“For Asia Market Only”: A Green Tattoo Ink between Safety and Regulations
Abstract
:1. Introduction
2. Materials and Methods
Extraction Procedures
3. Results and Discussion
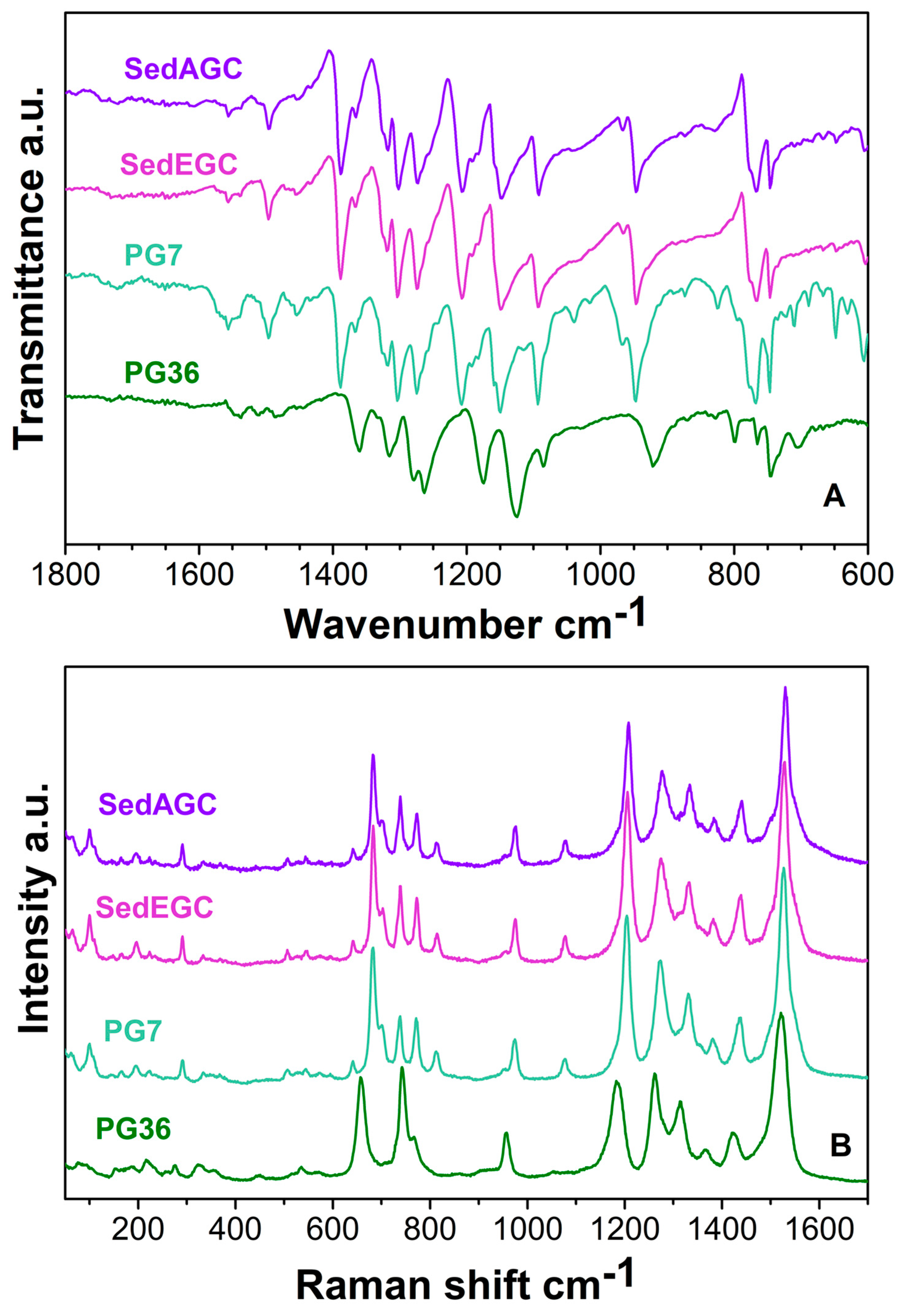
3.1. IR and Raman
3.2. Elemental Content by XRF
3.3. GC-Mass Spectrometry
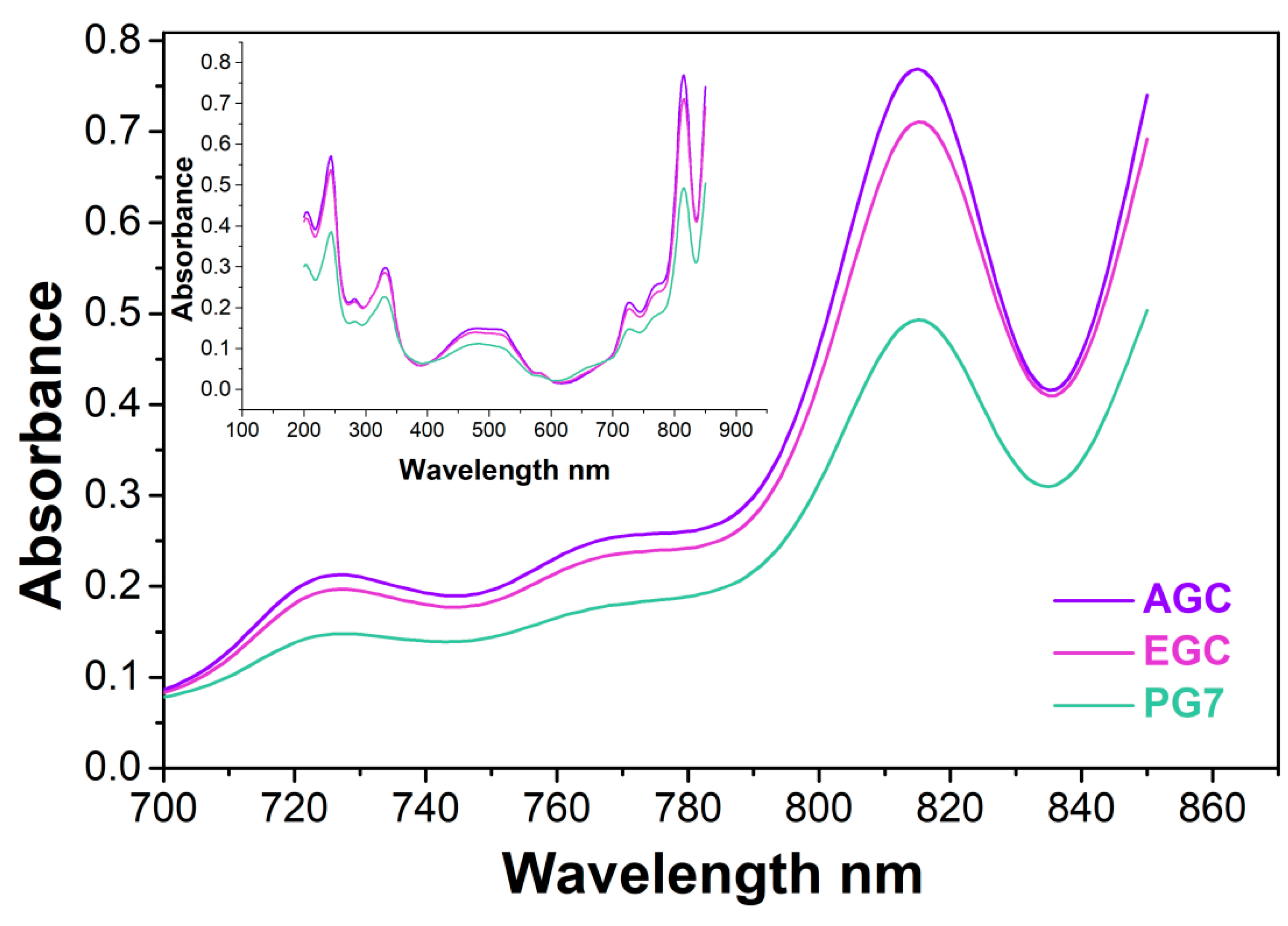
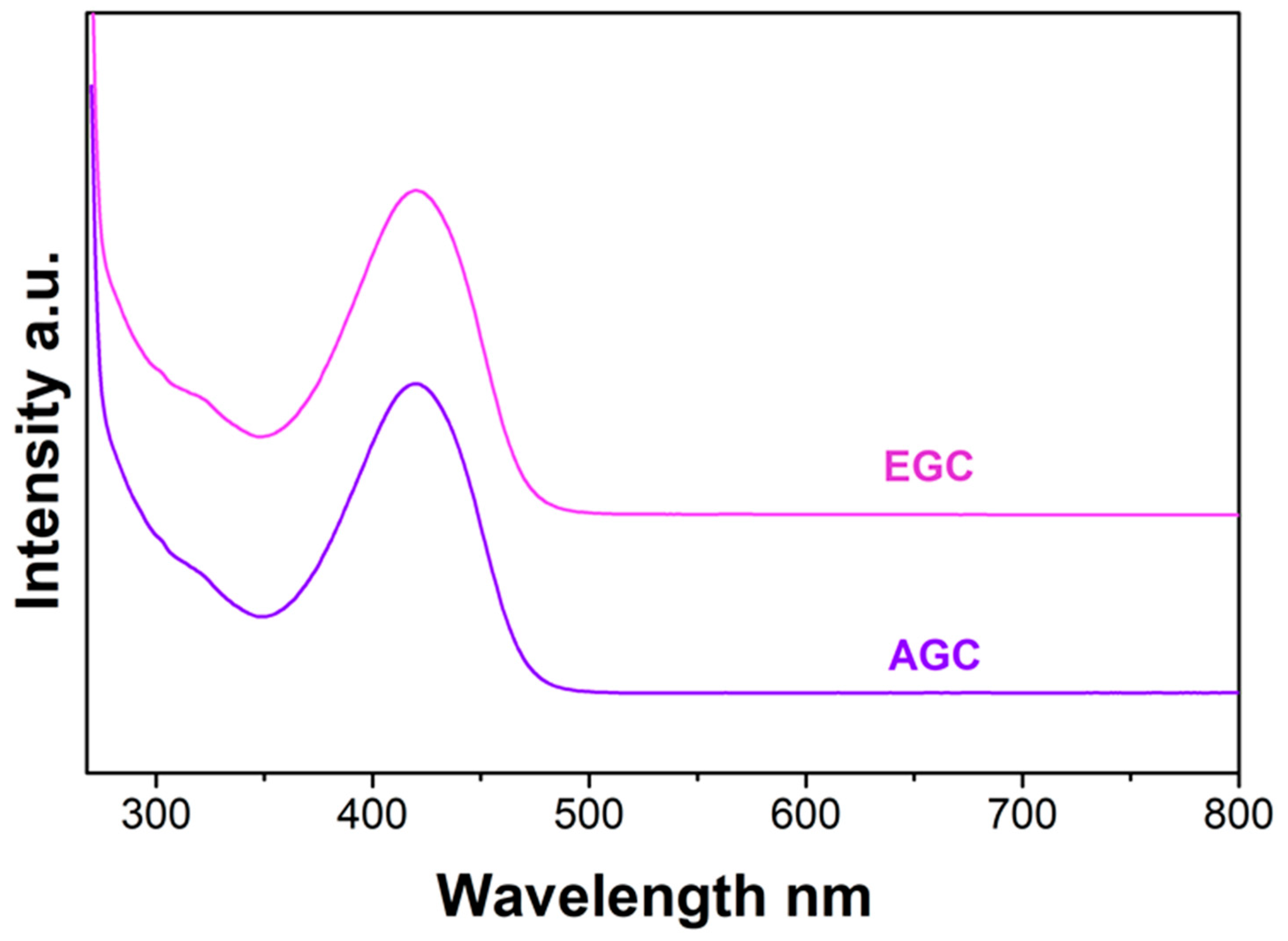
3.4. Analysis of the Extracts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Piccinini, P.; Pakalin, S.; Contor, L.; Bianchi, I.; Senaldi, C. “Safety of Tattoos and Permanent Make-Up: Final Report.” EUR 27947 EN; Publications Office of the European Union: Luxembourg, 2016. [Google Scholar] [CrossRef]
