Essential Oil from Eucalyptus globulus (Labill.) Activates Complement Receptor-Mediated Phagocytosis and Stimulates Podosome Formation in Human Monocyte-Derived Macrophages
Abstract
:1. Introduction
2. Results
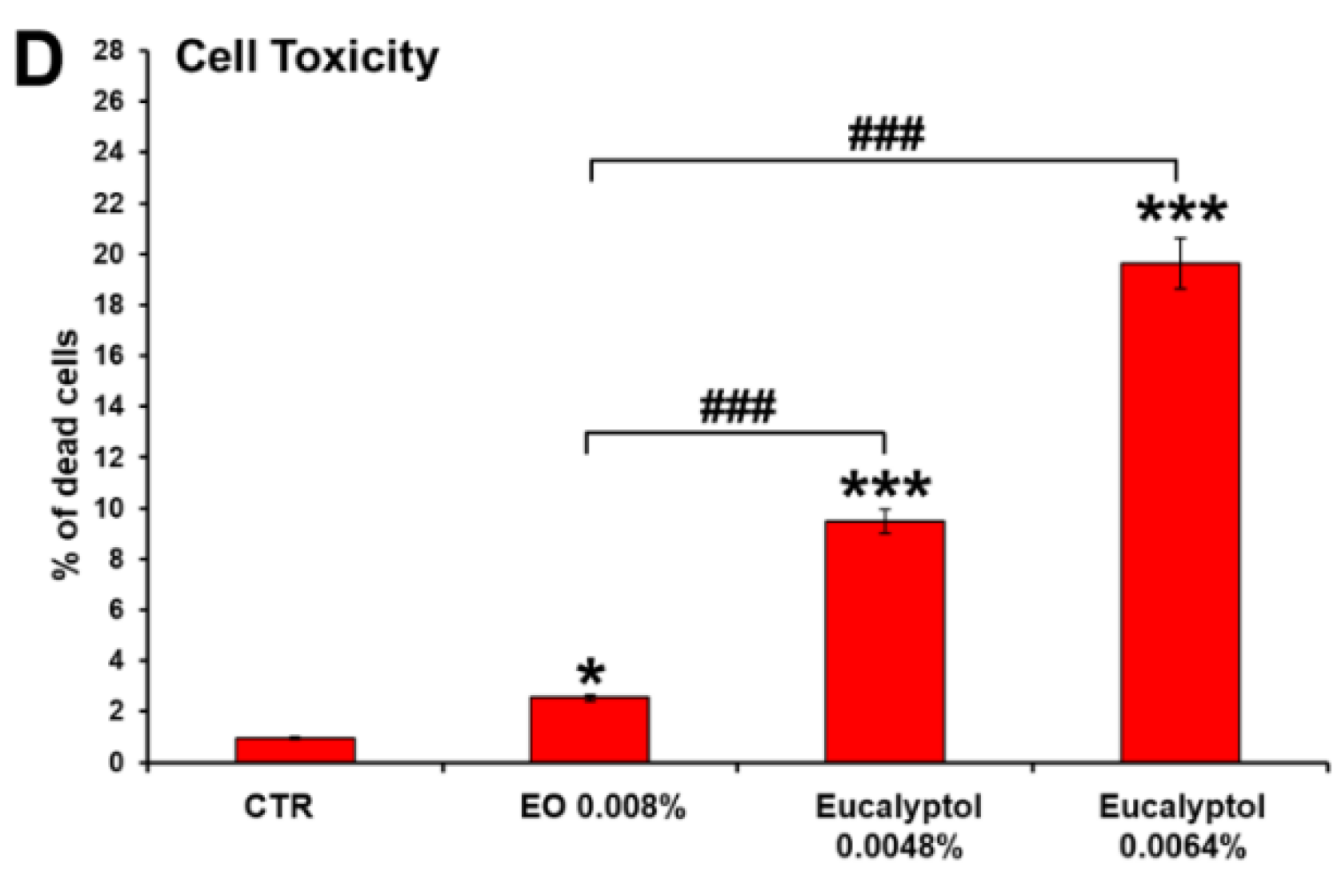
2.1. Comparative Efficacy of Eucalyptus Oil and Eucalyptol on Phagocytic Ability and Viability of MDMs
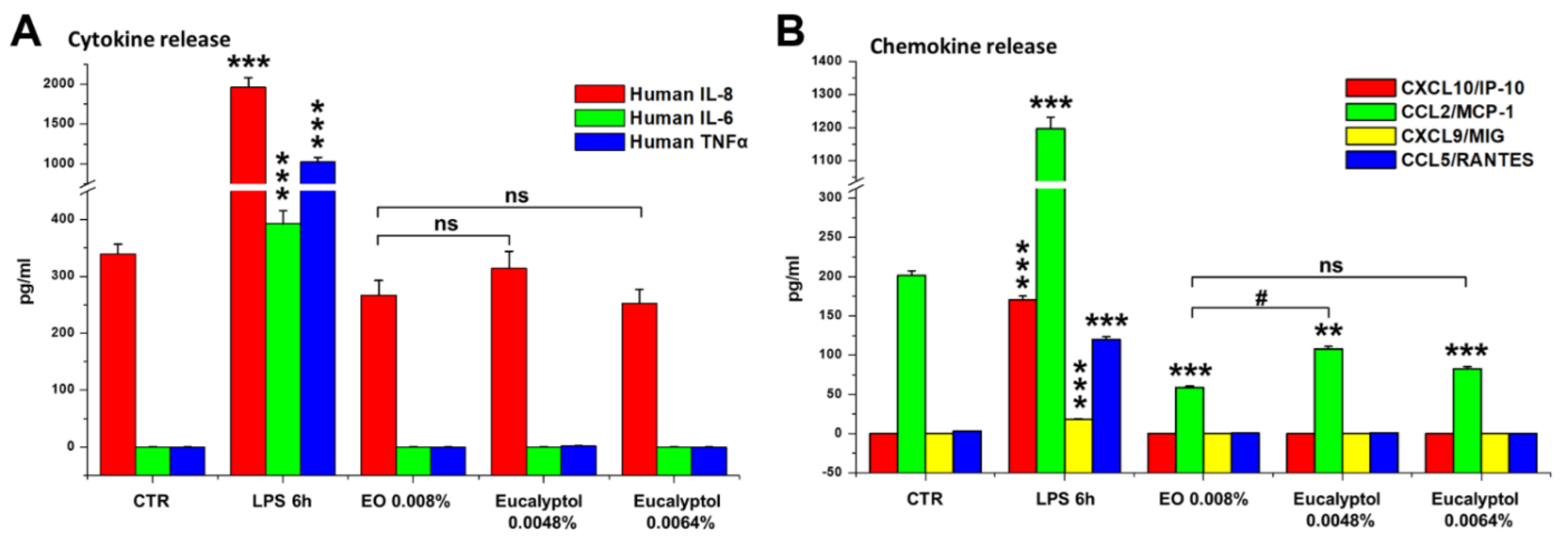
2.2. Effect of Eucalyptus Oil on Microorganism Clearance
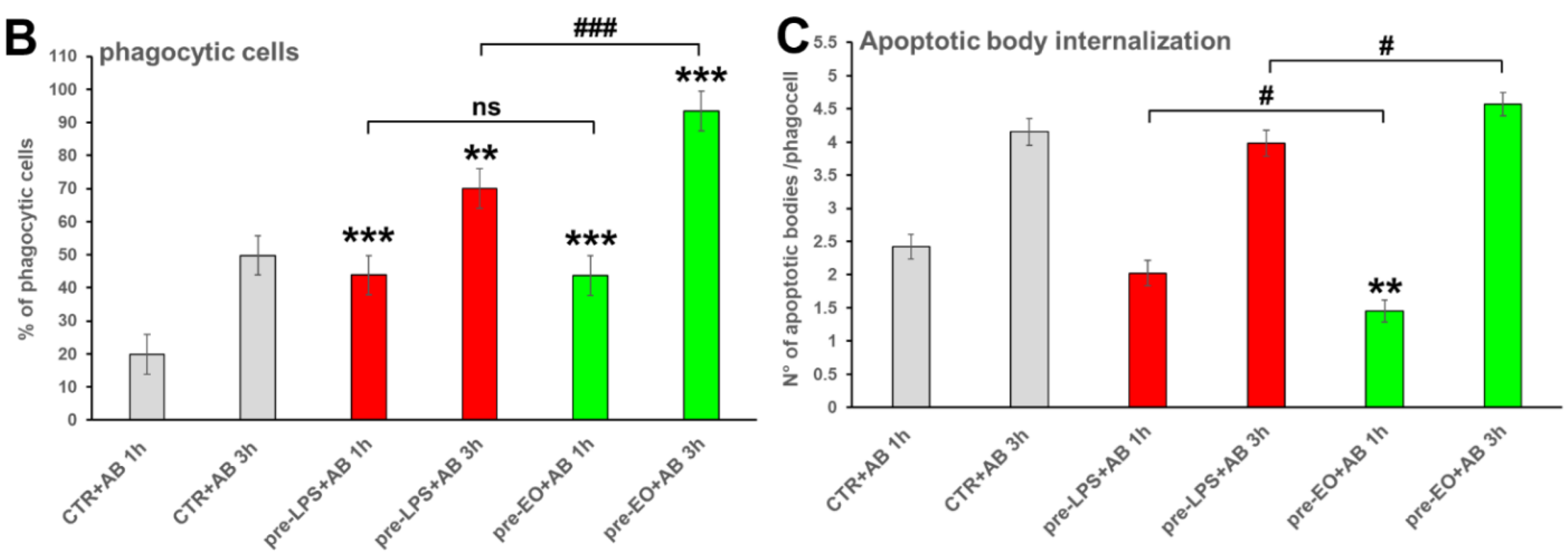
2.3. Effect of Eucalyptus Oil on Efferocytosis
2.4. Assessment of the Involvement of Complement Receptor in EO-Stimulated Phagocytosis
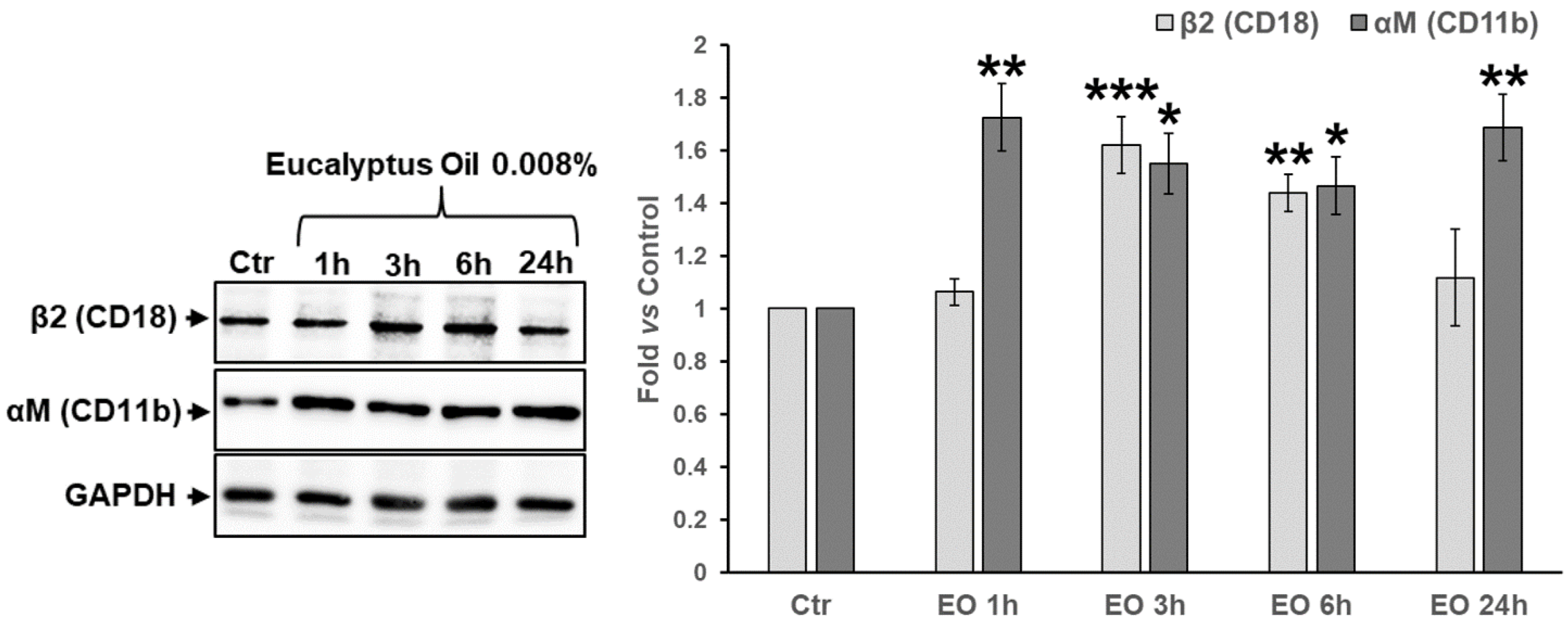
2.5. Effect of Eucalyptus Oil on Podosome Formation and Podosome Structural Components
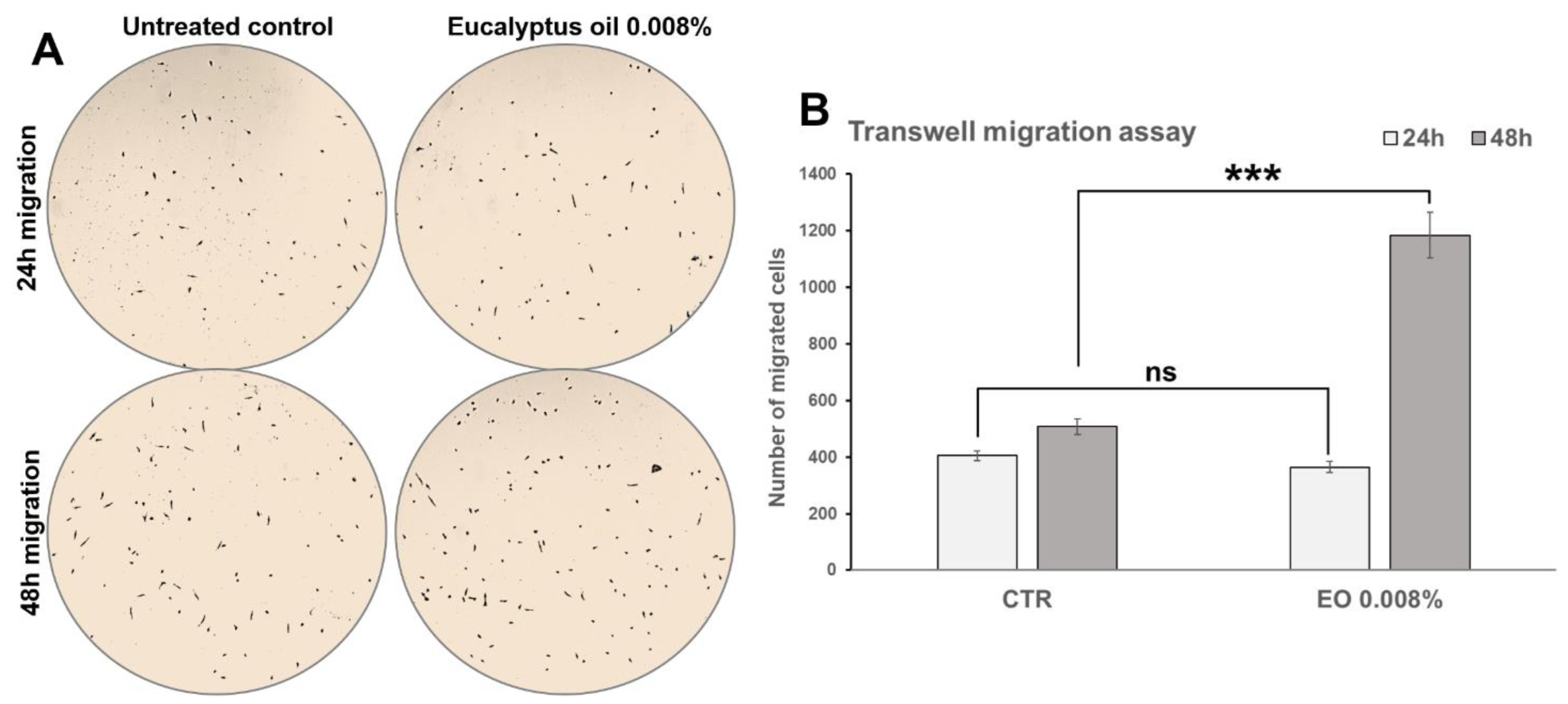
2.6. Effect of Eucalyptus Oil on Macrophage Motility
3. Discussion
4. Materials and Methods
4.1. Preparation of the Human MDM Cultures
4.2. MDM Treatments and Cell Viability Determination
4.3. Phagocytic Activity and Killing Ability Assays by Confocal Microscopy
4.4. Generation of Apoptotic Bodies and Evaluation of Their Internalization by MDMs
4.5. Evaluation of Cytokine and Chemokine Release by MDMs
4.6. Phagocytosis Assay Using Complement- or IgG-Opsonized Zymosan BioParticles
4.7. Western Blot (WB) Analysis
4.8. Transwell Migration Assay
