Quinine Esters with 1,2-Azole, Pyridine and Adamantane Fragments
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Evaluation of the Biological Activity
2.2.1. Antiviral Activity
2.2.2. Antimicrobial Activity
2.2.3. Analgesic Activity In Vivo
3. Materials and Methods
3.1. General Chemistry Section
3.2. In Vitro Biological Assays
3.2.1. Antiviral Activity
3.2.2. Antimicrobial Activity
3.3. Analgesic Activity In Vivo
3.4. Experimental Section
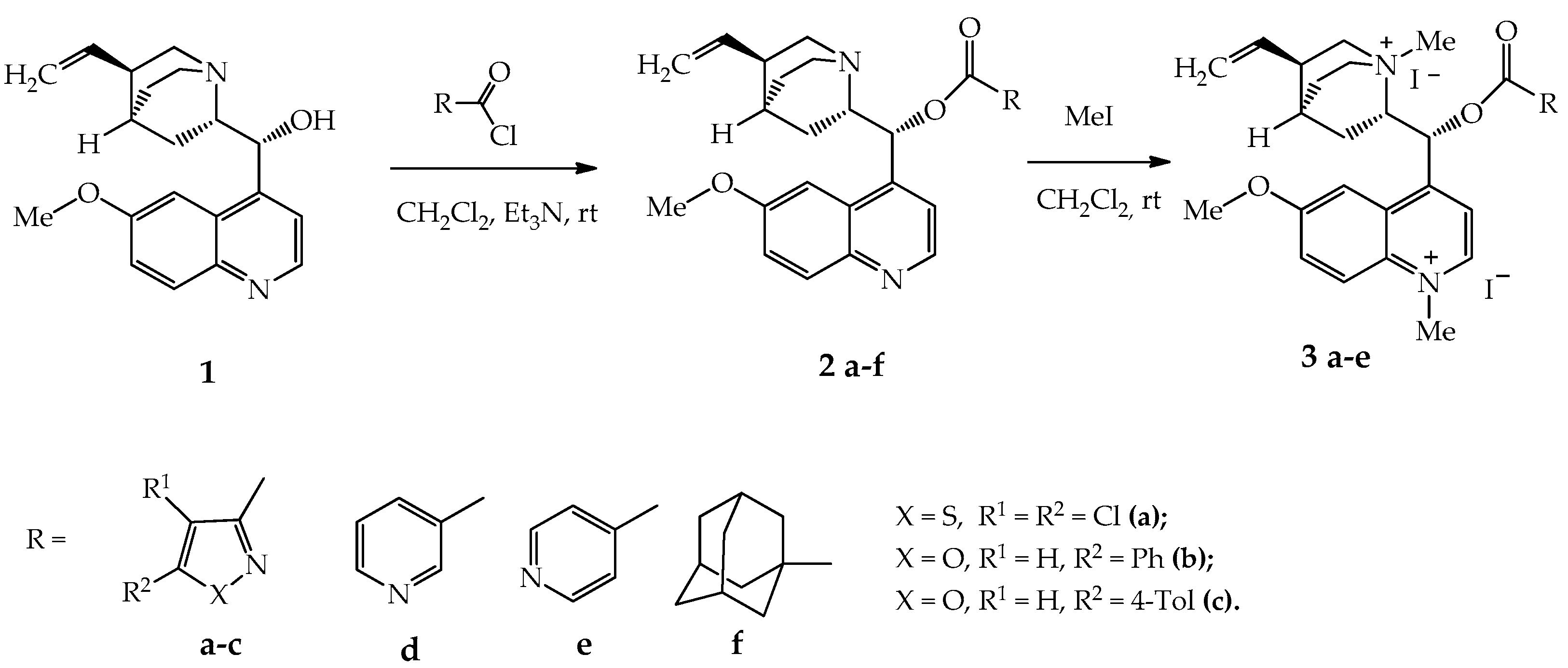
3.4.1. General Procedure for the Synthesis of Compounds 2a–f
3.4.2. General Procedure for the Synthesis of Compounds 3a–e
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Achan, J.; Talisuna, A.O.; Erhart, A.; Yeka, A.; Tibenderana, J.K.; Baliraine, F.N.; Rosenthal, P.J.; D’Alessandro, U. Quinine, an old anti-malarial drug in a modern world: Role in the treatment of malaria. Malar. J. 2011, 10, 144–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liles, N.W.; Page, E.E.; Liles, A.L.; Vesely, S.K.; Raskob, G.E.; George, J.N. Diversity and severity of adverse reactions to quinine: A systematic review. Am. J. Hematol. 2016, 91, 461–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, W.R.J.; White, N.J. Antimalarial Drug Toxicity. Drug Saf. 2004, 27, 25–61. [Google Scholar] [CrossRef] [PubMed]
- Heller, L.E.; Roepe, P.D. Artemisinin-Based Antimalarial Drug Therapy: Molecular Pharmacology and Evolving Resistance. Trop. Med. Infect. Dis. 2019, 4, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolf, R.; Baroni, A.; Greco, R.; Donnarumma, G.; Ruocco, E.; Tufano, M.A.; Ruocco, V. Quinine sulfate and bacterial invasion. Ann. Clin. Microbiol. Antimicrob. 2002, 1, 5. [Google Scholar] [CrossRef]
- Kharal, S.A.; Hussain, Q.; Ali, S.; Fakhuruddin. Quinine is bactericidal. J. Pak. Med. Assoc. 2009, 59, 208–212. [Google Scholar]
- Leanse, L.G.; Goh, X.S.; Dai, T. Quinine Improves the Fungicidal Effects of Antimicrobial Blue Light: Implications for the Treatment of Cutaneous Candidiasis. Lasers Surg. Med. 2019, 52, 569–575. [Google Scholar] [CrossRef]