- Weiβ, K.T.; Schreiver, I.; Siewert, K.; Luch, A.; Haslböck, B.; Berneburg, M.; Bäumler, W. Tattos—more than just colored skin? Searching for tattoo allergens. J. Dtsch. Dermatol. Ges. 2021, 19, 657–669. [Google Scholar]
- Alsing, K.K.; Johannesen, H.H.; Hansen, R.H.; Serup, J. Tattoo complications and magnetic resonance imaging: A comprehensive review of the literature. Acta Radiol. 2020, 61, 1695–1700. [Google Scholar] [CrossRef] [PubMed]
- Fraser, T.R.; Ross, K.E.; Alexander, U.; Lenehan, C.E. Current knowledge of the degradation products of tattoo pigments by sunlight, laser irradiation and metabolism: A systematic review. J. Expos. Sci. Environ. Epidemiol. 2022, 32, 343–355. [Google Scholar] [CrossRef]
- Karregat, J.J.P.; Rustemeyer, T.; van der Bent, S.A.S.; Spiekstra, S.W.; Thon, M.; Rivas, D.F.; Gibbs, S. Assessment of cytotoxicity and sensitization potential of intradermally injected tattoo inks in reconstructed human skin. Contact Dermat. 2021, 85, 324–339. [Google Scholar] [CrossRef] [PubMed]
- Donia, D.T.; Scibetta, E.V.; Tagliatesta, P.; Carbone, M. Chemistry through Tattoo Inks: A Multilevel Approach to a Practice on the Rise for Eliciting Interest in Chemical Education. J. Chem. Educ. 2021, 98, 1309–1320. [Google Scholar] [CrossRef]
- MacFarlane, M. Tattoos in East Asia: Conforming to Individualism; University of Puget Sound, Sound Ideas: Takoma, WA, USA, 2019; Available online: https://soundideas.pugetsound.edu/summer_research/343/ (accessed on 18 May 2022).