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Underhill, D.M.; Ozinsky, A. Phagocytosis of microbes: Complexity in action. Annu. Rev. Immunol. 2002, 20, 825–852. [Google Scholar] [CrossRef] [PubMed]
- Doran, A.C.; Yurdagul, A., Jr.; Tabas, I. Efferocytosis in health and disease. Nat. Reviews. Immunol. 2020, 20, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Rosales, C.; Uribe-Querol, E. Phagocytosis: A fundamental process in immunity. BioMed Res. Int. 2017, 2017, 9042851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aderem, A.; Underhill, D.M. Mechanisms of phagocytosis in macrophages. Annu. Rev. Immunol. 1999, 17, 593–623. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.A.; Aderem, A. Molecular definition of distinct cytoskeletal structures involved in complement- and Fc receptor-mediated phagocytosis in macrophages. J. Exp. Med. 1996, 184, 627–637. [Google Scholar] [CrossRef]
- Linder, S.; Aepfelbacher, M. Podosomes: Adhesion hot-spots of invasive cells. Trends Cell Biol. 2003, 13, 376–385. [Google Scholar] [CrossRef]
- Evans, J.G.; Matsudaira, P. Structure and dynamics of macrophage podosomes. Eur. J. Cell Biol. 2006, 85, 145–149. [Google Scholar] [CrossRef]
- Linder, S.; Nelson, D.; Weiss, M.; Aepfelbacher, M. Wiskott-Aldrich syndrome protein regulates podosomes in primary human macrophages. Proc. Natl. Acad. Sci. USA 1999, 96, 9648–9653. [Google Scholar] [CrossRef] [Green Version]
