigation. Biochem. Syst. Ecol. 2002, 30, 763–791. [Google Scholar] [CrossRef]
- Boskabady, M.H.; Farkhondeh, T. Antiinflammatory, Antioxidant, and Immunomodulatory Effects of Crocus sativus L. and Its Main Constituents: Crocus sativus L. Medicinal Properties. Phytother. Res. 2016, 30, 1072–1094. [Google Scholar] [CrossRef]
- Moratalla-López, N.; Bagur, M.J.; Lorenzo, C.; Martínez-Navarro, M.E.; Salinas, M.R.; Alonso, G.L. Bioactivity and Bioavailability of the Major Metabolites of Crocus sativus L. Flower. Molecules 2019, 24, 2827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardone, L.; Castronuovo, D.; Perniola, M.; Cicco, N.; Candido, V. Saffron (Crocus sativus L.), the King of Spices: An Overview. Sci. Hortic. 2020, 272, 109560. [Google Scholar] [CrossRef]
- Alhumaydhi, F.A.; Khan, I.; Rauf, A.; Qureshi, M.N.; Aljohani, A.S.M.; Khan, S.A.; Khalil, A.A.; El-Esawi, M.A.; Muhammad, N. Synthesis, Characterization, Biological Activities, and Catalytic Applications of Alcoholic Extract of Saffron (Crocus sativus) Flower Stigma-Based Gold Nanoparticles. Green Process. Synth. 2021, 10, 230–245. [Google Scholar] [CrossRef]
- Satybaldiyeva, D.; Mursaliyeva, V.; Rakhimbayev, I.; Zayadan, B.; Mammadov, R. Preliminary Phytochemical Analysis and Antioxidant, Antibacterial Activities of Crocus alatavicus from Kazakhstan. Not. Bot. Horti. Agrobot. Cluj Napoca 2015, 43, 343–348. [Google Scholar] [CrossRef] [Green Version]
- Satybaldiyeva, D.N.; Mursaliyeva, V.K.; Mammadov, R.; Zayadan, B.K. Phenolic profiles and brine shrimp cytotoxicity of the ethanolic extract from the aerial part of Crocus alatavicus L. Int. J. Biol. Chem. 2016, 9, 38–41. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y. Breeding System and Pollination Biology of Crocus alatavicus (Iridaceae), a Geocarpic Subalpine Plant of the Western Tianshan Mountains. Biodivers. Sci. 2009, 17, 468–475. [Google Scholar] [CrossRef]
- Imran, M.; Salehi, B.; Sharifi-Rad, J.; Aslam Gondal, T.; Saeed, F.; Imran, A.; Shahbaz, M.; Tsouh Fokou, P.V.; Umair Arshad, M.; Khan, H.; et al. Kaempferol: A Key Emphasis to Its Anticancer Potential. Molecules 2019, 24, 2277. [Google Scholar] [CrossRef] [Green Version]
- Akram, M.; Tahir, I.M.; Shah, S.M.A.; Mahmood, Z.; Altaf, A.; Ahmad, K.; Munir, N.; Daniyal, M.; Nasir, S.; Mehboob, H. Antiviral Potential of Medicinal Plants against HIV, HSV, Influenza, Hepatitis, and Coxsackievirus: A Systematic Review. Phytother. Res. 2018, 32, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Gezici, S.; Şekeroğlu, N. Current Perspectives in the Application of Medicinal Plants Against Cancer: Novel Therapeutic Agents. Anticancer Agents Med. Chem. 2019, 19, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Kokoska, L.; Kloucek, P.; Leuner, O.; Novy, P. Plant-Derived Products as Antibacterial and Antifungal Agents in Human Health Care. Curr. Med. Chem. 2019, 26, 5501–5541. [Google Scholar] [CrossRef] [PubMed]