- Majnooni, M.B.; Fakhri, S.; Bahrami, G.; Naseri, M.; Farzaei, M.H.; Echeverría, J. Alkaloids as Potential Phytochemicals against SARS-CoV-2: Approaches to the Associated Pivotal Mechanisms. Evid.-Based Complementary Altern. Med. 2021, 2021, 6632623. [Google Scholar] [CrossRef]
- Latarissa, I.R.; Barliana, M.I.; Meiliana, A.; Lestari, K. Potential of Quinine Sulfate for COVID-19 Treatment and Its Safety Profile: Review. Clin. Pharmacol. Adv. Appl. 2021, 13, 225–234. [Google Scholar]
- Colson, P.; Rolain, J.M.; Lagier, J.C.; Brouqui, P.; Raoult, D. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int. J. Antimicrob. Agents 2020, 55, 105932. [Google Scholar] [CrossRef]
- Younis, N.K.; Zareef, R.O.; Al Hassan, S.N.; Bitar, F.; Eid, A.H.; Arabi, M. Hydroxychloroquine in COVID-19 Patients: Pros and Cons. Front. Pharmacol. 2020, 11, 597985. [Google Scholar] [CrossRef] [PubMed]
- Antika, L.D.; Triana, D.; Ernawati, T. Antimicrobial activity of quinine derivatives against human pathogenic bacteria. IOP Conf. Ser. Earth Environ. Sci. 2020, 462, 012006. [Google Scholar] [CrossRef]
- Ernawati, T.; Minarti, M.; Lotulung, P.D.N. Structure Modification of Quinine on C-9 Hydroxyl Group via Esterification Reaction. J. Pure Appl. Chem. Res. 2020, 9, 32–39. [Google Scholar] [CrossRef]
- Che, Z.; Yang, J.; Sun, D.; Tian, Y.; Liu, S.; Lin, X.; Jiang, J.; Chen, G. Combinatorial Synthesis of Novel 9R-Acyloxyquinine Derivatives as Insecticidal Agents. Comb. Chem. High Throughput Screen. 2020, 23, 111–118. [Google Scholar] [CrossRef]
- Wang, L.; Yang, D.; Han, F.; Li, D.; Zhao, D.; Wang, R. Catalytic Asymmetric Construction of Pyrroloindolines via an in Situ Generated Magnesium Catalyst. Org. Lett. 2014, 17, 176–179. [Google Scholar] [CrossRef]
- Kletskov, A.V.; Bumagin, N.A.; Zubkov, F.I.; Grudinin, D.G.; Potkin, V.I. Isothiazoles in the design and synthesis of biologically active substances and ligands for metal complexes. Synthesis 2020, 52, 159–188. [Google Scholar] [CrossRef]
- Zhu, J.; Mo, J.; Lin, H.; Chen, Y.; Sun, H. The recent progress of isoxazole in medicinal chemistry. Bioorganic Med. Chem. 2018, 26, 3065–3075. [Google Scholar] [CrossRef]