- Linder, S.; Wintergerst, U.; Bender-Gotze, C.; Schwarz, K.; Pannicke, U.; Aepfelbacher, M. Macrophages of patients with X-linked thrombocytopenia display an attenuated Wiskott-Aldrich syndrome phenotype. Immunol. Cell Biol. 2003, 81, 130–136. [Google Scholar] [CrossRef]
- Juergens, U.R.; Stober, M.; Vetter, H. Inhibition of cytokine production and arachidonic acid metabolism by eucalyptol (1.8-cineole) in human blood monocytes in vitro. Eur. J. Med. Res. 1998, 3, 508–510. [Google Scholar]
- Dhakad, A.K.; Pandey, V.V.; Beg, S.; Rawat, J.M.; Singh, A. Biological, medicinal and toxicological significance of Eucalyptus leaf essential oil: A review. J. Sci. Food Agric. 2018, 98, 833–848. [Google Scholar] [CrossRef] [PubMed]
- Hendry, E.R.; Worthington, T.; Conway, B.R.; Lambert, P.A. Antimicrobial efficacy of eucalyptus oil and 1,8-cineole alone and in combination with chlorhexidine digluconate against microorganisms grown in planktonic and biofilm cultures. J. Antimicrob. Chemother. 2009, 64, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Schnitzler, P.; Schon, K.; Reichling, J. Antiviral activity of Australian tea tree oil and eucalyptus oil against herpes simplex virus in cell culture. Pharmazie 2001, 56, 343–347. [Google Scholar] [PubMed]
- Santos, F.A.; Rao, V.S. Antiinflammatory and antinociceptive effects of 1,8-cineole a terpenoid oxide present in many plant essential oils. Phytother. Res. 2000, 14, 240–244. [Google Scholar] [CrossRef]
