Jatrolignans C and D: New Neolignan Epimers from Jatropha curcas
Abstract
1. Introduction
2. Results and Discussion
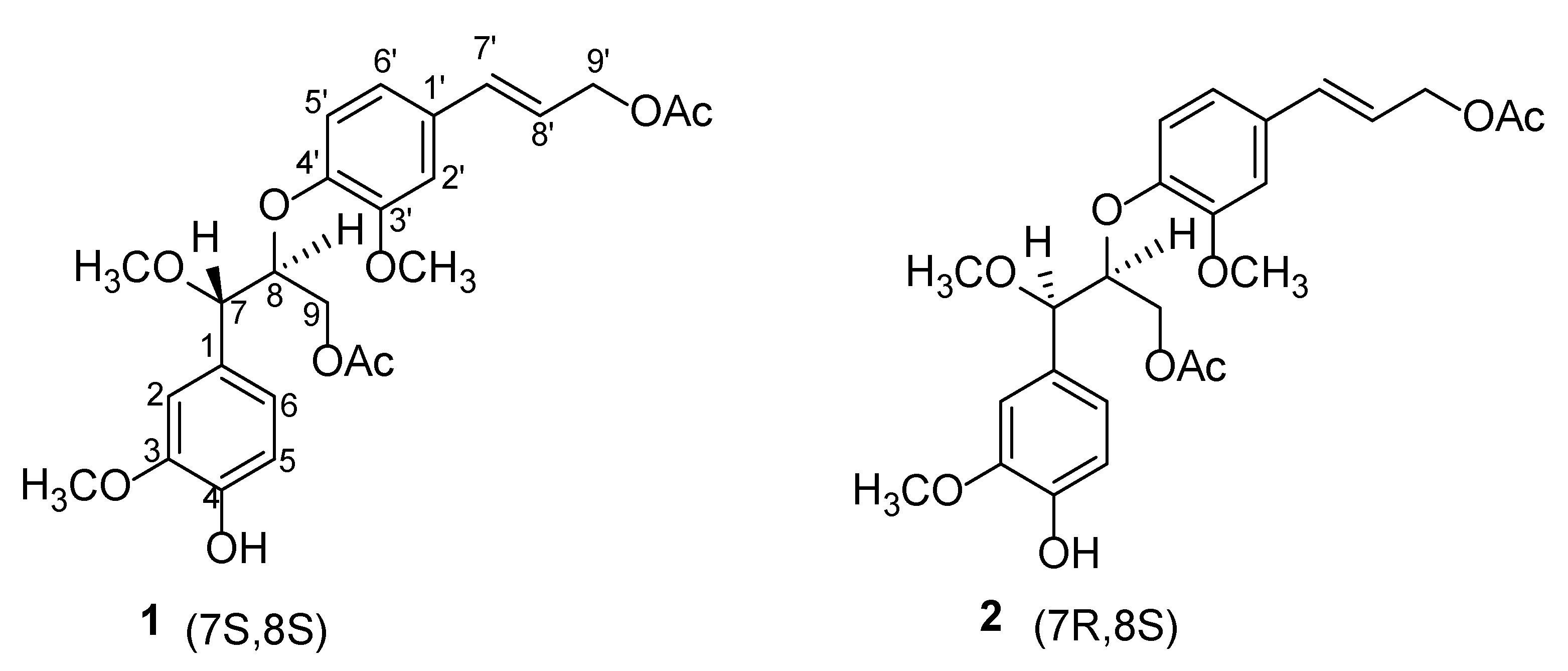
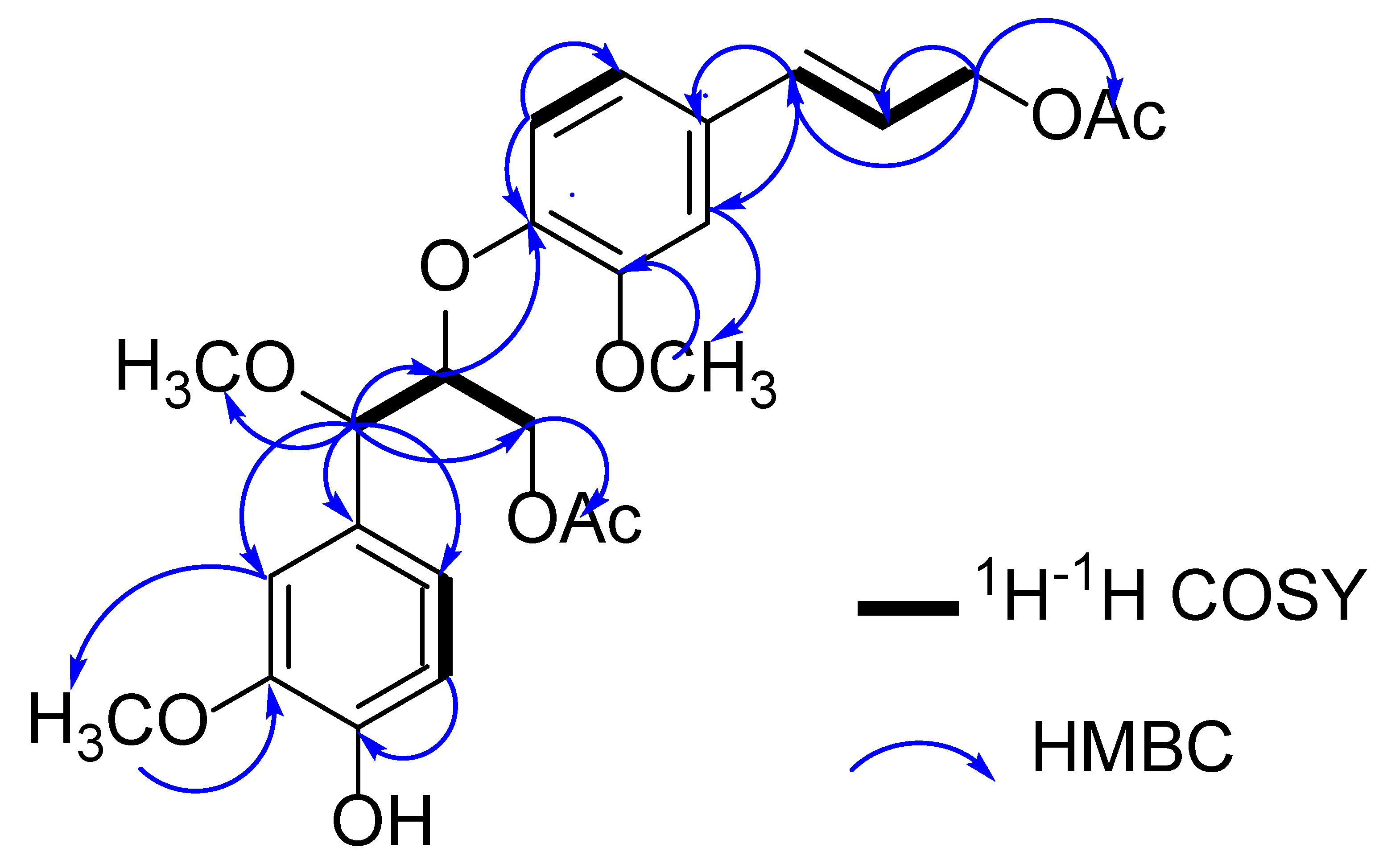
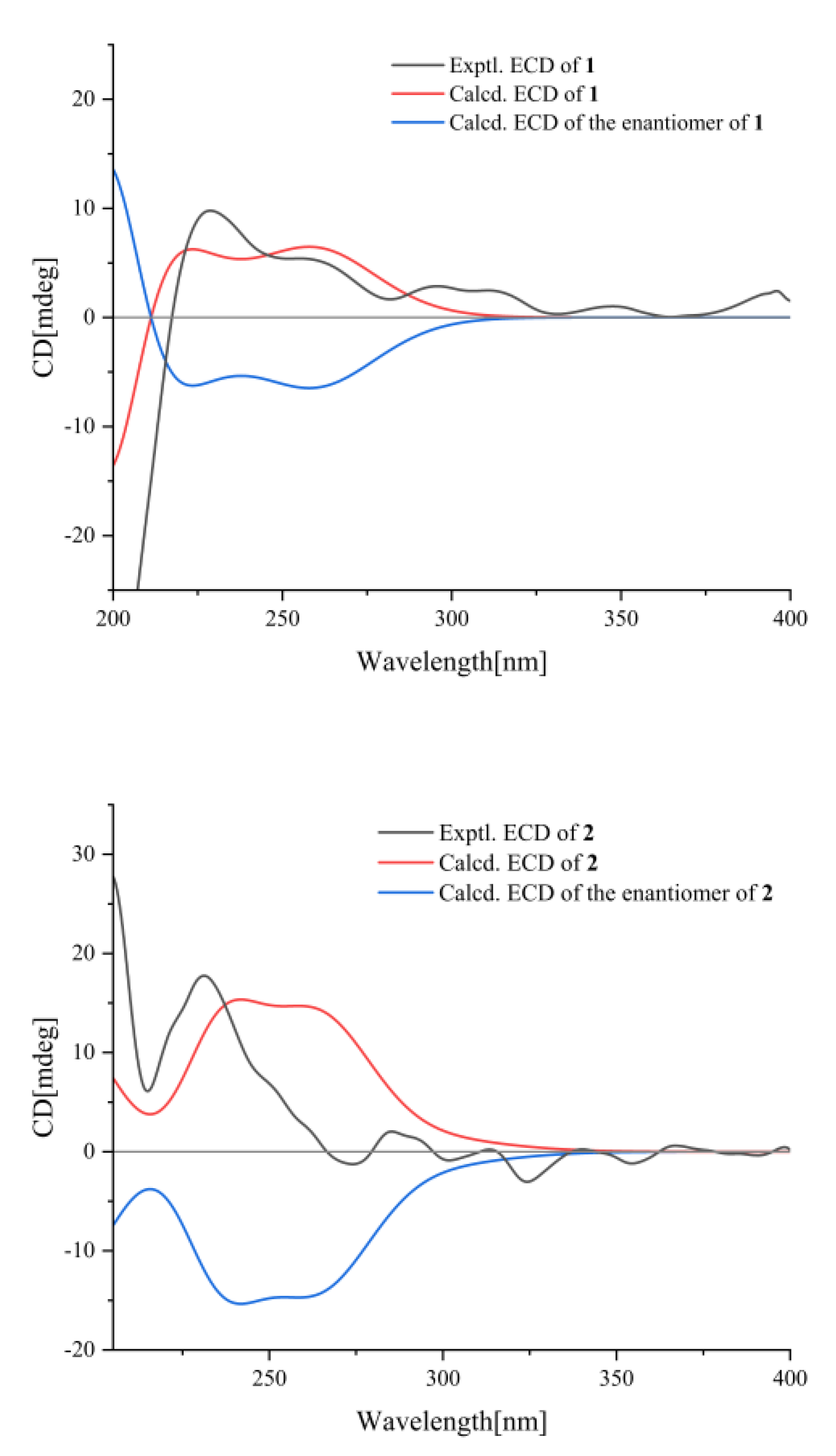
2.1. Structure Elucidation
2.2. The Antichlamydial Activity of Compounds
3. Experimental Section
3.1. General Experimental Procedures
3.2. Plant Materials
3.3. Extraction and Isolation
3.3.1. Jatrolignan C (1)
3.3.2. Jatrolignan D (2)
3.4. ECD Calculation
3.5. Chlamydia Strains and Cell Line
3.6. Antichlamydial Activity Screening