- White, K. Changing Views of Tattoos in Japan. Marshall Digital Scholar, 29 February 2019. Available online: https://mds.marshall.edu/colaconf/2019/day2/15/ (accessed on 18 May 2022).
- McCallum, D. Historical and Cultural Dimensions of the Tattoo in Japan. In Marks of Civilization: Artistic Transformations of the Human Body; Rubin, A., Ed.; Los Angeles Museum of Cultural History, University of California: Los Angeles, CA, USA, 1988. [Google Scholar]
- Bratt, M. A History of Japanese Body Suit Tattooing; KIT Publishers: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Yamada, M. Westernization and cultural resistance in tattooing practices in contemporary Japan. Int. J. Cult. Stud. 2009, 12, 319–338. [Google Scholar] [CrossRef] [Green Version]
- Theobald, U. Mo 墨, Penal Tattooing. An Encyclopaedia on Chinese History, Literature and Art. 2016. Available online: https://www.chinaknowledge.de/History/Terms/penal_mo.html (accessed on 18 May 2022).
- Reed, C.E. Tattoo in Early China. J. Am. Orient. Soc. 2000, 120, 360–376. [Google Scholar] [CrossRef]
- Park, J. Sign of social change on the bodies of youth: Tattoos in Korea. Vis. Commun. 2016, 15, 71–92. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.Y. A study on adult women’s cosmetic tattoo experiences and comparison of health concern and health practise between the cosmetic tattooed and non-cosmetic tattooed groups. J. Korean Acad. Community Health Nurs. 2017, 28, 69–77. [Google Scholar] [CrossRef]
- Kluger, N.; Seité, S.; Taineb, C. The prevalence of tattooing and motivations in five major countries over the world. J. Eur. Acad. Dermatol. 2019, 33, e484–e486. [Google Scholar] [CrossRef]
- Tizzard, D. Morality, Legality and Tattoos. 2020. Available online: https://www.koreatimes.co.kr/www/opinion/2020/11/197_298485.html (accessed on 18 May 2022).
- Private Email Exchange with the Corresponding Author, Assisted by the Experts of Chinese Language. Available online: https://www.cnas.org.cn/english/suspendingcanceling/index.shtml or http://www.ccaa.org.cn/ (accessed on 18 May 2022).
- Bauer, E.M.; De Caro, T.; Tagliatesta, P.; Carbone, M. Unraveling the real pigment composition of tattoo inks: The case of bi-components phthalocyanine based greens. Dyes Pigment. 2019, 167, 225–235. [Google Scholar] [CrossRef]
- National Regulations on Tattoo Ink and Permanent Make-Up Formulations in Spain Informaciόn Sobre Productos Para Maquillaje Permanente (Micropigmentaciόn) y Tatuaje. Available online: https://www.aemps.gob.es/informa/notasInformativas/cosmeticosHigiene/2008/NI-prodAutorizados-tatuaje_julio-2008.htm (accessed on 18 May 2022).
- National Regulations on Tattoo Ink and Permanent Make-Up Formulations in France—Arrêté du 6 Mars 2013 Fixant la Liste des Substances qui ne Peuvent pas Entrer dans la Composition des Produits de Tatouage. Available online: https://www.legifrance.gouv.fr/affichTexte.do?cidTexte=JORFTEXT000027167179&dateTexte=20200415 (accessed on 18 May 2022).
- National Regulations on Tattoo Ink and Permanent Make-Up Formulations in Sweden—Förordning (2012:503) om Tatueringsfärger. Available online: https://www.riksdagen.se/sv/dokument-lagar/dokument/svensk-forfattningssamling/forordning-2012503-omtatueringsfarger_sfs-2012-503 (accessed on 18 May 2022).
- National Regulations on Tattoo Ink and Permanent Make-Up Formulations in The Netherlands—Besluit van 24 April 2013, Houdende Wijziging van He Warenwetbesluit Tatoeagekleurstoffen in Verband Met Het Intrekken van Richtlijn 76/768/EEG. Available online: https://zoek.officielebekendmakingen.nl/stb-2013-177.html (accessed on 18 May 2022).
- Manso, M.; Pessanha, S.; Guerra, M.; Reinholz, U.; Afonso, C.; Radtke, M.; Lourenço, H.; Carvalho, M.L.; Buzanich, A.G. Assessment of toxic metals and hazardous substances in tattoo inks using Sy-XRF, AAS, and Raman spectroscopy. Biol. Trace Elem. Res. 2019, 187, 596–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bocca, B.; Senofonte, O.; Petrucci, F. Hexavalent chromium in tattoo inks: Dermal exposure and systemic risk. Contact Dermat. 2018, 79, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.-H.; Shin, H.-S. Identification and quantification of phthalates, PAHs, amines, phenols, and metals in tattoo. Bull. Korean Chem. Soc. 2015, 36, 2039–2050. [Google Scholar] [CrossRef]
- Schreiver, I.; Hutzler, C.; Luch, A. A Two-Step Pyrolysis-Gas Chromatography Method with Mass Spectrometric Detection for Identification of Tattoo Ink Ingredients and Counterfeit Products. Available online: https://www.jove.com/it/t/59689/a-two-step-pyrolysis-gas-chromatography-method-with-mass (accessed on 18 May 2022).
