Screening of Indanoyl-Type Compounds as Elicitors of Isoflavonoid Phytoalexins in Colombian Common Bean Cultivars
Abstract
:1. Introduction
2. Results
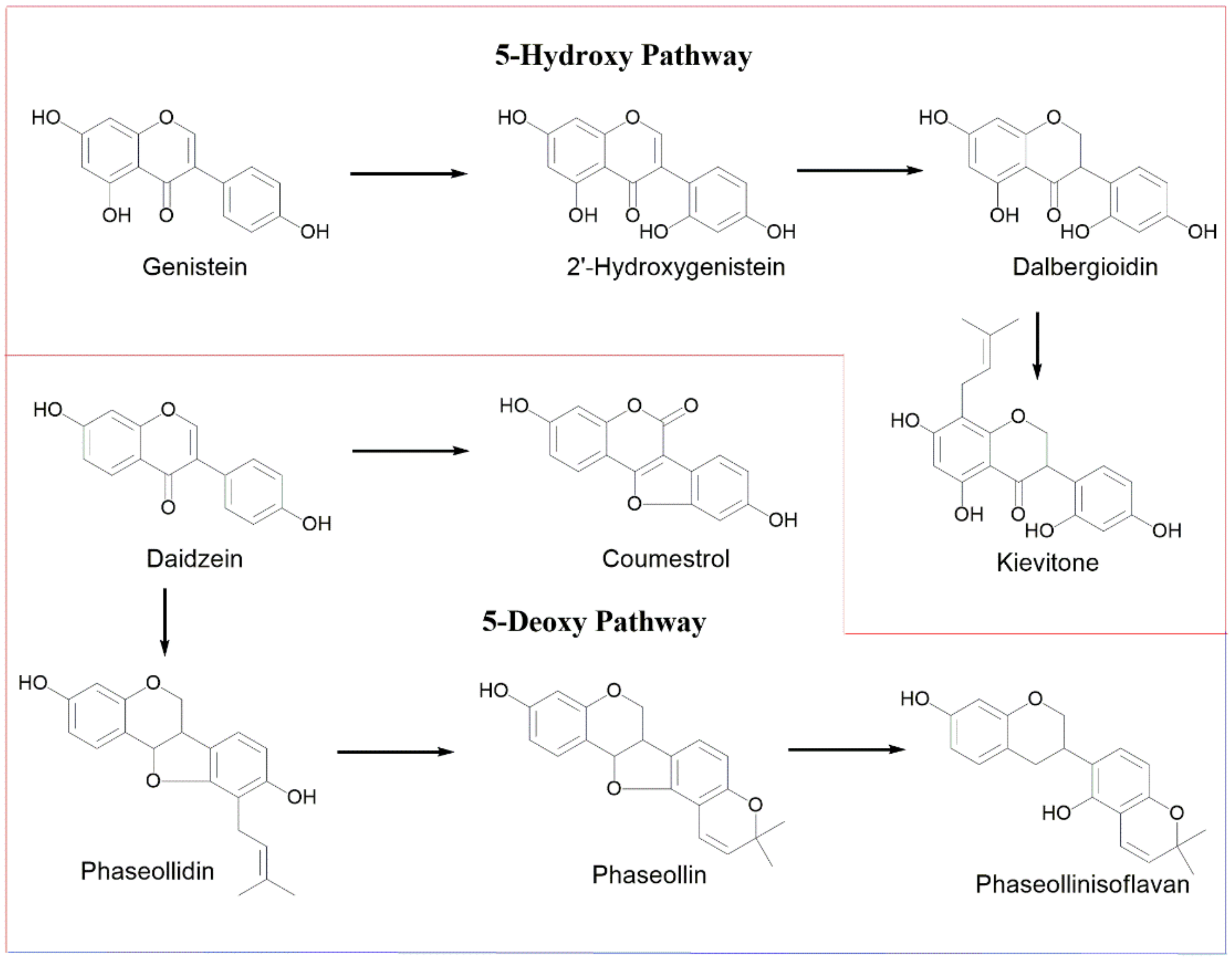
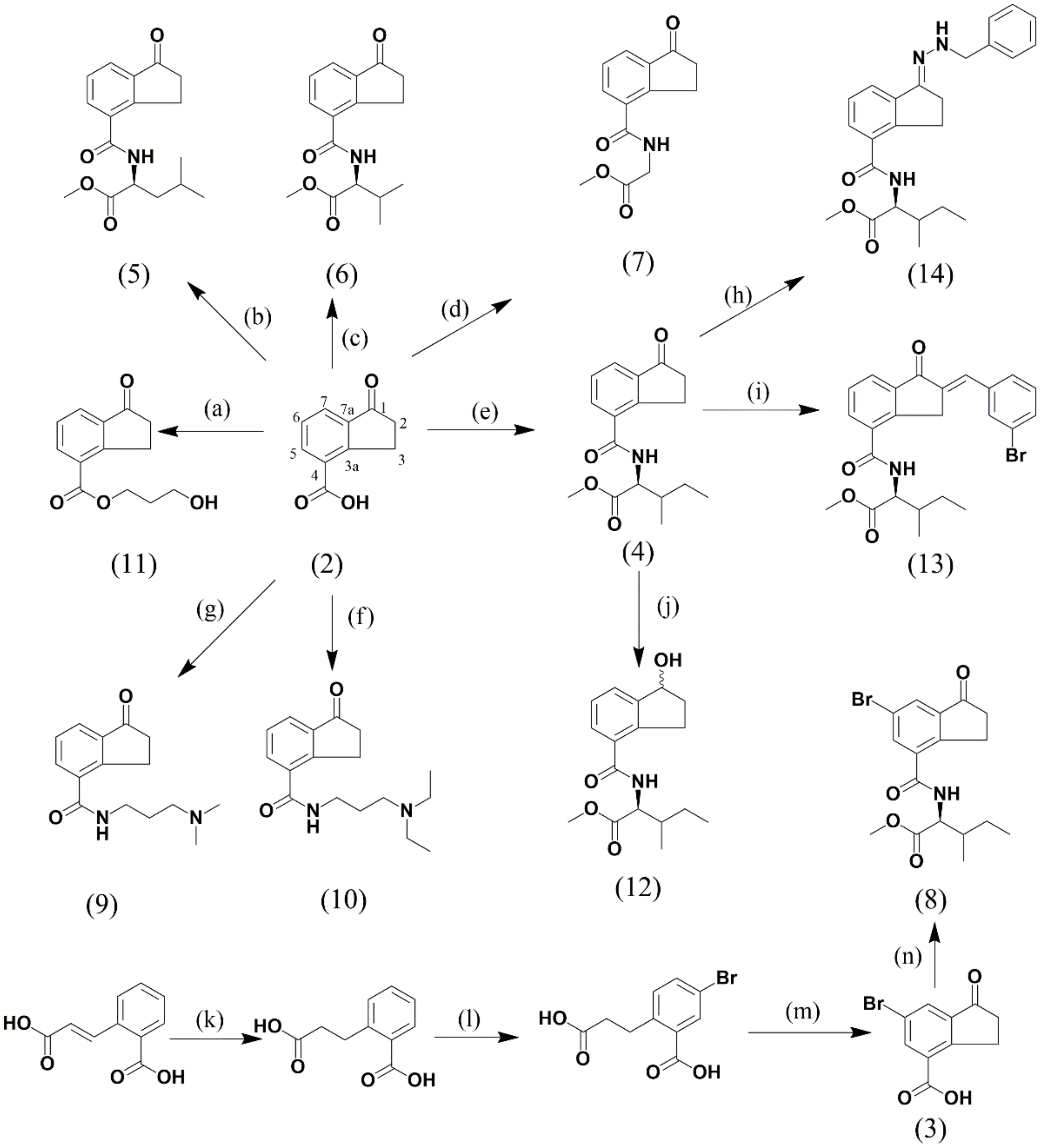
2.1. Influence of Indanoyl-Derivative Structure on Phytoalexin Accumulation
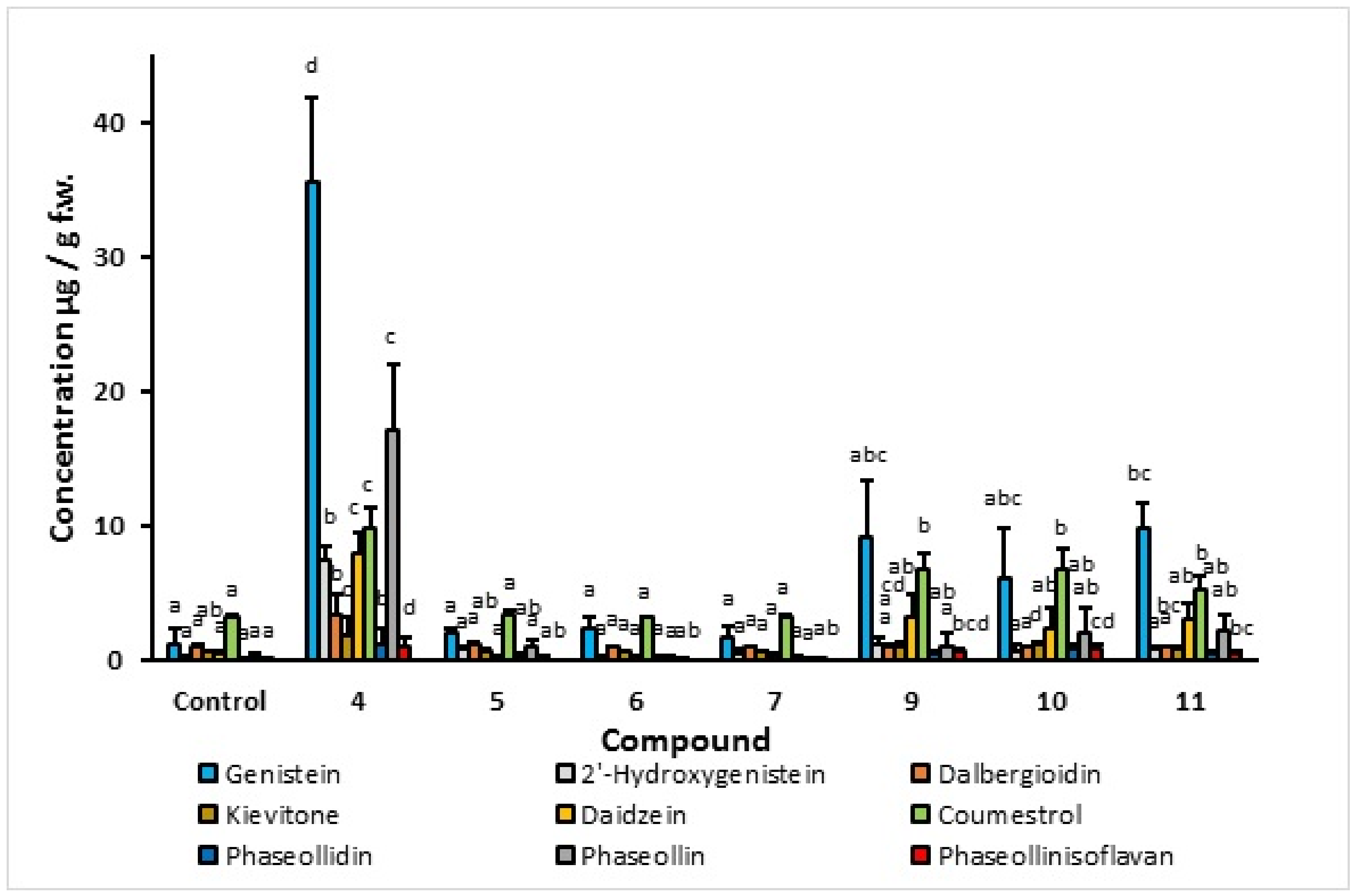
2.1.1. Effect of C4-Substituted Indanoyl Derivatives (4, 9, 10, and 11) on Phytoalexin Accumulation
2.1.2. Effect of Indanoyl-Amino Acid Conjugates (4, 5, 6, and 7) on Phytoalexin Accumulation
