Synthesis, Characterization and Antimicrobial Activity of Zinc Oxide Nanoparticles against Selected Waterborne Bacterial and Yeast Pathogens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Urea-Based Synthesis of Zinc Oxide Nanoparticles
2.2. Characterization of the Synthesized Zinc Oxide Nanoparticles
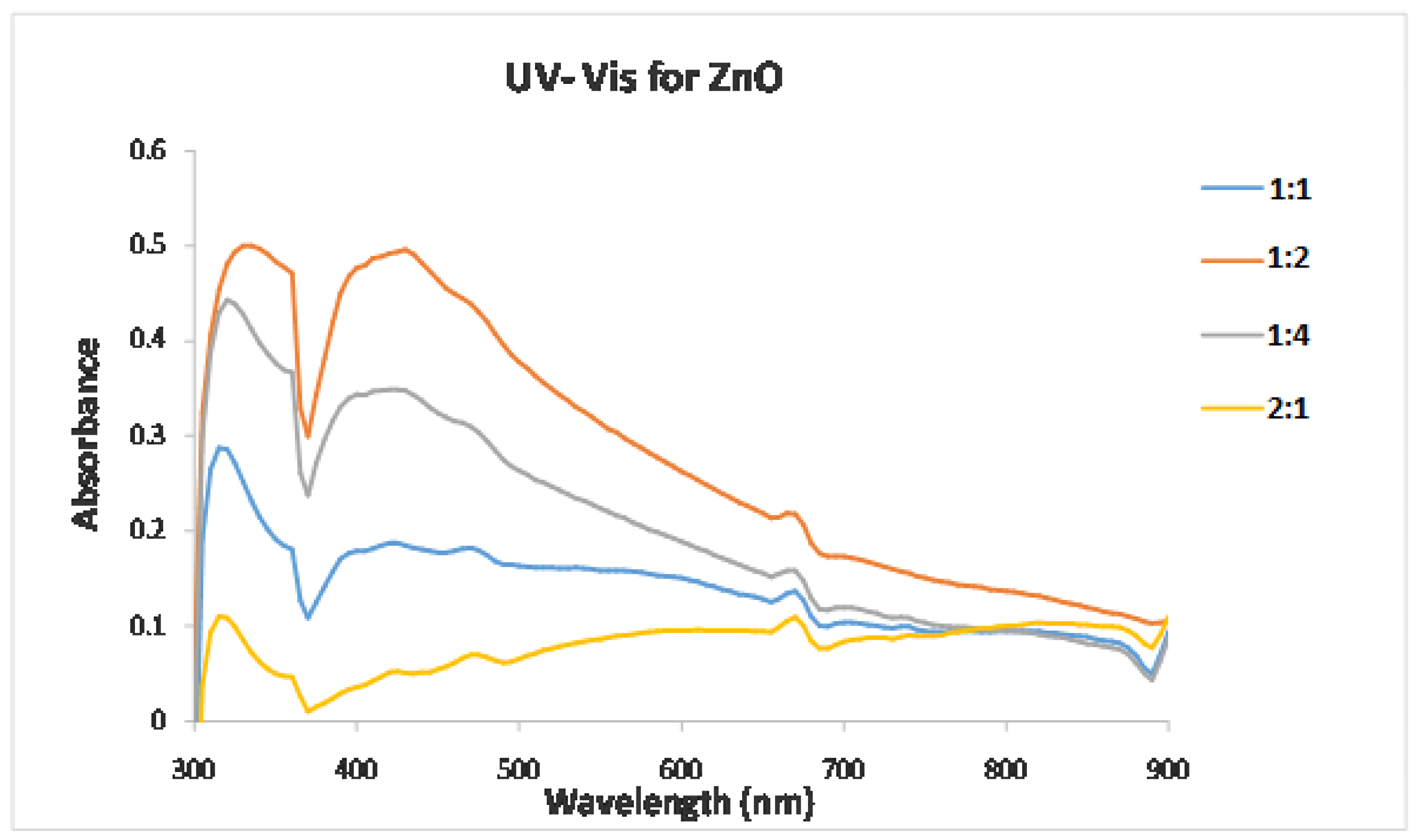
2.2.1. UV–Visible Spectroscopy
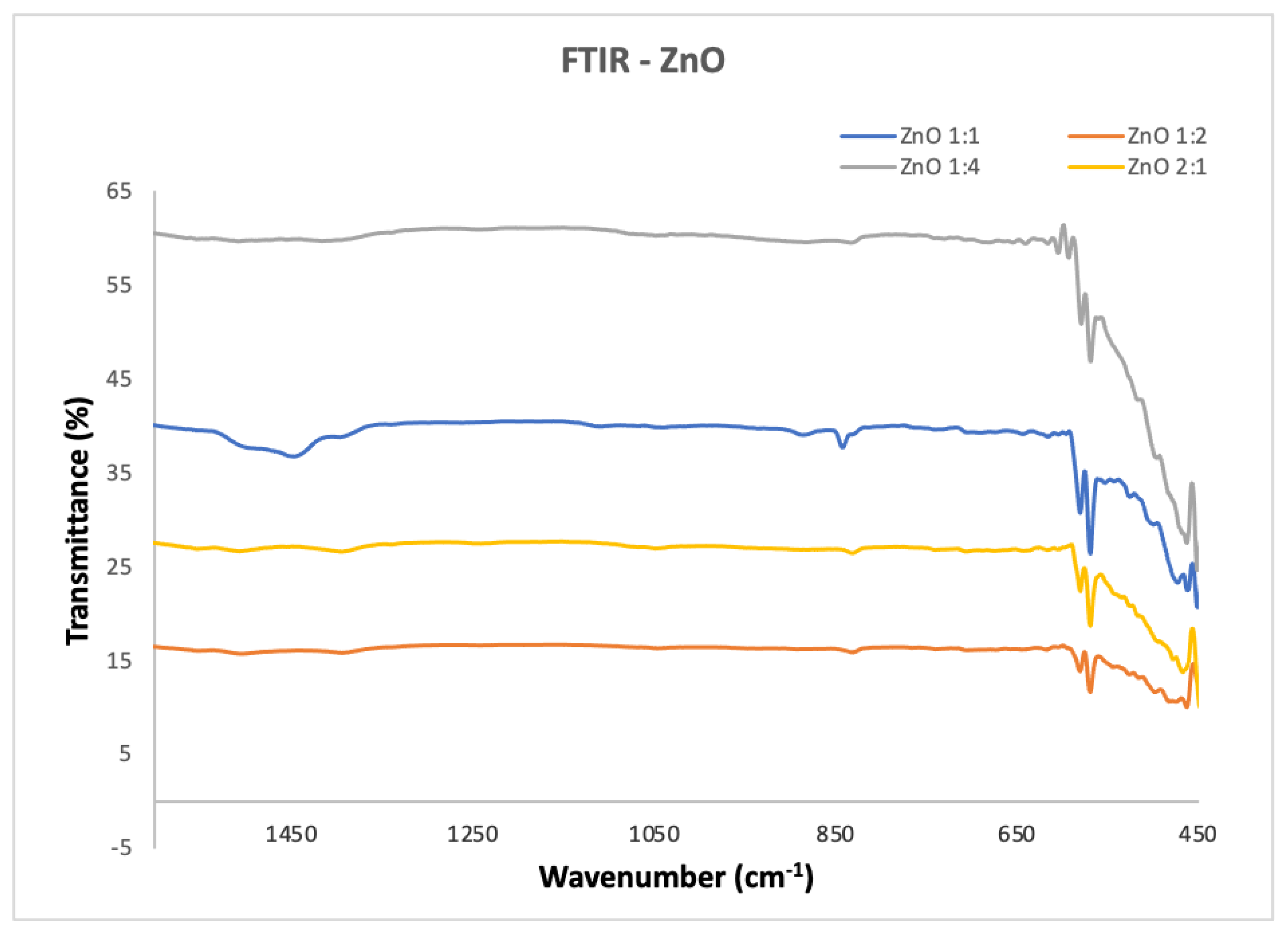
2.2.2. Fourier Transform Infrared Spectroscopy
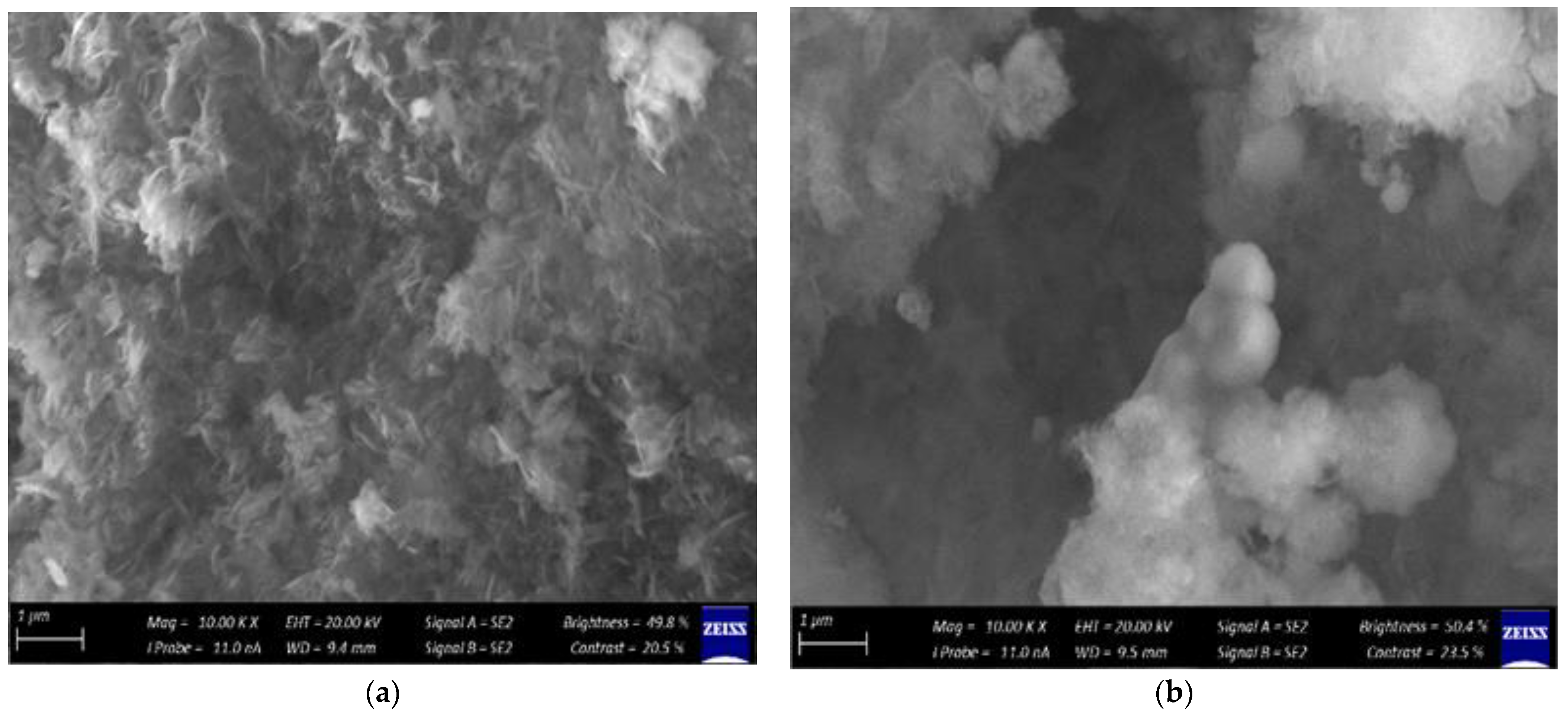
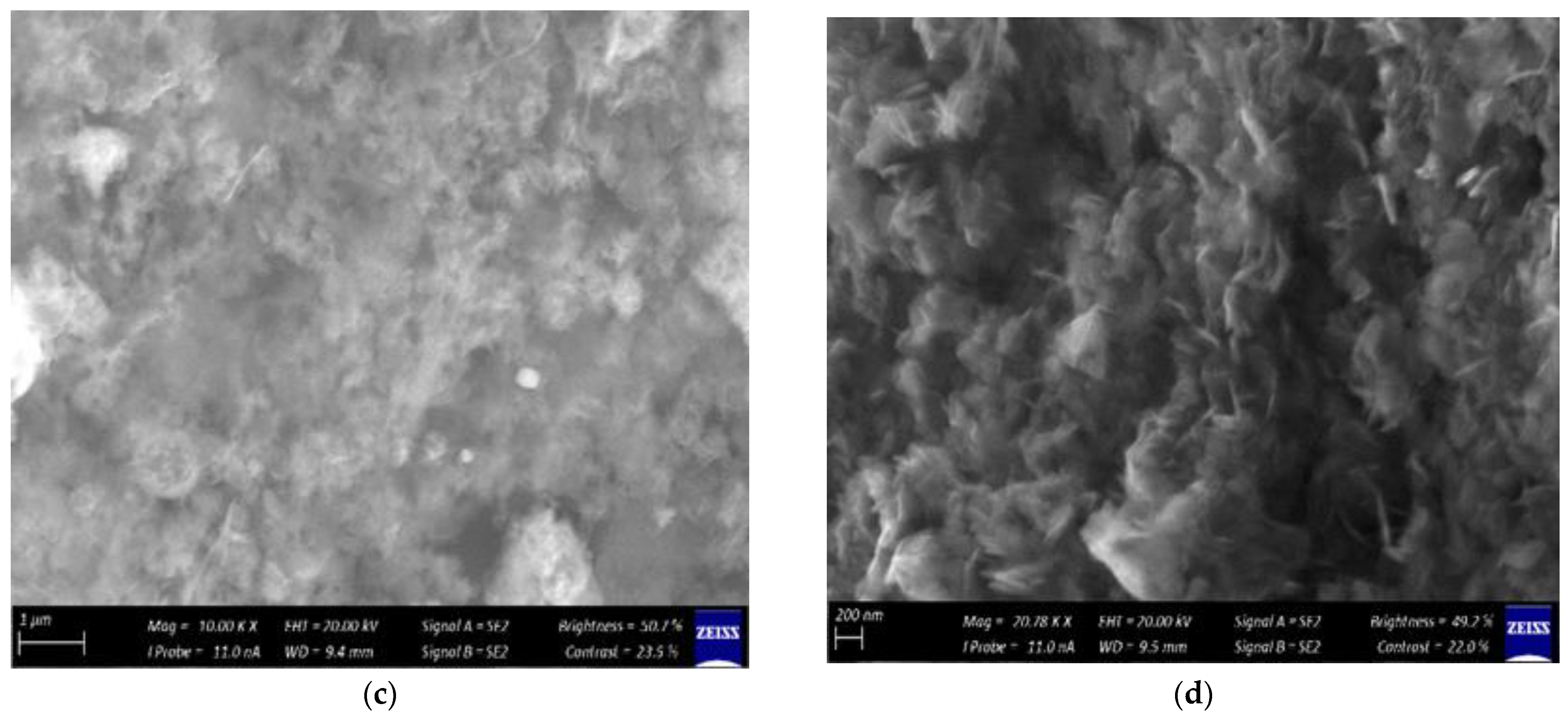
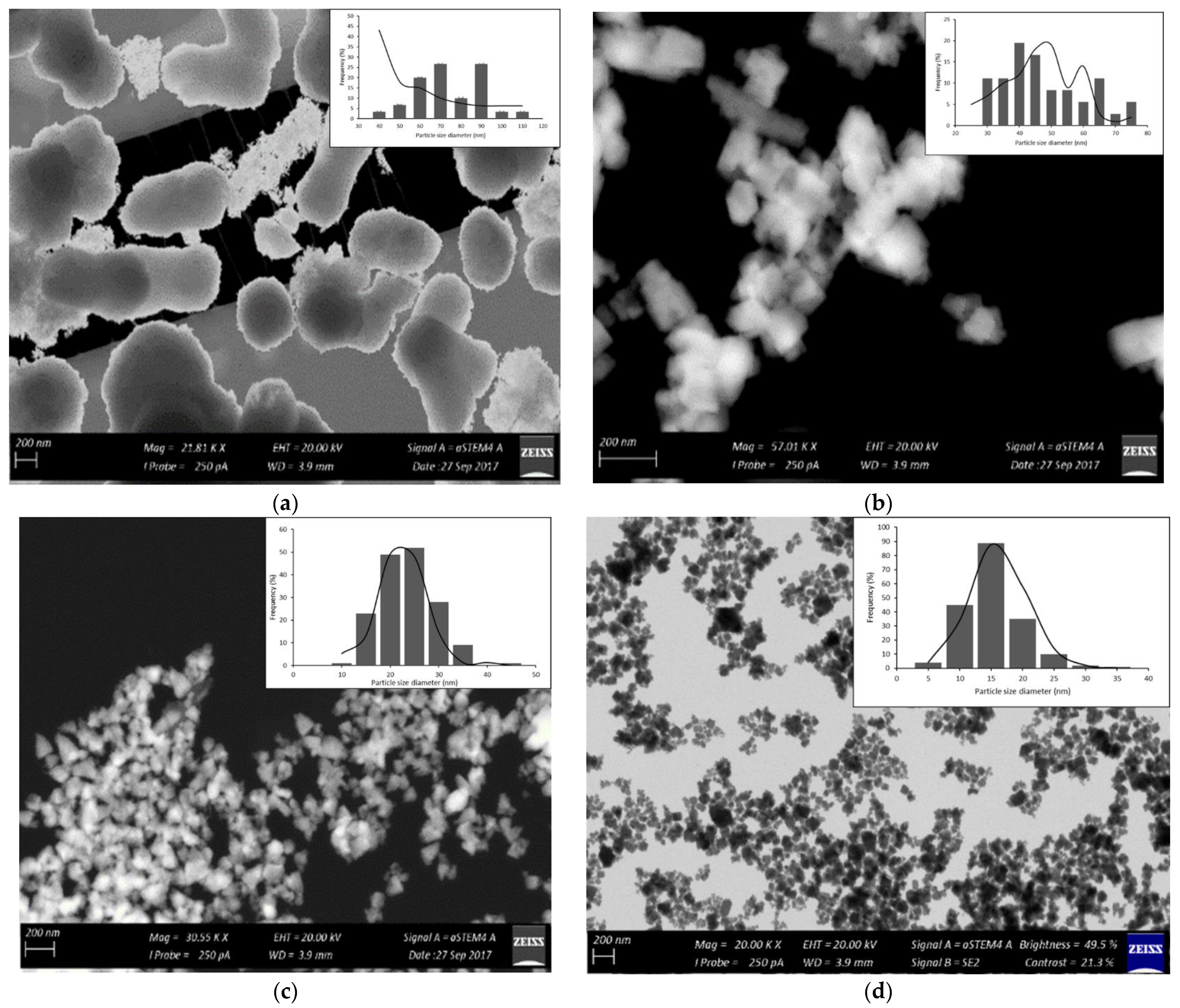
2.2.3. Scanning Electron Microscopy
2.2.4. X-ray Diffraction
2.2.5. Transmission Electron Microscopy
2.3. Antimicrobial Activity of ZnO Nanoparticles
2.3.1. Agar Well Diffusion Technique
2.3.2. Minimum Inhibitory Concentration (MIC) Assay
3. Results and Discussion
3.1. Synthesis of ZnO NPs
3.2. Characterization of the Synthesized ZnO Nanoparticles
3.3. Evaluation of Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The United Nations World Water Development Report. World Water Assessment Programme (Nations Unies). United Nations Educational, Scientific and Cultural Organization, New York, United States. 2018. Available online: www.unwater.org/publications/world-water-development-report-2018/ (accessed on 5 March 2021).