- Altaf, A.A.; Shahzad, A.; Gul, Z.; Rasool, N.; Badshah, A.; Lal, B.; Khan, E. Review on the Medicinal Importance of Pyridine Derivatives. J. Drug Des. Med. Chem. 2015, 1, 1–11. [Google Scholar]
- Kletskov, A.V.; Potkin, V.I.; Dikusar, E.A.; Zolotar, R.M. New Data on Vanillin-Based Isothiazolic Insecticides Synergists. Nat. Prod. Commun. 2017, 12, 105–106. [Google Scholar] [CrossRef] [Green Version]
- Kletskov, A.V.; Potkin, V.I.; Kolesnik, I.A.; Petkevich, S.K.; Kvachonak, A.V.; Dosina, M.O.; Loiko, D.O.; Larchenko, M.V.; Pashkevich, S.G.; Kulchitsky, V.A. Synthesis and biological activity of novel comenic acid derivatives containing isoxazole and isothiazole moieties. Nat. Prod. Commun. 2018, 13, 1507–1510. [Google Scholar] [CrossRef] [Green Version]
- Potkin, V.I.; Kletskov, A.V.; Petkevich, S.K.; Pashkevich, S.G.; Kazbanov, V.V.; Denisov, A.A.; Kulchitsky, V.A. Synthesis of water soluble isoxazol-3-yl(isothiazol-3-yl) carboxamides and ureas containing amino acid residues—Potential anticancer agents. Heterocycl. Lett. 2015, 1, 11–19. [Google Scholar]
- Klimochkin, Y.N.; Shiryaev, V.A.; Leonova, M.V. Antiviral properties of cage compounds. New prospects. Russ. Chem. Bull. 2015, 64, 1473–1496. [Google Scholar] [CrossRef]
- Malakar, S.; Sreelatha, L.; Dechtawewat, T.; Noisakran, S.; Yenchitsomanus, P.T.; Chu, J.J.H.; Limjindaporn, T. Drug repurposing of quinine as antiviral against dengue virus infection. Virus Res. 2018, 255, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Große, M.; Ruetalo, N.; Layer, M.; Hu, D.; Businger, R.; Rheber, S.; Setz, C.; Rauch, P.; Auth, J.; Fröba, M.; et al. Quinine Inhibits Infection of Human Cell Lines with SARS-CoV-2. Viruses 2021, 13, 647. [Google Scholar] [CrossRef]
- Beaumont, K.; Webster, R.; Gardner, I.; Dack, K. Design of Ester Prodrugs to Enhance Oral Absorption of Poorly Permeable Compounds: Challenges to the Discovery Scientist. Curr. Drug Metab. 2003, 4, 461–485. [Google Scholar] [CrossRef]
- Larsen, E.M.; Johnson, R.J. Microbial esterases and ester prodrugs: An unlikely marriage for combating antibiotic resistance. Drug Dev. Res. 2019, 80, 33–47. [Google Scholar] [CrossRef] [Green Version]