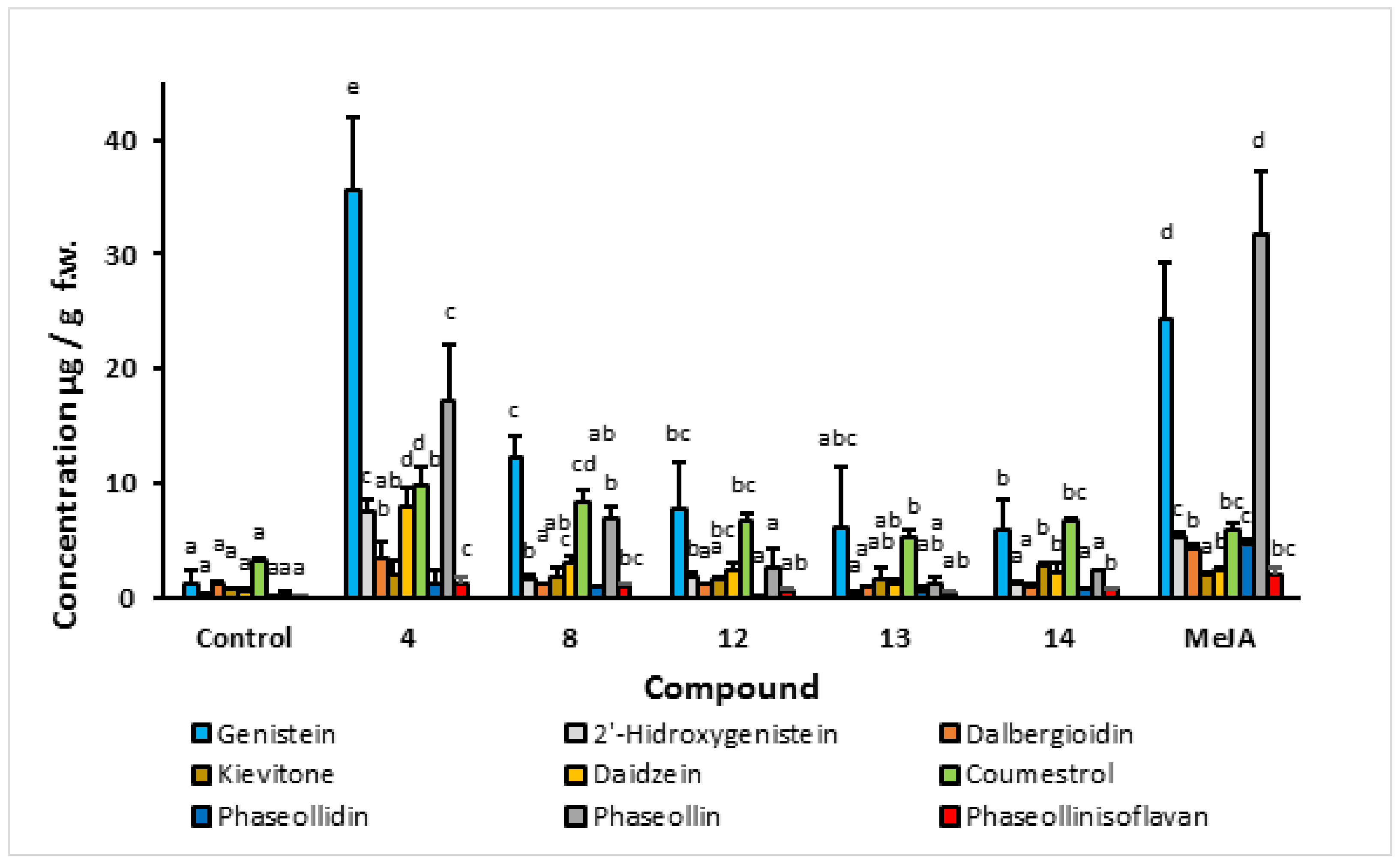
2.1.3. Effect of Indanoyl-L-Isoleucine Methyl Ester Derivatives (4, 8, 12, 13, and 14) on Phytoalexin Accumulation
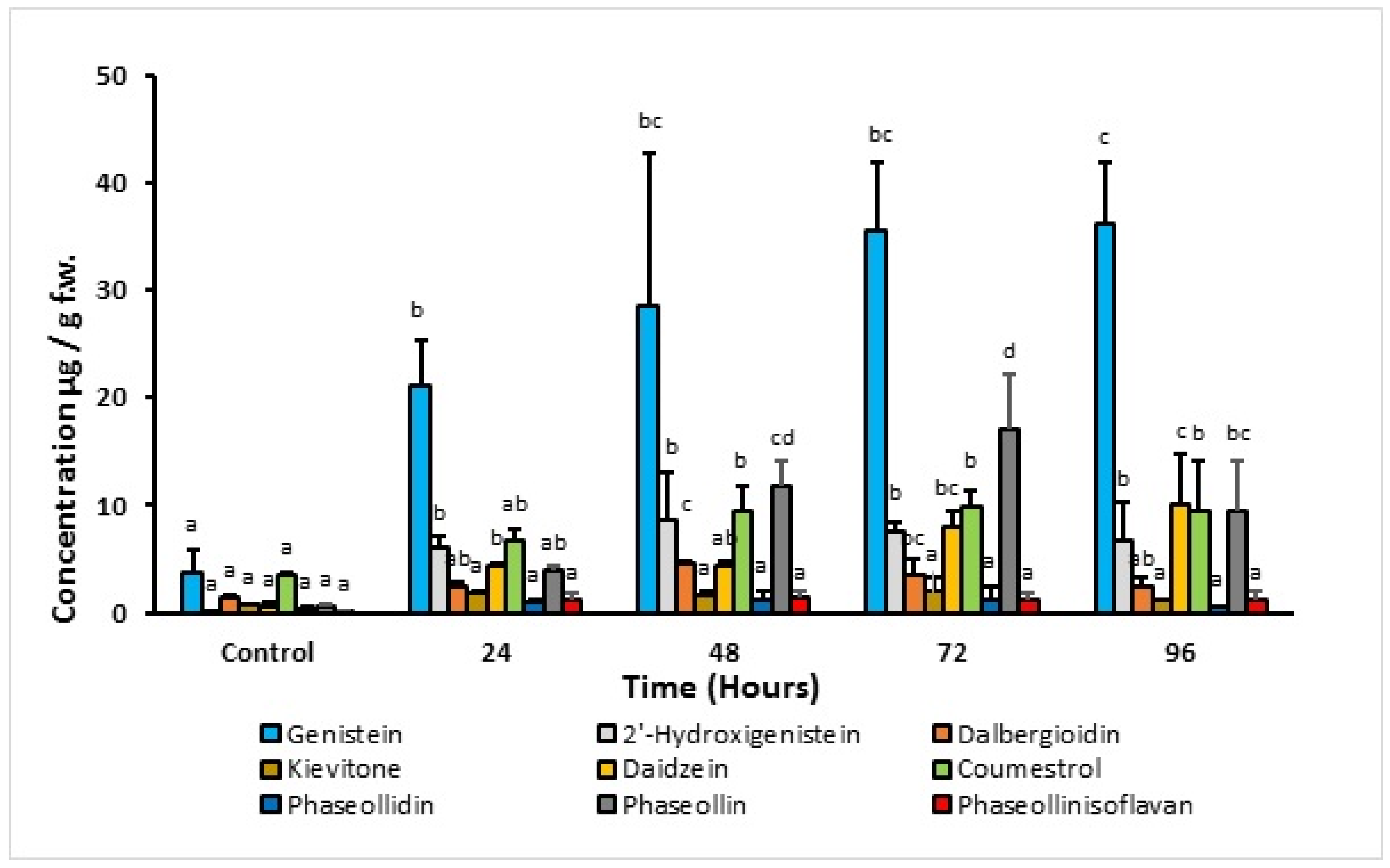
2.2. Time-Course Experiments with 1-Oxo-Indanoyl-L-Isoleucyl Methyl Ester
2.3. Phytotoxicity Effects of 1-Oxo-Indanoyl-L-Isoleucyl Methyl Ester on Bean Seedlings
2.3.1. Effect of 1-Oxo-Indanoyl-L-Isoleucyl Methyl Ester on Common Bean Seeds and Cotyledon Hardness
2.3.2. Effect of 1-Oxo-Indanoyl-L-Isoleucyl Methyl Ester on Common Bean Seedlings and Chlorophyll Content
3. Discussion
- (i)
- Structure of the elicitor. Systematic modifications of the 1-oxo-indane-4-carboxylic acid system showed the importance of different functional groups and substituents in inducing activity of isoflavonoid phytoalexins. First, a change at position 4 by another substituent different from the amino acid L-isoleucine methyl ester resulted in a significant loss of the abovementioned biological activity; second, the carbonyl group of the oxo-cyclopentyl system plays an important role in the elicitor activity; reduction of this group or nucleophilic addition for hydrazone formation had negative effects on the activity. Finally, incorporation of a bulky substituent, such as bromine, at position 6 significantly reduced the elicitor activity. It is clear that it is possible to modulate phytoalexin production in beans with the use of indanoyl-type elicitors.