- Szálkai, K. Water-Borne Diseases. In The Palgrave Encyclopedia of Global Security Studies; Romaniuk, S., Thapa, M., Marton, P., Eds.; Palgrave Macmillan: Cham, Switzerland, 2019. [Google Scholar]
- Edokpayi, J.N.; Odiyo, J.O.; Durowoju, O.S. Impact of Wastewater on Surface Water Quality in Developing Countries: A Case Study of South Africa. In Water Quality; INTECH: Vienna, Austria, 2017; pp. 401–416. [Google Scholar]
- United Nations Development Program (UNDP). Sustainable Development Goals (SDGs). 2015. Available online: https://www.undp.org/content/undp/en/home/sustainable-development-goals.html (accessed on 8 March 2021).
- Okello, C.; Tomasello, B.; Greggio, N.; Wambiji, N.; Antonellini, M. Impact of Population Growth and Climate Change on the Freshwater Resources of Lamu Island, Kenya. Water 2015, 7, 1264–1290. [Google Scholar] [CrossRef]
- Gwimbi, P.; George, M.; Ramphalile, M. Bacterial contamination of drinking water sources in rural villages of Mohale Basin, Lesotho: Exposures through neighbourhood sanitation and hygiene practices. Environ. Heal. Prev. Med. 2019, 24, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayukekbong, J.A.; Ntemgwa, M.; Atabe, A.N. The threat of antimicrobial resistance in developing countries: Causes and control strategies. Antimicrob. Resist. Infect. Control 2017, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- Albukhaty, S.; Al-Karagoly Dragh, M.A. Synthesis of Zinc Oxide Nanoparticles and Evaluated Its Activity Against Bacterial Isolates. J. Biotech Res. 2020, 11, 47–53. [Google Scholar]
- Parsonage, B.; Hagglund, P.K.; Keogh, L.; Wheelhouse, N.; Brown, R.E.; Dancer, S.J. Control of antimicrobial resistance requires an ethical approach. Front. Microbiol. 2017, 8, 2124. [Google Scholar] [CrossRef] [Green Version]
- Prathna, T.C.; Sharma, S.K.; Kennedy, M. Nanoparticles in household level water treatment: An overview. Sep. Purif. Technol. 2018, 199, 260–270. [Google Scholar] [CrossRef]
- Mo, D.; Hu, L.; Zeng, G.; Chen, G.; Wan, J.; Yu, A.; Huang, Z.; He, K.; Zhang, C.; Cheng, M. Cadmium-containing quantum dots: Properties, applications, and toxicity. Appl. Microbiol. Biotechnol. 2017, 101, 2713–2733. [Google Scholar] [CrossRef]