- Silva, J.; Abebe, W.; Sousa, S.M.; Duarte, V.G.; Machado, M.I.; Matos, F.J. Analgesic and anti-inflammatory effects of essential oils of Eucalyptus. J. Ethnopharmacol. 2003, 89, 277–283. [Google Scholar] [CrossRef]
- Yadav, N.; Chandra, H. Suppression of inflammatory and infection responses in lung macrophages by eucalyptus oil and its constituent 1,8-cineole: Role of pattern recognition receptors TREM-1 and NLRP3, the MAP kinase regulator MKP-1, and NFkappaB. PLoS ONE 2017, 12, e0188232. [Google Scholar] [CrossRef] [Green Version]
- Mieres-Castro, D.; Ahmar, S.; Shabbir, R.; Mora-Poblete, F. Antiviral activities of eucalyptus essential oils: Their effectiveness as therapeutic targets against human viruses. Pharmaceuticals 2021, 14, 1210. [Google Scholar] [CrossRef]
- Moreira, P.; Sousa, F.J.; Matos, P.; Brites, G.S.; Goncalves, M.J.; Cavaleiro, C.; Figueirinha, A.; Salgueiro, L.; Batista, M.T.; Branco, P.C.; et al. Chemical composition and effect against skin alterations of bioactive extracts obtained by the hydrodistillation of Eucalyptus globulus leaves. Pharmaceutics 2022, 14, 561. [Google Scholar] [CrossRef]
- Nakamura, T.; Yoshida, N.; Yamanoi, Y.; Honryo, A.; Tomita, H.; Kuwabara, H.; Kojima, Y. Eucalyptus oil reduces allergic reactions and suppresses mast cell degranulation by downregulating IgE-FcepsilonRI signalling. Sci. Rep. 2020, 10, 20940. [Google Scholar] [CrossRef]