- (ii)
- Elicitation process. Phytoalexin accumulation depends on the common bean cultivar, the post-induction time, and the elicitor structure. The maximum concentrations of isoflavonoid phytoalexins were elicited after treatment with the compound 1-oxo-indanoyl-L-isoleucine methyl ester (4) and a post-induction time of 72 h. Additionally, the major phytoalexins in the elicitation process were genistein and phaseollin, which are recognized for their antimicrobial effect. Likewise, the application of indanoyl derivatives showed that the 5-deoxyisoflavonoid production pathway, which uses daidzein as a precursor, was preferentially channeled to the pterocarpans biosynthetic pathway, specifically phaseollin. In the case of the 5-hydroxyisoflavonoid biosynthetic pathway, a high concentration of genistein was observed but a low concentration of 2′-hydroxygenistein, with considerably reduced levels of dalbergioidin and kievitone; this may suggest that the 5-hydroxyisoflavonoid phytoalexin pathway is repressed or blocked in the tissues of the cultivars ICA Cerinza and Uribe Rosado.
- (iii)
- Phytotoxicity test. The compound 1-oxo-indanoyl-L-isoleucine methyl ester exhibited a low phytotoxic effect on different bean tissues. The elicitor should be applied during a later stage after germination because inhibition of root growth was evidenced. The insignificant or null effects on seedling size, stem and leaves, chlorophyll content, number of secondary roots, and root length, along with the strong phytoalexin-elicitor capacity of 1-oxo-indanoyl-L-isoleucine methyl ester, make it a potential elicitor for disease control that could be applied in the field.
4. Materials and Methods
4.1. General Methods
4.2. Chemicals
4.3. Plant Material
4.4. Synthesis and Identification of Indanoyl Derivatives
4.4.1. Indanoyl-Amino Acid Conjugates and Indanoyl Amides
4.4.2. Indanoyl Ester
4.4.3. Carbonyl Group Reduction
4.4.4. 2-Benzylidene
4.4.5. Imine
4.5. Induction Experiments
4.5.1. Inducer Effect of Indanoyl Derivatives
4.5.2. Time-Course Experiments
4.6. Extraction, Detection, and Quantification of Isoflavonoid Compounds
4.7. Phytotoxicity Tests
4.7.1. Assays on Common Bean Seeds and Cotyledons Hardness
4.7.2. Assays on Common Bean Seedlings and Chlorophyll Content
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Durango, D.; Quiñones, W.; Torres, F.; Rosero, Y.; Gil, J.; Echeverri, F. Phytoalexin accumulation in colombian bean varieties and aminosugars as elicitors. Molecules 2002, 7, 817–832. [Google Scholar] [CrossRef] [Green Version]
- Durango, D.; Pulgarin, N.; Echeverri, F.; Escobar, G.; Quiñones, W. Effect of salicylic acid and structurally related compounds in the accumulation of phytoalexins in cotyledons of common bean (Phaseolus vulgaris L.) cultivars. Molecules 2013, 18, 10609–10628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bektas, Y.; Eulgem, T. Synthetic plant defense elicitors. Front. Plant Sci. 2015, 5, 804. [Google Scholar] [CrossRef] [PubMed]
- Walker, T.; Pal-Bais, H.; Vivanco, J. Jasmonic acid-induced hypericin production in cell suspension cultures of Hypericum perforatum L. (St. John’s wort). Phytochemistry 2002, 60, 289–293. [Google Scholar] [CrossRef]