- Vassallo, A.; Silletti, M.F.; Faraone, I.; Milella, L. Nanoparticulate Antibiotic Systems as Antibacterial Agents and Antibiotic Delivery Platforms to Fight Infections. J. Nanomater. 2020, 2020, 6905631. [Google Scholar] [CrossRef]
- Nair, S.; Sasidharan, A.; Rani, V.V.D.; Menon, D.; Nair, S.; Manzoor, K.; Raina, S. Role of size scale of ZnO nanoparticles and microparticles on toxicity toward bacteria and osteoblast cancer cells. J. Mater. Sci. Mater. Med. 2009, 20, 235–241. [Google Scholar] [CrossRef]
- Lee, N.-Y.; Ko, W.-C.; Hsueh, P.-R. Nanoparticles in the Treatment of Infections Caused by Multidrug-Resistant Organisms. Front. Pharmacol. 2019, 10, 1153. [Google Scholar] [CrossRef] [Green Version]
- Sabir, S.; Arshad, M.; Chaudhari, S.K. Zinc Oxide Nanoparticles for Revolutionizing Agriculture: Synthesis and Applications. Sci. World J. 2014, 2014, 925494. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; Pi, J.; Cai, J. The Advancing of Zinc Oxide Nanoparticles for Biomedical Applications. Bioinorg. Chem. Appl. 2018, 2018, 1062562. [Google Scholar] [CrossRef] [PubMed]