- Shao, J.; Yin, Z.; Wang, Y.; Yang, Y.; Tang, Q.; Zhang, M.; Jiao, J.; Liu, C.; Yang, M.; Zhen, L.; et al. Effects of different doses of eucalyptus oil from Eucalyptus globulus labill on respiratory tract immunity and immune function in healthy rats. Front. Pharmacol. 2020, 11, 1287. [Google Scholar] [CrossRef]
- Sandner, G.; Heckmann, M.; Weghuber, J. Immunomodulatory activities of selected essential oils. Biomolecules 2020, 10, 1139. [Google Scholar] [CrossRef] [PubMed]
- Silveira, D.; Prieto-Garcia, J.M.; Boylan, F.; Estrada, O.; Fonseca-Bazzo, Y.M.; Jamal, C.M.; Magalhaes, P.O.; Pereira, E.O.; Tomczyk, M.; Heinrich, M. COVID-19: Is there evidence for the use of herbal medicines as adjuvant symptomatic therapy? Front. Pharmacol. 2020, 11, 581840. [Google Scholar] [CrossRef] [PubMed]
- Serafino, A.; Sinibaldi Vallebona, P.; Andreola, F.; Zonfrillo, M.; Mercuri, L.; Federici, M.; Rasi, G.; Garaci, E.; Pierimarchi, P. Stimulatory effect of eucalyptus essential oil on innate cell-mediated immune response. BMC Immunol. 2008, 9, 17. [Google Scholar] [CrossRef] [Green Version]
- Byard, R. Traditional medicine of aboriginal Australia. CMAJ 1988, 139, 792–794. [Google Scholar] [PubMed]
- Akhtar, M.A.; Raju, R.; Beattie, K.D.; Bodkin, F.; Munch, G. Medicinal plants of the Australian Aboriginal Dharawal people exhibiting anti-inflammatory activity. Evid.-Based Complement. Altern. Med. 2016, 2016, 2935403. [Google Scholar] [CrossRef] [PubMed]