- Kim, H.; Chen, F.; Wang, X.; Rajapakse, N. Effect of methyl jasmonate on secondary metabolites of sweet basil (Ocimum basilicum L.). J. Agric. Food Chem. 2006, 54, 2327–2332. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Tao, Z.; Jin, Y.; Chen, S.; Zhou, Z.; Gong, A.; Yuan, Y.; Dong, T.; Tsim, K. Jasmonate-elicited stress induces metabolic change in the leaves of Leucaena leucocephala. Molecules 2018, 23, 188. [Google Scholar] [CrossRef] [Green Version]
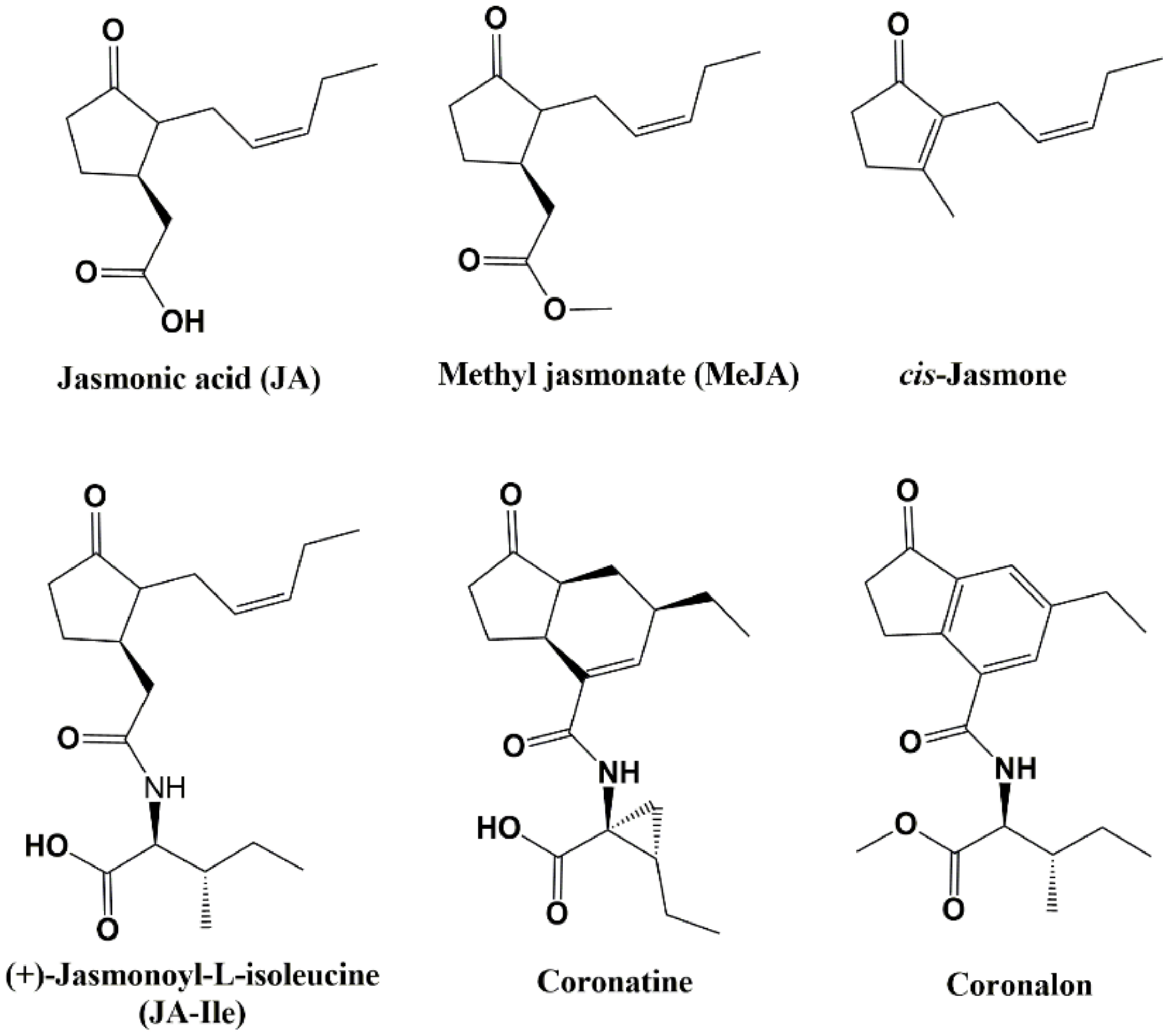
- Fliegmann, J.; Schüler, G.; Boland, W.; Ebel, J.; Mithofer, A. The role of octadecanoids and functional mimics in soybean defense responses. Biol. Chem. 2003, 384, 437–446. [Google Scholar] [CrossRef] [Green Version]
- Schüler, G.; Mithöfer, A.; Baldwin, I.; Berger, S.; Ebel, J.; Santos, J.G.; Herrmann, G.; Hölscher, D.; Kramell, R.; Kutchan, T.; et al. Coronalon: A powerful tool in plant stress physiology. FEBS Lett. 2004, 563, 17–22. [Google Scholar] [CrossRef] [Green Version]
- Krumm, T.; Katja, B.; Boland, W. Induction of volatile biosynthesis in the Lima bean (Phaseolus lunatus) by leucine-and isoleucine conjugates of 1-oxo- and 1-hydroxyindan-4-carboxylic acid: Evidence for amino acid conjugates of jasmonic acid as intermediates in the octadecanoid signalling pathway. FEBS Lett. 1995, 377, 523–529. [Google Scholar]
- Hu, Q.; Boland, W.; Liu, J.-K. 6-Substituted indanoyl isoleucine conjugate induces tobacco plant responses in secondary metabolites. Z. Nat. C 2005, 60, 1–4. [Google Scholar] [CrossRef]
- Cai, Z.; Knorr, D.; Smetanska, I. Enhanced anthocyanins and resveratrol production in Vitis vinifera cell suspension culture by indanoyl-isoleucine, N-linolenoyl-L-glutamine and insect saliva. Enzym. Microb. Technol. 2012, 50, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Tamogami, S.; Kodama, O. Coronatine elicits phytoalexin production in rice leaves (Oryza sativa L.) in the same manner as jasmonic acid. Phytochemistry 2000, 54, 689–694. [Google Scholar] [CrossRef]
- Botero, L.; Vizcaíno, S.; Quiñones, W.; Echeverri, F.; Gil, J.; Durango, D. Increased accumulation of isoflavonoids in common bean (Phaseolus vulgaris L.) tissues treated with 1-oxo-indane-4-carboxylic acid derivatives. Biotechnol. Rep. 2021, 29, e00601. [Google Scholar] [CrossRef]
- Bansal, P.; Rawal, V.; Bansal, V. Pulses of Phaseolus and Vigna genera. In The Global Economy of Pulses, 1st ed.; Rawal, V., Navarro, D., Eds.; FAO: Rome, Italy, 2019; pp. 39–67. [Google Scholar]
- Cortés, A.; Monserrate, F.; Ramírez-Villegas, J.; Madriñán, S.; Blair, M. Drought tolerance in wild plant populations: The case of common beans (Phaseolus vulgaris L.). PLoS ONE 2013, 8, e62898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayat, I.; Ahmad, A.; Masud, T.; Ahmed, A.; Bashir, S. Nutritional and health perspectives of beans (Phaseolus vulgaris L.): An overview. Crit. Rev. Food Sci. Nutr. 2014, 54, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Aktar, M.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perrin, D.R.; Cruickshank, A.M. The antifungal activity of pterocarpans towards Monilinia fructicola. Phytochemistry 1969, 8, 971–978. [Google Scholar] [CrossRef]