- Colon, G.; Ward, B.C.; Webster, T.J. Increased osteoblast and decreasedStaphylococcus epidermidis functions on nanophase ZnO and TiO2. J. Biomed. Mater. Res. Part A 2006, 78A, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Padmavathy, N.; Vijayaraghavan, R. Enhanced bioactivity of ZnO nanoparticles—An antimicrobial study. Sci. Technol. Adv. Mater. 2008, 9, 035004. [Google Scholar] [CrossRef] [PubMed]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, H.; Kumar, S.V.; RajeshKumar, S. A review on green synthesis of zinc oxide nanoparticles—An eco-friendly approach. Resour. Technol. 2017, 3, 406–413. [Google Scholar] [CrossRef]
- Kołodziejczak-Radzimska, A.; Jesionowski, T. Zinc Oxide—From Synthesis to Application: A Review. Materials 2014, 7, 2833–2881. [Google Scholar] [CrossRef] [Green Version]
- Dimapilis, E.A.S.; Hsu, C.-S.; Mendoza, R.M.O.; Lu, M.-C. Zinc oxide nanoparticles for water disinfection. Sustain. Environ. Res. 2018, 28, 47–56. [Google Scholar] [CrossRef]
- Ojha, A. Nanomaterials for removal of waterborne pathogens: Opportunities and challenges. In Waterborne Pathogens: Detection and Treatment; Narasimha, M., Prasad, V., Grobelak, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 385–432. [Google Scholar]