- Worth, H.; Schacher, C.; Dethlefsen, U. Concomitant therapy with cineole (eucalyptole) reduces exacerbations in COPD: A placebo-controlled double-blind trial. Respir Res. 2009, 10, 69. [Google Scholar] [CrossRef] [Green Version]
- Juergens, U.R.; Dethlefsen, U.; Steinkamp, G.; Gillissen, A.; Repges, R.; Vetter, H. Anti-inflammatory activity of 1.8-cineol (eucalyptol) in bronchial asthma: A double-blind placebo-controlled trial. Respir Med. 2003, 97, 250–256. [Google Scholar] [CrossRef] [Green Version]
- Sadlon, A.E.; Lamson, D.W. Immune-modifying and antimicrobial effects of eucalyptus oil and simple inhalation devices. Altern. Med. Rev. 2010, 15, 33–47. [Google Scholar]
- Juergens, U.R. Anti-inflammatory properties of the monoterpene 1.8-cineole: Current evidence for co-medication in inflammatory airway diseases. Drug Res. 2014, 64, 638–646. [Google Scholar] [CrossRef]
- Alatawi, K.A.; Ravishankar, D.; Patra, P.H.; Bye, A.P.; Stainer, A.R.; Patel, K.; Widera, D.; Vaiyapuri, S. 1,8-Cineole affects agonists-induced platelet activation, thrombus formation and haemostasis. Cells 2021, 10, 2616. [Google Scholar] [CrossRef]
- van den Dries, K.; Schwartz, S.L.; Byars, J.; Meddens, M.B.; Bolomini-Vittori, M.; Lidke, D.S.; Figdor, C.G.; Lidke, K.A.; Cambi, A. Dual-color superresolution microscopy reveals nanoscale organization of mechanosensory podosomes. Mol. Biol. Cell 2013, 24, 2112–2123. [Google Scholar] [CrossRef] [PubMed]