- Ríos, J.L.; Recio, M.C. Medicinal Plants and Antimicrobial Activity. J. Ethnopharmacol. 2005, 100, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Treml, J.; Gazdová, M.; Šmejkal, K.; Šudomová, M.; Kubatka, P.; Hassan, S.T.S. Natural Products-Derived Chemicals: Breaking Barriers to Novel Anti-HSV Drug Development. Viruses 2020, 12, 154. [Google Scholar] [CrossRef] [Green Version]
- Šudomová, M.; Berchová-Bímová, K.; Mazurakova, A.; Šamec, D.; Kubatka, P.; Hassan, S.T.S. Flavonoids Target Human Herpesviruses That Infect the Nervous System: Mechanisms of Action and Therapeutic Insights. Viruses 2022, 14, 592. [Google Scholar] [CrossRef]
- Ürményi, F.G.G.; Saraiva, G.d.N.; Casanova, L.M.; dos Matos, A.S.; de Magalhães Camargo, L.M.; Romanos, M.T.V.; Costa, S.S. Anti-HSV-1 and HSV-2 Flavonoids and a New Kaempferol Triglycoside from the Medicinal Plant Kalanchoe daigremontiana. Chem. Biodivers. 2016, 13, 1707–1714. [Google Scholar] [CrossRef]
- Castanares, M.A.; Copeland, B.T.; Chowdhury, W.H.; Liu, M.M.; Rodriguez, R.; Pomper, M.G.; Lupold, S.E.; Foss, C.A. Characterization of a Novel Metastatic Prostate Cancer Cell Line of LNCaP Origin: Characterization of JHU-LNCaP-SM. Prostate 2016, 76, 215–225. [Google Scholar] [CrossRef] [Green Version]
- Halimah, E.; Diantini, A.; Destiani, D.P.; Pradipta, I.S.; Sastramihardja, H.S.; Lestari, K.; Subarnas, A.; Abdulah, R.; Koyama, H. Induction of Caspase Cascade Pathway by Kaempferol-3-O-Rhamnoside in LNCaP Prostate Cancer Cell Lines. Biomed. Rep. 2015, 3, 115–117. [Google Scholar] [CrossRef]
- Gemejiyeva, N.G.; Grudzinskaya, L.M. Current State and Prospects for Studies on the Diversity of Medicinal Flora in Kazakhstan. In Vegetation of Central Asia and Environs; Egamberdieva, D., Öztürk, M., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 239–262. ISBN 978-3-319-99728-5. [Google Scholar]
- Tojibaev, K.S.; Beshko, N.Y.; Turginov, O.T.; Lyskov, D.F.; Ukrainskaja, U.A.; Kljuykov, E.V. An Annotated Checklist of the Endemic Apiaceae of Uzbekistan. Phytotaxa 2020, 455, 70–94. [Google Scholar] [CrossRef]
- Zhumakanova, B.S.; Korona-Głowniak, I.; Skalicka-Woźniak, K.; Ludwiczuk, A.; Baj, T.; Wojtanowski, K.K.; Józefczyk, A.; Zhaparkulova, K.A.; Sakipova, Z.B.; Malm, A. Phytochemical Fingerprinting and In Vitro Antimicrobial and Antioxidant Activity of the Aerial Parts of Thymus marschallianus Willd. and Thymus seravschanicus Klokov Growing Widely in Southern Kazakhstan. Molecules 2021, 26, 3193. [Google Scholar] [CrossRef] [PubMed]