- Joyce, M.D.; Jennings, M.C.; Santiago, C.N.; Fletcher, M.H.; Wuest, W.M.; Minbiole, K.P. Natural product-derived quaternary ammonium compounds with potent antimicrobial activity. J. Antibiot. 2015, 69, 344–347. [Google Scholar] [CrossRef]
- Patel, P.R.; Joshi, H.; Shah, U.; Bapna, M.; Patel, B. New generation of quinazolinone derivatives as potent antimicrobial agents. Asian Pac. J. Health Sci. 2021, 8, 61–66. [Google Scholar] [CrossRef]
- Badshah, S.L.; Ullah, A. New developments in non quinolone-based antibiotics for the inhibition of bacterial gyrase and topoisomerase IV. Eur. J. Med. Chem. 2018, 152, 393–400. [Google Scholar] [CrossRef]
- Potkin, V.I.; Gadzhily, R.A.; Dikusar, E.A.; Petkevich, S.K.; Zhukovskaya, N.A.; Aliev, A.G.; Nagieva, S.F. Synthesis of hydroxybenzaldehyde derivatives containing an isoxazole heteroring. Russ. J. Org. Chem. 2012, 48, 127–136. [Google Scholar] [CrossRef]
- Nechai, N.I.; Dikusar, E.A.; Potkin, V.I.; Kaberdin, R.V. Synthesis of 4,5-Dichloroisothiazole-3-carboxylic Acid Amides and Esters. Russ. J. Org. Chem. 2004, 40, 1009–1014. [Google Scholar] [CrossRef]
- Makarova, N.V.; Boreko, E.I.; Moiseev, I.K.; Pavlova, N.I.; Nikolaeva, S.N.; Zemtsova, M.N.; Vladyko, G.V. Antiviral activity of adamantane-containing heterocycles. Pharm. Chem. J. 2002, 36, 5–7. [Google Scholar] [CrossRef]
- Rajtar, B.; Szacoń, E.; Świątek, Ł.; Rządkowska, M.; Matosiuk, D.; Polz-Dacewicz, M. Antiviral activity of 1-(1-arylimidazolidine-2-ylidene)-3-(4-chlorobenzyl)urea derivatives. J. Pre-Clin. Clin. Res. 2013, 7, 104–106. [Google Scholar] [CrossRef]
- Filippova, E.I.; Kukushkina, T.A.; Lobanova, I.E.; Vysochina, G.I.; Mazurkova, N.A. Antiviral properties of the preparation based on the amount of flavonoids of the cuff (Alchemilla vulgaris L.) in relation to the influenza virus. Fundam. Res. 2015, 2, 5139–5144. [Google Scholar]
- State Pharmacopoeia of the Republic of Kazakhstan; Publishing House “Zhibek Zholy”: Almaty, Kazakhstan, 2015; Volume I, 720p.
- Guidelines for Conducting Preclinical Studies of Drugs. Mironov, A.N., Ed.; GRIF-K: Moscow, Russia, 2012; Part 1; p. 206.