- Moukangoe, G.P.; Laloo, N.; Pakade, V.; Mtunzi, F.; Monapathi, M.E.; Modise, J.S.; Klink, M.J. Synthesis and antibacterial activity of Cobalt-thiourea nanoparticles on selected waterborne bacterial pathogens. Dig. J. Nanomater. Biostructures 2020, 15, 757–766. [Google Scholar]
- Zhang, L.L.; Chen, B.; Xie, L.L.; Li, Z.F. Study on the Antimicrobial Properties of ZnO Suspension against Gram-Positive and Gram-Negative Bacteria Strains. Adv. Mat. Res. 2011, 393–395, 1488–1491. [Google Scholar]
- Marinho, J.; Romeiro, F.D.C.; Lemos, S.; Motta, F.V.; Riccardi, C.D.S.; Li, M.S.; Longo, E.; Lima, R.C. Urea-Based Synthesis of Zinc Oxide Nanostructures at Low Temperature. J. Nanomater. 2012, 2012, 427172. [Google Scholar] [CrossRef]
- El-Naggar, M.E.; Shaheen, T.I.; Abdelgawad, A.M.; Hebeish, A. Durable antibacterial and UV protections of in situ synthesized Zincoxide nanoparticles onto cotton fabrics. Int. J. Biol. Macromol. 2016, 83, 426. [Google Scholar]
- Kumar, H.; Poonam Sangwan, M. Synthesis and Characterization of cobalt oxide nanoparticles by sol-gel method. In Applied Physical and Chemical Sciences—A Sustainable Approach; 2011; pp. 99–104. Available online: https://krishisanskriti.org/vol_image/07Sep201509093715.pdf (accessed on 25 May 2022)ISBN 978-93-83083-72-5.