- Xing, B.; Li, S.; Yang, J.; Lin, D.; Feng, Y.; Lu, J.; Shao, Q. Phytochemistry, Pharmacology, and Potential Clinical Applications of Saffron: A Review. J. Ethnopharmacol. 2021, 281, 114555. [Google Scholar] [CrossRef] [PubMed]
- Kakouri, E.; Hatziagapiou, K.; Bethanis, K.; Nikola, O.A.; Lambrou, G.I.; Tarantilis, P.A. Tumor-Suppressing Properties of Crocus sativus L.: Nature as an Anti-Cancer Agent. Crit. Rev. Oncog. 2017, 22, 263–273. [Google Scholar] [CrossRef]
- Lambrianidou, A.; Koutsougianni, F.; Papapostolou, I.; Dimas, K. Recent Advances on the Anticancer Properties of Saffron (Crocus sativus L.) and Its Major Constituents. Molecules 2021, 26, 86. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, B.; Marasini, B.P.; Rayamajhee, B.; Bhattarai, B.R.; Lamichhane, G.; Khadayat, K.; Adhikari, A.; Khanal, S.; Parajuli, N. Potential Roles of Medicinal Plants for the Treatment of Viral Diseases Focusing on COVID-19: A Review. Phytother. Res 2021, 35, 1298–1312. [Google Scholar] [CrossRef] [PubMed]
- Crocetto, F.; di Zazzo, E.; Buonerba, C.; Aveta, A.; Pandolfo, S.D.; Barone, B.; Trama, F.; Caputo, V.F.; Scafuri, L.; Ferro, M.; et al. Kaempferol, Myricetin and Fisetin in Prostate and Bladder Cancer: A Systematic Review of the Literature. Nutrients 2021, 13, 3750. [Google Scholar] [CrossRef]
- Kadyrbayeva, G.; Zagórska, J.; Grzegorczyk, A.; Gaweł-Bęben, K.; Strzępek-Gomółka, M.; Ludwiczuk, A.; Czech, K.; Kumar, M.; Koch, W.; Malm, A.; et al. The Phenolic Compounds Profile and Cosmeceutical Significance of Two Kazakh Species of Onions: Alliumgalanthum and A. turkestanicum. Molecules 2021, 26, 5491. [Google Scholar] [CrossRef]
- Sakipova, Z.; Giorno, T.B.S.; Bekezhanova, T.; Siu Hai Wong, N.; Shukirbekova, A.; Fernandes, P.D.; Boylan, F. Pharmacological Evaluation of Artemisia cina Crude CO2 Subcritical Extract After the Removal of Santonin by Means of High Speed Countercurrent Chromatography. Molecules 2020, 25, 2728. [Google Scholar] [CrossRef]
- Sitpayeva, G.; Yerekeyeva, S.; Grudzinskaya, L.; Gemejieva, N.; Anarbekova, G.; Saikenov, B. Bringing Medicinal Plants of the Native Flora of the Northern Tien Shan Into Cultivation. J. Water Land Dev. 2021, 50, 207–219. [Google Scholar] [CrossRef]
- National Institute of Intellectual Property. Available online: https://kazpatent.kz/en (accessed on 5 April 2022).
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID). Determination of Minimum Inhibitory Concentrations (MICs) of Antibacterial Agents by Broth Dilution. Clin. Microbiol. Infect. 2003, 9, IX–XV. [Google Scholar] [CrossRef] [Green Version]