- Schreiver, I.; Hutzler, C.; Andree, S.; Laux, P.; Luch, A. Identification and hazard prediction of tattoo pigments by means of pyrolysis—gas chromatography/mass spectrometry. Arch. Toxicol. 2016, 90, 1639–1650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battistini, B.; Petrucci, F.; De Angelis, I.; Failla, C.M.; Bocca, B. Quantitative analysis of metals and metal-based nano- and submicron-particles in tattoo inks. Chemosphere 2020, 245, 125667. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Josefsson, L.; Meschnark, S.; Lind, M.-L.; Emmer, Å.; Goessler, W.; Hedberg, Y.S. Analytical survey of tattoo inks—A chemical and legal perspective with focus on sensitizing substances. Contact Dermat. 2021, 85, 340–353. [Google Scholar] [CrossRef]
- Ali, A.; Al-Easawi, N. Determination of heavy metals in tattoo inks form the local market in Baghdad City. Plant Arch. 2020, 20, 1289–1292. [Google Scholar]
- Eghbali, K.; Mousavi, Z.; Ziarati, P. Determination of Heavy Metals in Tattoo Ink. Biosci. Biotechnol. Res. Asia 2014, 11, 941–946. [Google Scholar] [CrossRef]
- Karbowska, B.; Rębiś, T.; Zembrzuska, J.; Nadolska, K. Thallium in color tattoo inks: Risk associated with tattooing. Med. Pr. 2020, 71, 405–411. [Google Scholar] [CrossRef]
- Donia, D.T.; Carbone, M. Fate of the nanoparticles in environmental cycles. Int. J. Environ. Sci. Technol. 2019, 16, 583–600. [Google Scholar] [CrossRef]
- Resolution ResAP(2008)1 on Requirements and Criteria for the Safety of Tattoos Permanent Make-Up. Available online: https://search.coe.int/cm/Pages/result_details.aspx?ObjectID=09000016805d3dc4 (accessed on 18 May 2022).
- Resolution Amendment ResAP(2008)1 Commission Regulation (EU) 2020/2081 of December 14th, 2020 Amending Annex XVII to Regulation (EC) No 1907/2006 of the European Parliament and of the Council. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32020R2081&qid=1620664323883 (accessed on 18 May 2022).
- Lim, H.-H.; Shin, H.-S. Sensitive Determination of Volatile Organic Compounds and Aldehydes in Tattoo Inks. J. Chromatogr. Sci. 2017, 55, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Bauer, E.M.; Scibetta, E.V.; Cecchetti, D.; Piccirillo, S.; Antonaroli, S.; Sennato, S.; Cerasa, M.; Tagliatesta, P.; Carbone, M. Treatments of a phthalocyanine-based green ink for tattoo removal purposes: Generation of toxic fragments and potentially harmful morphologies. Arch. Toxicol. 2020, 94, 2359–2375. [Google Scholar] [CrossRef] [PubMed]
- Schulte, F.; Brzezinka, K.W.; Lutzenberger, K.; Stege, H.; Panne, U. Raman spectroscopy of synthetic organic pigments used in 20th century works of art. J. Raman Spectrosc. 2008, 39, 1455–1463. [Google Scholar] [CrossRef]
- Anghelone, M.; Jembrih-Simbürger, D.; Pintus, V.; Schreiner, M. Photostability and influence of phthalocyanine pigments on the photodegradation of acrylic paints under accelerated solar radiation. Polym. Degrad. Stabil. 2017, 146, 13–23. [Google Scholar] [CrossRef]
- Bosi, A.; Ciccola, A.; Serafini, I.; Guiso, M.; Ripanti, F.; Postorino, P.; Curini, R.; Bianco, A. Street art graffiti: Discovering their composition and alteration by FTIR and micro-Raman spectroscopy. Spectrochim. Acta Part A 2020, 225, 117474. [Google Scholar] [CrossRef]