- Piasecka, A.; Jedrzejczak-Rey, N.; Bednarek, P. Secondary metabolites in plant innate immunity: Conserved function of divergent chemicals. New Phytol. 2015, 206, 948–964. [Google Scholar] [CrossRef]
- García, R.; Pérez, R. Fitoalexinas: Mecanismos de defensa de las plantas. Rev. Chapingo Ser. Cienc. Ambiente 2003, 9, 5–10. [Google Scholar]
- Lauchli, R.; Schüler, G.; Boland, W. Selective induction of secondary metabolism in Phaseolus lunatus by 6-substituted indanoyl isoleucine conjugates. Phytochemistry 2002, 61, 807–817. [Google Scholar] [CrossRef]
- Goossens, J.F.; Stabel, A.; Vendrig, J.C. Relationships between kievitone and phaseollin accumulation in different tissues of Phaseolus vulgaris in response to treatment with mercuric chloride, a fungal cell wall elicitor and abscisic acid. Physiol. Mol. Plant Pathol. 1987, 30, 1–12. [Google Scholar] [CrossRef]
- Skipp, R.; Selby, C.; Bailey, J. Toxic effects of phaseollin on plant cells. Physiol. Plant Pathol. 1977, 10, 221–227. [Google Scholar] [CrossRef]
- Pancheva, T.; Popova, L.; Uzunova, A. Effect of salicylic acid on growth and photosynthesis in barley plants. J. Plant Physiol. 1996, 149, 57–63. [Google Scholar] [CrossRef]
- Rajasekaran, L.; Stiles, A.; Caldwell, C. Stand establishment in processing carrots-effects of various temperature regimes on germination and the role of salicylates in promoting germination at low temperatures. Can. J. Plant Sci. 2002, 82, 443–450. [Google Scholar] [CrossRef]
- Peña-Valdivia, C.; García, N.; Aguirre, R.; Ybarra-Moncada, M.; López, H. Variation in physical and chemical characteristics of common bean (Phaseolus vulgaris L.) grain along a domestication gradient. Chem. Biodivers. 2011, 8, 2211–2225. [Google Scholar] [CrossRef]
- Rao, S.; Ayoubi, P.; Weng, H.; Palmer, D.; Mitchell, R.; Jones, W.; Bender, C. The phytotoxin coronatine and methyl jasmonate impact multiple phytohormone pathways in tomato. Plant J. 2005, 42, 201–217. [Google Scholar]
- Jităreanu, A.; Tataringa, G.; Zbancioc, A.; Stǎnescu, U. Toxicity of some cinnamic acid derivatives to common bean (Phaseolus vulgaris). Not. Bot. Horti. Agrobot. Cluj-Napoca 2011, 39, 130–134. [Google Scholar] [CrossRef] [Green Version]
- Zalewski, K.; Nitkiewicz, B.; Stolarski, M.; Amarowicz, R.; Okorski, A. The influence of exogenous methyl jasmonate on the germination and, content and composition of flavonoids in extracts from seedlings of yellow and narrow-leafed lupine. J. Food Compos. Anal. 2020, 87, 103398. [Google Scholar] [CrossRef]
- Tetley, R.; Thimann, K. The metabolism of oat leaves during senescence: I. respiration, carbohydrate metabolism and the action of cytokinins. Plant Physiol. 1974, 54, 294–303. [Google Scholar] [CrossRef] [Green Version]
- Xie, Z.; Duan, L.; Li, Z.; Wang, X.; Liu, X. Dose-dependent effects of coronatine on cotton seedling growth under salt stress. J. Plant Growth Regul. 2015, 34, 651–664. [Google Scholar] [CrossRef]