- Serafino, A.; Pica, F.; Andreola, F.; Gaziano, R.; Moroni, N.; Moroni, G.; Zonfrillo, M.; Pierimarchi, P.; Sinibaldi-Vallebona, P.; Garaci, E. Thymosin alpha1 activates complement receptor-mediated phagocytosis in human monocyte-derived macrophages. J. Innate Immun. 2014, 6, 72–88. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zonfrillo, M.; Andreola, F.; Krasnowska, E.K.; Sferrazza, G.; Pierimarchi, P.; Serafino, A. Essential Oil from Eucalyptus globulus (Labill.) Activates Complement Receptor-Mediated Phagocytosis and Stimulates Podosome Formation in Human Monocyte-Derived Macrophages. Molecules 2022, 27, 3488. https://doi.org/10.3390/molecules27113488
Zonfrillo M, Andreola F, Krasnowska EK, Sferrazza G, Pierimarchi P, Serafino A. Essential Oil from Eucalyptus globulus (Labill.) Activates Complement Receptor-Mediated Phagocytosis and Stimulates Podosome Formation in Human Monocyte-Derived Macrophages. Molecules. 2022; 27(11):3488. https://doi.org/10.3390/molecules27113488
Chicago/Turabian StyleZonfrillo, Manuela, Federica Andreola, Ewa K. Krasnowska, Gianluca Sferrazza, Pasquale Pierimarchi, and Annalucia Serafino. 2022. "Essential Oil from Eucalyptus globulus (Labill.) Activates Complement Receptor-Mediated Phagocytosis and Stimulates Podosome Formation in Human Monocyte-Derived Macrophages" Molecules 27, no. 11: 3488. https://doi.org/10.3390/molecules27113488