- Ledson, L.D.; Twigg, M.V. Acid-base behaviour of phthalocyanine. Inorg. Chim. Acta 1975, 13, 43–46. [Google Scholar] [CrossRef]
- Wöhrle, D.; Hündorf, U. Polymeric phthalocyanines and their precursors, 6. Synthesis and analytical characterization of some octasubstituted phthalocyanines. Makromolekul. Chem. 1985, 186, 2177–2187. [Google Scholar] [CrossRef]
- Seelan, S.; Agashe, M.S.; Srinivas, D.; Sivasanker, S. Effect of peripheral substitution on spectral and catalytic properties of copper phthalocyanine complexes. J. Mol. Catal. A Chem. 2001, 168, 61–68. [Google Scholar] [CrossRef]
- Gebel, T. Arsenic and antimony: Comparative approach on mechanistic toxicology. Chem. Biol. Interact. 1997, 107, 131–144. [Google Scholar] [CrossRef]
- Rahimzadeh, M.R.; Rahimzadeh, M.R.; Kazemi, S.; Moghadamnia, A.A. Cadmium toxicity and treatment: An update. Casp. J. Intern. Med. 2017, 8, 135–145. [Google Scholar]
- Das, K.K.; Reddy, R.C.; Bagoji, I.B.; Das, S.; Bagali, S.; Mullur, L.; Khodnapur, J.P.; Biradar, M.S. Primary concept of nickel toxicity—An overview. J. Basic Clin. Physiol. Pharmacol. 2018, 30, 141–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, M.D.; Kargacin, B.; Klein, C.B.; Costa, M. Mechanisms of chromium carcinogenicity and toxicity. Crit. Rev. Toxicol. 2008, 23, 255–281. [Google Scholar] [CrossRef] [PubMed]
- Cecchetti, D.; Bauer, E.M.; Guerriero, E.; Sennato, S.; Tagliatesta, P.; Tagliaferri, M.; Cerri, L.; Carbone, M. Comparative treatments of a green tattoo ink with Ruby, Nd:YAG nano- and picosecond lasers in normal and array mode. Sci. Rep. 2022, 12, 3571. [Google Scholar] [CrossRef]
- Anastas, P.; Hammond, D. Chapter 4—Case Studied—Green Chemistry in Practice. In Inherent Safety at Chemical Sites, Reducing Vulnerability to Accidents and Terrorism Through Green Chemistry; Elsevier: Amsterdam, The Netherlands, 2015; pp. 23–118. [Google Scholar]
- Moyer, E. 1,4-Dioxane: Regulatory Developments, Uses, Properties, Assessment, and Remediation. 2008. Available online: https://www.lspa.org/assets/documents/lspa-doc-1-4-dioxane-summary.pdf (accessed on 18 May 2022).
- McCarthy, J. 1,4-Dioxane Essentially Banned in Cosmetics, Personal Care, and Cleaning Products in New York State. 2019. Available online: https://delltech.com/blog/1-4-dioxane-essentially-banned-in-cosmetics-personal-care-and-cleaning-products-in-new-york-state/ (accessed on 18 May 2022).
- Kano, H.; Umeda, Y.; Kasai, T.; Sasaki, T.; Matsumoto, M.; Yamazaki, K.; Nagano, K.; Arito, H.; Fukushima, S. Carcinogenicity studies of 1,4-dioxane administered in drinking-water to rats and mice for 2 years. Food Chem. Toxicol. 2009, 47, 2776–2784. [Google Scholar] [CrossRef]
- Schut, D.M. Derivatization of Dyes/PIGMENTS with Crown Ethers and Inkjet Printing Fluids Containing the Same (Patent United States n. US 2002/0144626 A1) Hewlett-Packard Company Intellectual Property Administration. 2002. Available online: https://patentimages.storage.googleapis.com/79/45/d0/70afcc05cffca7/US20020144626A1.pdf (accessed on 18 May 2022).