- Radu, G.L.; Truica, G.I.; Penu, R.; Moroeanu, V.; Litescu, S.C. Use of the Fourier Transform Infrared Spectroscopy in Characterization of Specific Samples. U.P.B. Sci. Bull. 2012, 74, 139. [Google Scholar]
- Joshi, M.; Bhattacharyya, A.; Ali, S.W. Characterization Techniques for Nanotechnology Applications in Textiles. Indian J. Fibre Text. Res. 2008, 3, 304–317. [Google Scholar]
- Navale, G.R.; Thripuranthaka, M.; Late, D.J.; Shinde, S.S. Antimicrobial Activity of ZnO Nanoparticles against Pathogenic Bacteria and Fungi. JSM Nanotechnol. Nanomed. 2015, 3, 1033–1035. [Google Scholar]
- Alias, S.; Ismail, A.; Mohamad, A.A. Effect of pH on ZnO nanoparticle properties synthesized by sol–gel centrifugation. J. Alloy. Compd. 2010, 499, 231–237. [Google Scholar] [CrossRef]
- Guzman, M.G.; Dille, J.; Godet, S. Synthesis of silver nanoparticles by chemical reduction method and their antibacterial activity. Int. J. Chem. Biomol. Eng. 2009, 2, 108. [Google Scholar]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Kakiuchi, K.; Hosono, E.; Kimura, T.; Imai, H.; Fujihara, S. Fabrication of mesoporous ZnO nanosheets from precursor templates grown in aqueous solutions. J. Sol-Gel Sci. Technol. 2006, 39, 63–72. [Google Scholar] [CrossRef]
- Hong, S.H.; Kim, S.; Choi, S.; Lee, K.J.; Lee, H. Optical Characterization of Si Nanocrystals in Si-rich SiOX and SiOX/SiO2 Multilayers Grown by Ion Beam Sputtering. J. Korean Phys. Soc. 2004, 45, 116–119. [Google Scholar]
- Chandra, S.; Kumar, A.; Tomar, P.K. Synthesis and characterization of copper nanoparticles by reducing agent. J. Saudi Chem. Soc. 2012, 18, 149–153. [Google Scholar] [CrossRef] [Green Version]
- Jamali-Sheini, F. Chemical solution deposition of ZnO nanostructure films: Morphology and substrate angle dependency. Ceram. Int. 2012, 38, 3649–3657. [Google Scholar] [CrossRef]
- Giri, P.K.; Bhattacharyya, S.; Singh, D.K.; Kesavamoorthy, R.; Panigrahi, B.K.; Nair, K.G.M. Correlation between microstructure and optical properties of ZnO nanoparticles synthesized by ball milling. J. Appl. Phys. 2007, 102, 093515. [Google Scholar] [CrossRef] [Green Version]
- Xiong, H.-M.; Shchukin, D.G.; Möhwald, H.; Xu, Y.; Xia, Y.-Y. Sonochemical Synthesis of Highly Luminescent Zinc Oxide Nanoparticles Doped with Magnesium(II). Angew. Chem. Int. Ed. 2009, 48, 2727–2731. [Google Scholar] [CrossRef] [PubMed]
- Ghamsari, M.S.; Alamdari, S.; Razzaghi, D.; Pirlar, M.A. ZnO nanocrystals with narrow-band blue emission. J. Lumin. 2019, 205, 508–518. [Google Scholar] [CrossRef]
- Narayanan, P.M.; Wilson, W.S.; Abraham, A.T.; Sevanan, M. Synthesis, Characterization, and Antimicrobial Activity of Zinc Oxide Nanoparticles Against Human Pathogens. BioNanoScience 2012, 2, 329–335. [Google Scholar] [CrossRef]
- Emami-Karvani, Z.; Chehrazi, P. Antibacterial activity of ZnO nanoparticle on Gram-positive and Gram-negative bacteria. Afr. J. Microbiol. Res. 2012, 5, 1368–1373. [Google Scholar] [CrossRef]
- Sharma, R.K.; Ghose, R. Synthesis of zinc oxide nanoparticles by homogeneous precipitation method and its application antifungal activity against Candida Albicans. Ceram. Int. 2015, 41, 967–975. [Google Scholar] [CrossRef]
- Taufiq, A.; Ulya, H.N.; Utomo, J.; Sunaryono Hidayat, N.; Susanto, H.; Mufti, N.; Munasir Soontaranon, S. IOP Conference Series. J. Phys. 2018, 1093, 012001. [Google Scholar]
- Singh, B.R. Evaluation of Antibacterial Activity of Salvia Officinalis L. Sage Oil on Veterinary Clinical Isolates of Bacteria. Medicine 2013, 11–27. Available online: https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.403.8444&rep=rep1&type=pdf (accessed on 5 March 2021).