- Diaz, C.; Santana, G. Revisión de las Fichas Técnicas de las Variedades Comerciales de Frijol (Phaseolus vulgaris) en Colombia, 1st ed.; CORPOICA: Medellín, Colombia, 2004; pp. 1–184. [Google Scholar]
- Arias, J.H.; Jaramillo, M.; Rengifo, T. Manual: Buenas Prácticas Agrícolas (BPA) en la Producción de Fríjol Voluble; Corpoica y FAO: La Selva, Colombia, 2007. [Google Scholar]
- Nakamura, Y.; Paetz, C.; Brandt, W.; David, A.; Rendón-Anaya, M.; Herrera-Estrella, A.; Mithöfer, A.; Boland, W. Synthesis of 6-substituted 1-oxoindanoyl isoleucine conjugates and modeling studies with the COI1-JAZ co-receptor complex of Lima bean. J. Chem. Ecol. 2014, 40, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Chu, J. Effect of Cultivar and Growth Region on the Mechanical and Biochemical Properties of Canned Baked Beans. Ph.D. Thesis, University of Leeds, Leeds, UK, 2014. [Google Scholar]
- Yasar, F.; Ellialtioglu, S.; Yildiz, K. Effect of salt stress on antioxidant defense systems, lipid peroxidation, and chlorophyll content in green bean. Russ. J. Plant Physiol. 2008, 55, 782–786. [Google Scholar] [CrossRef]
| % Seed Germination | Force (N) | Roots | |||||
|---|---|---|---|---|---|---|---|
| Treatment | Day 3 | Day 4 | Day 5 | Puncture | Compression | Radicle Length (cm) | Number of Secondary Roots |
| Negative Control | 21.0 ± 11.9 a | 95.5 ± 6.2 a | 97.5 ± 5.6 a | 10.6 ± 1.0 b | 167.2 ± 21.9 b | 6.7 ± 1.2 b | 11.3 ± 2.7 b |
| MeJA | 56.9 ± 21.1 b | 81.1 ± 15.9 a | 92.8 ± 11.0 a | 10.9 ± 1.3 b | 152.2 ± 36.8 b | 6.3 ± 0.8 b | 10.1 ± 1.8 b |
| (4) | 71.7 ± 21.6 b | 90.0 ± 16.3 a | 100.0 ± 0.0 a | 8.8 ± 1.2 a | 114.5 ± 19.1 a | 4.6 ± 0.5 a | 1.9 ± 1.8 a |
| Day 9 | Day 13 | |||||
|---|---|---|---|---|---|---|
| Treatment | Plant Size (cm) | Hypocotyl Size (cm) | Leaf Size (cm) | Plant Size (cm) | Hypocotyl Size (cm) | Leaf Size (cm) |
| Negative Control | 38.0 ± 1.0 a | 19.3 ± 1.2 a | 5.7 ± 0.8 a | 38.0 ± 1.0 a | 21.8 ± 0.8 a | 6.7 ± 0.3 a |
| MeJA | 36.0 ± 2.0 a | 18.2 ± 1.0 a | 6.0 ± 0.5 a | 38.2 ± 0.8 a | 19.0 ± 1.0 a | 6.3 ± 0.3 a |
| (4) | 35.7 ± 1.5 a | 17.3 ± 0.6 a | 5.3 ± 0.3 a | 38.2 ± 1.0 a | 19.2 ± 1.3 a | 6.2 ± 0.3 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aristizábal, D.; Gil, J.; Quiñones, W.; Durango, D. Screening of Indanoyl-Type Compounds as Elicitors of Isoflavonoid Phytoalexins in Colombian Common Bean Cultivars. Molecules 2022, 27, 3500. https://doi.org/10.3390/molecules27113500
Aristizábal D, Gil J, Quiñones W, Durango D. Screening of Indanoyl-Type Compounds as Elicitors of Isoflavonoid Phytoalexins in Colombian Common Bean Cultivars. Molecules. 2022; 27(11):3500. https://doi.org/10.3390/molecules27113500
Chicago/Turabian StyleAristizábal, Diego, Jesús Gil, Winston Quiñones, and Diego Durango. 2022. "Screening of Indanoyl-Type Compounds as Elicitors of Isoflavonoid Phytoalexins in Colombian Common Bean Cultivars" Molecules 27, no. 11: 3500. https://doi.org/10.3390/molecules27113500
APA StyleAristizábal, D., Gil, J., Quiñones, W., & Durango, D. (2022). Screening of Indanoyl-Type Compounds as Elicitors of Isoflavonoid Phytoalexins in Colombian Common Bean Cultivars. Molecules, 27(11), 3500. https://doi.org/10.3390/molecules27113500