- Herbst, W.; Hunger, K. Industrial Organic Pigments: Production, Properties, Applications; John Wiley & Sons: Weinheim, Germany, 2004. [Google Scholar]
- Bauer, E.M.; Cecchetti, D.; Nisticò, S.; Germinario, G.; Sennato, S.; Gontrani, L.; Tagliatesta, P.; Carbone, M. Laser vs. thermal treatments of green pigment PG36: Coincidence and toxicity of process. Arch. Toxicol. 2021, 95, 2367–2383. [Google Scholar] [CrossRef]
- Kamrin, M.A. Phthalate risks, phthalate regulation, and public health: A review. J. Toxicol. Environ. Health B 2009, 12, 157–174. [Google Scholar] [CrossRef]
| Metal | EGC | AGC | [29] * | [24] | [26] | [32] * | [31] * | [30] | EU limits * |
|---|---|---|---|---|---|---|---|---|---|
| Chromium | <0.1 | <0.1 | 0.22 | <DL | 6.1 ± 7.7 | 170 | AA | 0.5 | |
| Cobalt | <0.1 | <0.1 | ND | 1.7 ± 4.6 | OA | 0.5 | |||
| Nickel | 12.0 | 11.7 | 0.14 | <DL | 5 ± 8.7 | 6.8 | 3/59 | 5 | |
| Copper | 38610 | 33440 | 3882 | 4400 ± 200 | 1840 ± 5040 | 63 | 1 | 250 | |
| Zinc | 9.9 | <0.1 | 0.98 | 8.7 ± 23.6 | 5.23 | <RC | 2000 | ||
| Arsenic | 2.4 | 2.3 | ND | 2.7 ± 6 | OA | 0.5 | |||
| Selenium | <0.1 | <0.1 | 1.66 | 2 | |||||
| Cadmium | 0.5 | 0.7 | 0.06 | <DL | 0.6 ± 1.9 | 0.83 | 1.617 | <RC | 0.5 |
| Antimony | 1.9 | 2.6 | ND | 1.6 ± 4.5 | <RC | 0.5 | |||
| Barium | 19.3 | 12.2 | 18.1 | 9.8 ± 18.8 | 500 | ||||
| Mercury | <0.2 | <0.2 | 0.06 | <DL | 0.0027 ± 0.0034 | AF | 0.5 | ||
| Lead | <0.8 | <0.8 | 0.17 | 0.80 ± 0.04 | 1.6 ± 5.2 | 6.3 | 2.27 | AF | 0.7 |
| RT min | Compound | Hazards | EGC | AGC |
|---|---|---|---|---|
| Extraction in H20 | ||||
| 3.87 | Octamethylcyclotetrasiloxane (D4) | H361f(2) |  | |
| 4.90 | 2-methyl-1-propanol | H302(4) H332(4) H350(1B) |  | |
| Extraction in Acetone | ||||
| 2.09 | 1,4-dioxane | H319(2) H335(3)H351(2) |  | |
| 3.37 | Styrene | H315(2) H319 (2) H332(4) H372(1) H361d (2) |  |  |
| 3.83 | Octamethylcyclotetrasiloxane (D4) | H361f (2) |  |  |
| 8.40 | 3,3,5-trimethyl-2-cyclohexen-1-one | H302(4) H312(4) H319(2) H335(3) H351(2) |  | |
| 9.56 | Naphthalene | H302(4)H351 (2) |  | |
| 11.67 | 1-methylnaphthalene | H302(4) H319(2) H335(3) H336/ H373(2) |  | |
| 11.80 | 1,3-dioxolane | H319(2) H360(1B) |  | |
| 19.17 | Pentachloro aniline | H301(3) H311(3) H331(3) 373(2) |  |  |
| 19.27 | 12-crown-4 | H330(1) |  | |
| 21.27 | 4,5,6,7-tetrachloro-1,3-isobenzofuranedione | H317(1) H318(1) H334(1) H350(1A) H373(2) |  | |
| Extraction in CH2Cl2 | ||||
| 3.90 | Octamethylcyclotetrasiloxane (D4) | H361f(2) |  |  |
| 18.65 | Dibutyl phthalate | H360 DF (1B) |  | |
| 19.22 | Pentachloro aniline | H301(3) H311(3) H331(3) H373(2) |  |  |
| 24.87 | Diisoocyl phthalate | H360 DF (1B) |  |
| RT Min | Compound | Hazards | EGC | AGC | EGC/AGC Ratio |
|---|---|---|---|---|---|
| 3.07 | Tetrachloroethene | H315(2) H319(2) H317(1)H336(3) H351(2) |  |  | |
| 3.55 | 1,1,2,2-tetrachloroethane | H330(2) H310(1) |  |  | |
| 4.14 | Pentachloroethane | H351(2) H372(1) |  |  | 14.86 |
| 5.88 | Hexachloroethane | H319(2)H351(2) |  |  | 0.93 |
| 8.29 | 4-ethyl benzaldehyde | NDAS |  | ||
| 9.01 | 1-isocyanato-2-methoxy benzene | NA |  | ||
| 9.06 | 1,3-di-tert-butyl benzene | NDAS |  | ||
| 9.29 | Terbuthylazine | H302(4) H373(2) |  |  | |
| 9.43 | 1,4-dichloro-2-ethenyl benzene | NA |  |  | |
| 10.32 | 3,4,6-trichloro-2-methyl phenol | NA |  |  | 1.55 |
| 10.33 | 1-(dichloromethyl)-3-methyl benzene * | NA |  | ||
| 10.57 | 2-chloro-4-(chloromethyl)-1-methylbenzene | NA |  | ||