- EUCAST Definitive Document. Methods for the determination of susceptibility of bacteria to antimicrobial agents. Terminol. Clin. Microbiol. Infect. 1998, 4, 291–296. [Google Scholar] [CrossRef] [Green Version]
- Rampersad, S.N. Multiple Applications of Alamar Blue as an Indicator of Metabolic Function and Cellular Health in Cell Viability Bioassays. Sensors 2012, 12, 12347–12360. [Google Scholar] [CrossRef] [PubMed]
- Elshikh, M.; Ahmed, S.; Funston, S.; Dunlop, P.; McGaw, M.; Marchant, R.; Banat, I.M. Resazurin-based 96-well plate method for the determination of minimum inhibitory concentration of biosurfactants. Biotechnol. Lett. 2016, 38, 1015–1019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padiyara, P.; Inoue, H.; Sprenger, M. Global Governance Mechanisms to Address Antimicrobial Resistance. Infect. Dis. Res. Treat. 2018, 11, 1–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patil, M.; Poyil, A.N.; Joshi, S.D.; Patil, S.A.; Patil, S.A.; Bugarin, A. Synthesis, molecular docking studies, and evaluation of new structurally diverse ureas. Bioorganic Chem. 2019, 87, 302–311. [Google Scholar] [CrossRef]
- Toche, R.B.; Janrao, R.A. Synthesis, characterization and antimicrobial evaluation of novel urea, sulfonamide and acetamide 3,4-dihydropyrazino[1,2-a]indol-1(2H)-one derivatives. Arab. J. Chem. 2019, 12, 3406–3416. [Google Scholar] [CrossRef] [Green Version]
- Patil, P.P.; Meshram, J.V.; Bohara, R.A.; Nanaware, S.G.; Pawar, S.H. ZnO nanoparticle-embedded silk fibroin–polyvinyl alcohol composite film: A potential dressing material for infected wounds. New J. Chem. 2018, 42, 14620–14629. [Google Scholar] [CrossRef]


| Microorganisms | Agar Well Diffusion Method | ||||
|---|---|---|---|---|---|
| ZnO NPs (mm) | Antibiotic (Neomycin/Amphotericin B) (mm) | ||||
| 1:1 | 1:2 | 1:4 | 2:1 | ||
| Staphylococcus aureus | 4 | 3 | 5 | 4 | 10 |
| Escherichia coli | 0 | 0 | 0 | 0 | 10 |
| Salmonella enterica | 0 | 0 | 0 | 0 | 10 |
| Shigella sonnei | 0 | 0 | 0 | 0 | 13 |
| Candida albicans | 0 | 0 | 0 | 0 | (Positive control measurement) |
| Microorganisms | Minimum Inhibitory Concentrations (MICs) | ||||
|---|---|---|---|---|---|
| ZnO NPs | Antibiotic (Neomycin/Amphotericin B) | ||||
| 1:1 | 1:2 | 1:4 | 2:1 | ||
| Staphylococcus aureus | 6.25 | 6.25 | 12.5 | 12.5 | 3.125 |
| Escherichia coli | 25 | 25 | 25 | 25 | 1.5623 |
| Salmonella enterica | 12.5 | 12.5 | 12.5 | 25 | 1.5623 |
| Shigella sonnei | 6.25 | 12.5 | 12.5 | 12.5 | 1.5623 |
| Candida albicans | 6.25 | 12.5 | 12.5 | 12.5 | 3.125 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klink, M.J.; Laloo, N.; Leudjo Taka, A.; Pakade, V.E.; Monapathi, M.E.; Modise, J.S. Synthesis, Characterization and Antimicrobial Activity of Zinc Oxide Nanoparticles against Selected Waterborne Bacterial and Yeast Pathogens. Molecules 2022, 27, 3532. https://doi.org/10.3390/molecules27113532
Klink MJ, Laloo N, Leudjo Taka A, Pakade VE, Monapathi ME, Modise JS. Synthesis, Characterization and Antimicrobial Activity of Zinc Oxide Nanoparticles against Selected Waterborne Bacterial and Yeast Pathogens. Molecules. 2022; 27(11):3532. https://doi.org/10.3390/molecules27113532
Chicago/Turabian StyleKlink, Michael John, Neelan Laloo, Anny Leudjo Taka, Vusumzi Emmanuel Pakade, Mzimkhulu Ephraim Monapathi, and Johannes Sekomeng Modise. 2022. "Synthesis, Characterization and Antimicrobial Activity of Zinc Oxide Nanoparticles against Selected Waterborne Bacterial and Yeast Pathogens" Molecules 27, no. 11: 3532. https://doi.org/10.3390/molecules27113532