- Council, N.R. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2011; 220p. [Google Scholar]
- Réus, G.Z.; Stringari, R.B.; de Souza, B.; Petronilho, F.; Dal-Pizzol, F.; Hallak, J.E.; Zuardi, A.W.; Crippa, J.A.; Quevedo, J. Harmine and Imipramine Promote Antioxidant Activities in Prefrontal Cortex and Hippocampus. Oxidative Med. Cell. Longev. 2010, 3, 325–331. [Google Scholar] [CrossRef] [Green Version]
| Compound | Virus-Inhibiting Activity (%) at a Dose of mg/Chick Embryo | |||
|---|---|---|---|---|
| 0.003 | 0.02 | 0.08 | 0.4 | |
| 2a | 0 | 0 | 0 | 0 |
| 2b | 0 | 0 | 0 | 0 |
| 2d | 0 | 2.1 | 3.5 | 5.6 |
| 2e | 3.6 | 5.5 | 7.4 | 11.3 |
| 2f | 1.8 | 2.4 | 3.0 | 3.6 |
| 3a | 0 | 0 | 2.1 | 4.5 |
| 3b | 0 | 0 | 5.2 | 10.1 |
| 3c | 0 | 1.7 | 4.9 | 10.8 |
| 3d | 0 | 0 | 4.5 | 10.6 |
| 3e | 1.6 | 2.9 | 6.7 | 11.1 |
| Amizon | 1.9 | 3.4 | 5.2 | 9.8 |
| Compound | Virus-Inhibiting Activity (%) at a Dose of Mg/Chick Embryo | |||
|---|---|---|---|---|
| 0.003 | 0.02 | 0.08 | 0.4 | |
| 2a | 0 | 0 | 0 | 0 |
| 2b | 0 | 1.7 | 3.5 | 4.7 |
| 2d | 0 | 2.1 | 3.9 | 5.6 |
| 2e | 0 | 3.5 | 7.3 | 16.5 |
| 2f | 0 | 0 | 0 | 0 |
| 3a | 0 | 0 | 0 | 0 |
| 3b | 0 | 5.4 | 7.1 | 11.2 |
| 3c | 0 | 2.6 | 4.2 | 5.3 |
| 3d | 0 | 4.6 | 8.5 | 10.4 |
| 3e | 0 | 5.8 | 9.3 | 14.7 |
| Amizon | 0 | 4.3 | 7.2 | 8.6 |
| Compound | Virus-Inhibiting Activity (%) at a Dose of Mg/Chick Embryo | |||
|---|---|---|---|---|
| 0.003 | 0.02 | 0.08 | 0.4 | |
| 2a | 0 | 0 | 6.8 | 8.5 |
| 2b | 3.2 | 5.0 | 7.0 | 9.2 |
| 2d | 2.5 | 4.9 | 8.2 | 10.5 |
| 2e | 3.4 | 5.5 | 20.6 | 35.2 |
| 2f | 0 | 5.2 | 7.2 | 8.1 |
| 3a | 0 | 5.3 | 7.5 | 9.6 |
| 3b | 3.4 | 5.4 | 16.3 | 21.6 |
| 3c | 3.4 | 5.5 | 19.2 | 28.5 |
| 3d | 2.9 | 4.1 | 8.5 | 11.7 |
| 3e | 3.5 | 5.6 | 23.3 | 36.4 |
| Amizon | 3.6 | 5.8 | 7.5 | 10.9 |
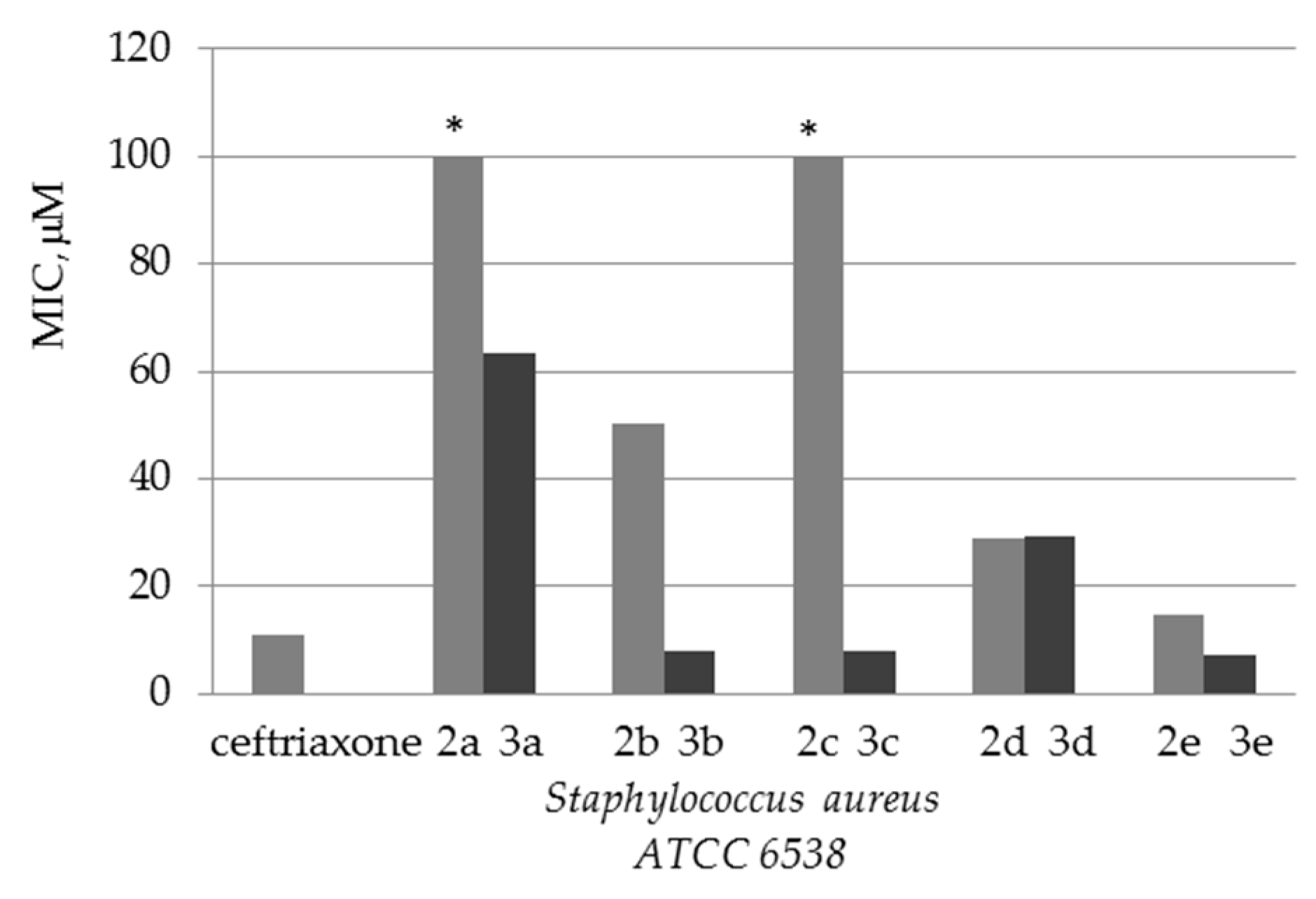
| Compound | MIC (μM) | ||||
|---|---|---|---|---|---|
| Staphylococcus aureus ATCC 6538 | Bacillus subtilis ATCC 6633 | Escherichia coli ATCC 25922 | Pseudomonas aeruginosa ATCC 27853 | Candida albicans ATCC 10231 | |
| 2a | - | - | 99 | - | - |