| 10.70 | 1,2-dichloro-4-(1-chloroethyl) benzene | NA |  |  | |
| 11.86 | Tetradecamethyl cycloheptasiloxane (D7) | H319(2) |  |  | |
| 11.93 | 1,3,5-trichloro-2,4,6-trimethyl benzene | H315(2) H319(2) H335(3) |  |  | |
| 12.40 | 3,4-dichlorophenyl thiocyanate | NA |  |  | |
| 12.87 | 2,6-di-t-butyl-1,4-benzoquinone | H315(2) H319(2) H335(3) |  | ||
| 13.19 | 3-chlorobenzamide | H302(4) H312(4) H315(2) H319(2)H332(4) H335(3) |  | ||
| 13.21 | 2,5-di-t-butylphenol | H315(2)H319(2) H335(3) |  | ||
| 13.44 | 3,3-dimethyl-1-(3H)-isobenzofuranone | H315(2)H319(2) H335(3) |  | ||
| 13.46 | Hexamethyl benzene | NDAS |  | ||
| 13.93 | Hexadecamethyl cyclooctasiloxane (D8) | H319(1) |  |  | |
| 14.03 | Pentachlorobenzene | H302(4) |  |  | 0.57 |
| 14.04 | 3,4-dichloro benzamine | H301(3) H311(3) H317(1)H318(1)H331(3) |  | ||
| 14.65 | Diethyl phthalate | NDAS |  |  | 2.33 |
| 15.81 | 2,3-diphenyl-2-butene | NA |  | ||
| 15.99 | 2,4-dichloro-1,1′-biphenyl | H373(2) |  | ||
| 16.48 | Hexachlorobenzene | H350(1B) H372(1) |  |  | 0.13 |
| 16.78 | 2,6-dibromo-4-chloroaniline | H315(2) H319(2) H335(3) |  | ||
| 17.04 | 1,4-dimethyl anthracene | NA |  | ||
| 17.53 | Anthracene D10 | Internal standard | |||
| 17.63 | Butyl tridecyl phthalate | NA |  |  | |
| 17.65 | Pentachlorobenzonitrile | H315(2)H319(2) H335(3) |  |  | |
| 17.80 | 1,1-(4,4′-diethyl)diphenylethane | NA |  | ||
| 18.01 | 4,5-dichlorophthalimide | H315(2)H319(2) H335(3) |  | ||
| 18.11 | Pentachloroaniline | H301(3) H311(3)H331(3) H373(2) |  |  | 0.12 |
| 18.24 | 7,9-Di-tert-butyl-1-oxaspiro[4,5]deca-6,9-diene-2,8-dione | H315(2) H319(2A) H335(3) |  |  | 0.56 |
| 18.28 | Trichlorobenzenamide | NA |  |  | 6.00 |
| 18.63 | 2,3,4,5-tetrachloro aniline | H302(4) H315(2) H317(1) H318(1) H335(3) |  | ||
| 18.68 | Butyl 2-pentyl phthalate | NA |  |  | 1.60 |
| 18.71 | 2,3,4,5,6-pentachloro-N-(dichloromethylene)-benzenamine | NA |  | ||
| 18.96 | Pentachlorophenol | H301(3) H311(3) H315(2)H319(2)H330(2) H335(3) H351(2) |  |  | 0.77 |
| 19.93 | Tetrachlorobenzamide | NA |  |  | 1.46 |
| 22.04 | Pentachlorobenzamide | H302(4) H312(4) H315(2) H319(2) H332(4) H335(3) |  |  | 1.05 |
| 22.61 | 3,4,5,6-tetrachloro phthalimide | H315(2)H319(2) H335(3) |  |  | 0.46 |
| 23.79 | Diisoctyl phthalate | H360(1B) |  |  | |
| 27.75 | Perylene D12 | Internal standard |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bauer, E.M.; Cecchetti, D.; Guerriero, E.; Quaranta, S.; Ripanti, F.; Postorino, P.; Tagliatesta, P.; Carbone, M. “For Asia Market Only”: A Green Tattoo Ink between Safety and Regulations. Molecules 2022, 27, 3491. https://doi.org/10.3390/molecules27113491
Bauer EM, Cecchetti D, Guerriero E, Quaranta S, Ripanti F, Postorino P, Tagliatesta P, Carbone M. “For Asia Market Only”: A Green Tattoo Ink between Safety and Regulations. Molecules. 2022; 27(11):3491. https://doi.org/10.3390/molecules27113491
Chicago/Turabian StyleBauer, Elvira M., Daniele Cecchetti, Ettore Guerriero, Simone Quaranta, Francesca Ripanti, Paolo Postorino, Pietro Tagliatesta, and Marilena Carbone. 2022. "“For Asia Market Only”: A Green Tattoo Ink between Safety and Regulations" Molecules 27, no. 11: 3491. https://doi.org/10.3390/molecules27113491
APA StyleBauer, E. M., Cecchetti, D., Guerriero, E., Quaranta, S., Ripanti, F., Postorino, P., Tagliatesta, P., & Carbone, M. (2022). “For Asia Market Only”: A Green Tattoo Ink between Safety and Regulations. Molecules, 27(11), 3491. https://doi.org/10.3390/molecules27113491