- Malm, A.; Grzegorczyk, A.; Biernasiuk, A.; Baj, T.; Rój, E.; Tyśkiewicz, K.; Dębczak, A.; Stolarski, M.J.; Krzyżaniak, M.; Olba-Zięty, E. Could Supercritical Extracts from the Aerial Parts of Helianthus salicifolius A. Dietr. and Helianthus tuberosus L. Be Regarded as Potential Raw Materials for Biocidal Purposes? Agriculture 2020, 11, 10. [Google Scholar] [CrossRef]
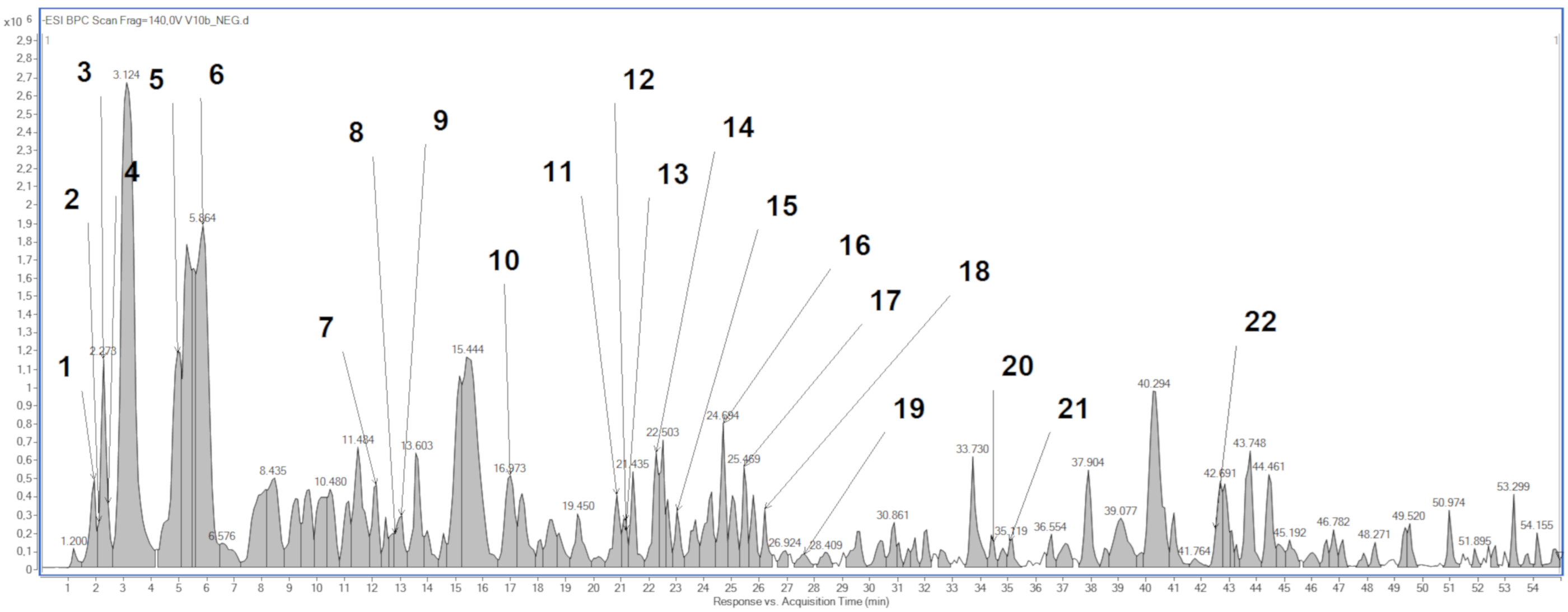



| No | Compound | Retention Time (min) | Formula | Molecular Ion [M − H]− | Fragmentation Ions |
|---|---|---|---|---|---|
| 1. | Gluconic acid | 1.917 | C6H12O7 | 195.0512 | 177.0421; 129.0195; 99.0094; 75.0095 |
| 2. | Malic acid | 2.041 | C4H6O5 | 133.0145 | 115.0043; 71.0148 |
| 3. | 2-Deoxy-2,3-dehydro-n-acetyl- neuraminic acid | 2.273 | C11H17NO8 | 290.0891 | 200.0561; 170.0454; 128.0352 |
| 4. | Citric acid | 2.459 | C₆H₈O₇ | 191.0200 | 129.0191; 111.0091; 87.0095 |
| 5. | DH-Crocusatin F | 4.994 | C10H16O4 | 199.0973 | 155.1072; 137.0969; 125.0977; 111.0821 |
| 6. | Crocusatin F | 5.864 | C10H14O4 | 197.0840 | 153.0939; 137.0985; 125.0987; 111.0831 |
| 7. | Kaempferol 7-O-tetrahexoside | 12.109 | C39H50O26 | 933.2560 | 771.1928; 609.1475; 446.0885; 284.0339 |
| 8. | Kaempferol 3-O-acyltetrahexoside | 12.799 | C43H54O26 | 973.2846 | 771.2038; 609.1481; 446.0870; 284.0340 |
| 9. | 2,4,4-Trimethyl-3-formyl-6-hydroxy-2,5-cyclohexadien-1-one | 13.029 | C10H12O3 | 179.0722 | 135.0801; 120.0578; 109.0305 |
| 10. | Kaempferol 7-O-dihexosidee | 16.973 | C27H30O16 | 609.1454 | 447.0840; 285.0351 |
| 11. | Kaempferol 7-O-trihexoside | 20.847 | C33H40O21 | 771.1996 | 447.0847; 285.0373 |
| 12. | Quercetin 3-O-dihexoside | 20.997 | C27H30O17 | 625.1438 | 300.0292; 271.0247 |
| 13. | Kaempferol 3,7-rutinoside, dihexoside | 21.331 | C39H50O25 | 917.2568 | 771.1673; 755.1738; 609.1419; 593.1419; 285.0337; 284.0281 |
| 14. | Kaempferol 3-O-dihexoside | 22.335 | C27H30O16 | 609.1470 | 284.0335; 255.0297 |
| 15. | Rutoside | 23.172 | C27H30O16 | 609.1486 | 300.0269; 284.0319; 271.0245 |
| 16. | Kaempferol 7-O-rutinoside | 24.677 | C27H30O15 | 593.1540 | 285.0372 |
| 17. | Astragalin | 25.514 | C21H20O11 | 447.0944 | 284.0322; 255.0299; 227.0343; 151.0036 |
| 18. | Nicotiflorin | 26.267 | C27H30O15 | 593.1479 | 284.0311; 255.0292 |
| 19. | Carboxyvanilic acid | 27.609 | C9H8O6 | 211.0240 | 167.0353; 123.0454; 108.0227 |
| 20. | Kaempferol | 34.420 | C15H10O6 | 285.0425 | 229.0476; 185.0605; 135.0077; 109.0288 |
| 21. | Endocrocin | 35.119 | C16H10O7 | 313.0379 | 241.0506; 225.0565; 213.0555; 201.0576; 197.0604 |
| 22. | Acacetin | 42.691 | C16H12O5 | 283.0614 | 268.0382; 240.0433; 212.0482 |
| Compound | Retention Time (min) | Content (μg/mg Dry Extract) | ||
|---|---|---|---|---|