| 2b | 50 | - | 101 | - | - |
| 2c | - | - | - | - | - |
| 2d | 29 | 116 | 116 | - | - |
| 2e | 15 | 58 | 58 | - | - |
| 2f | 51 | - | - | - | 103 |
| 3a | 63 | - | - | - | - |
| 3b | 8 | 64 | - | - | - |
| 3c | 8 | 63 | 32 | - | - |
| 3d | 29 | - | 59 | - | 59 |
| 3e | 7 | - | 15 | - | - |
| Ceftriaxone | 11 | 22 | 11 | 22 | - |
| Nystatin | - | - | - | - | 14 |
| Compound | Dose, mg/kg | Writhing Number | Decrease in the Vinegar Writhing Number (%) |
|---|---|---|---|
| control | - | 106.2 ± 11.2 | 100 |
| sodium diclofenac | 8 | 49.5 ± 10.7 | 53.3 |
| 2a | 25 | 95.4 ± 10.9 | 10.2 |
| 2b | 25 | 55.6 ± 11.3 * | 47.6 |
| 2d | 25 | 62.2 ± 11.2 * | 41.4 |
| 2e | 25 | 53.7 ± 11.6 * | 49.4 |
| 3a | 25 | 96.8 ± 11.2 | 8.8 |
| 3b | 25 | 83.8 ± 10.4 | 21.1 |
| 3c | 25 | 57.2 ± 7.5 * | 46.1 |
| 3d | 25 | 75.4 ± 10.5 | 29.0 |
| 3e | 25 | 50.2 ± 10.3 * | 52.7 |
| 2f | 25 | 54.4 ± 12.3 | 48.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukusheva, G.K.; Zhasymbekova, A.R.; Seidakhmetova, R.B.; Nurkenov, O.A.; Akishina, E.A.; Petkevich, S.K.; Dikusar, E.A.; Potkin, V.I. Quinine Esters with 1,2-Azole, Pyridine and Adamantane Fragments. Molecules 2022, 27, 3476. https://doi.org/10.3390/molecules27113476
Mukusheva GK, Zhasymbekova AR, Seidakhmetova RB, Nurkenov OA, Akishina EA, Petkevich SK, Dikusar EA, Potkin VI. Quinine Esters with 1,2-Azole, Pyridine and Adamantane Fragments. Molecules. 2022; 27(11):3476. https://doi.org/10.3390/molecules27113476
Chicago/Turabian StyleMukusheva, Gulim K., Aigerym R. Zhasymbekova, Roza B. Seidakhmetova, Oralgazy A. Nurkenov, Ekaterina A. Akishina, Sergey K. Petkevich, Evgenij A. Dikusar, and Vladimir I. Potkin. 2022. "Quinine Esters with 1,2-Azole, Pyridine and Adamantane Fragments" Molecules 27, no. 11: 3476. https://doi.org/10.3390/molecules27113476
APA StyleMukusheva, G. K., Zhasymbekova, A. R., Seidakhmetova, R. B., Nurkenov, O. A., Akishina, E. A., Petkevich, S. K., Dikusar, E. A., & Potkin, V. I. (2022). Quinine Esters with 1,2-Azole, Pyridine and Adamantane Fragments. Molecules, 27(11), 3476. https://doi.org/10.3390/molecules27113476