| Kaempferol 3-O-tetrahexoside | 2.11 | 14.14 | 0.09 a | 0.6 b |
| Kaempferol 3-O-acyltetrahexoside | 2.47 | 80.04 | 0.51 a | 0.6 b |
| Kaempferol 7-O-trihexoside | 9.10 | 9.32 | 0.22 a | 2.4 b |
| Quercetin 3-O-dihexoside | 9.40 | 6.89 | 0.07 a | 1.0 b |
| Kaempferol 3,7–rutinoside, dihexoside | 9.54 | 10.51 | 0.09 a | 0.9 b |
| Kaempferol 3-O-dihexoside | 12.77 | 150.76 | 0.16 a | 0.1 b |
| Rutoside | 13.50 | 5.12 | 0.04 a | 0.7 b |
| Kaempferol 7-O-rutinoside | 13.97 | 4.71 | 0.10 a | 2.2 b |
| Astragalin | 14.47 | 26.87 | 0.25 a | 0.9 b |
| Nicotiflorin | 15.83 | 30.83 | 0.26 a | 0.9 b |
| TOTAL (μg/mg dry extract) | 339.19 | |||
| Bacteria | MIC 1 (mg/mL) | MBC 2 (mg/mL) | MBC/MIC Ratio |
| Escherichia coli ATCC 25922 | 20 | >20 | Nd 4 |
| Staphylococcus aureus ATCC 25923 | 20 | 20 | 1 |
| Staphylococcus aureus ATCC BAA-1707 | 20 | 20 | 1 |
| Staphylococcus epidermidis ATCC 12228 | 20 | >20 | Nd |
| Bacillus cereus ATCC 10876 | 20 | 20 | 1 |
| Cutibacterium acnes ATCC 11827 | 20 | 20 | 1 |
| Fungi (Yeasts) | MIC (mg/mL) | MFC 3 (mg/mL) | MFC/MIC Ratio |
| Candida albicans ATCC 10231 | 10 | 10 | 1 |
| Candida glabrata ATCC 90030 | 10 | 20 | 2 |
| Concentration (mg/mL) | Reduction in Infectious Titer (Δlog) 1 | Reduction in Viral Load (Δlog’) 2 | ||
|---|---|---|---|---|
| CVB3 | HSV-1 | CVB3 | HSV-1 | |
| 2 | 1.14 ± 0.38 | 1.96 ± 0.28 | Nd 3 | 0.85 ± 0.10 |
| 1 | 0.55 ± 0.31 | 0.90 ± 0.06 | nd | 0.24 ± 0.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allambergenova, Z.; Kasela, M.; Adamczuk, G.; Humeniuk, E.; Iwan, M.; Świątek, Ł.; Boguszewska, A.; Rajtar, B.; Józefczyk, A.; Baj, T.; et al. Phytochemical Profile and Biological Activity of the Ethanolic Extract from the Aerial Part of Crocus alatavicus Regel & Semen Growing Wildly in Southern Kazakhstan. Molecules 2022, 27, 3468. https://doi.org/10.3390/molecules27113468
Allambergenova Z, Kasela M, Adamczuk G, Humeniuk E, Iwan M, Świątek Ł, Boguszewska A, Rajtar B, Józefczyk A, Baj T, et al. Phytochemical Profile and Biological Activity of the Ethanolic Extract from the Aerial Part of Crocus alatavicus Regel & Semen Growing Wildly in Southern Kazakhstan. Molecules. 2022; 27(11):3468. https://doi.org/10.3390/molecules27113468
Chicago/Turabian StyleAllambergenova, Zoya, Martyna Kasela, Grzegorz Adamczuk, Ewelina Humeniuk, Magdalena Iwan, Łukasz Świątek, Anastazja Boguszewska, Barbara Rajtar, Aleksandra Józefczyk, Tomasz Baj, and et al. 2022. "Phytochemical Profile and Biological Activity of the Ethanolic Extract from the Aerial Part of Crocus alatavicus Regel & Semen Growing Wildly in Southern Kazakhstan" Molecules 27, no. 11: 3468. https://doi.org/10.3390/molecules27113468