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Lin, J.; Zhou, X.W.; Tang, K.X.; Chen, F. A survey of the studies on the resources of Jatropha curcas. J. Trop. Subtrop. Bot. 2004, 12, 285–290. [Google Scholar]
- Hirota, M.; Suttajit, M.; Suguri, H.; Endo, Y.; Shudo, K.; Wongchai, V.; Hecker, E.; Fujiki, H. A new tumor promoter from the seed oil of Jatropha curcas L., an intramolecular diester of 12-deoxy-16-hydroxyphorbol. Cancer Res. 1988, 48, 5800–5804. [Google Scholar] [PubMed]
- Mitra, C.; Bhatnagar, S.; Sinha, M.K. Chemical examination of Jatropha curcas. Indian J. Chem. 1970, 8, 1047–1048. [Google Scholar]
- Suzuki, T.; Eto, K.; Kubota, Y.; Katayama, T.; Pankasemsuk, T. Antioxidative catechol lignans/neolignans isolated from defatted kernel of Jatropha curcas. J. Wood Sci. 2016, 62, 339–348. [Google Scholar] [CrossRef]
- Chen, M.J.; Hou, L.L.; Zhang, G.W. The diterpenoids from Jatropha curcas L. Acta Bot. Sin. 1988, 30, 308–311. [Google Scholar]
- Zhang, X.Q.; Li, F.; Zhao, Z.G.; Liu, X.L.; Tang, Y.X.; Wang, M.K. Diterpenoids from the root bark of Jatropha curcas and their cytotoxic activities. Phytochem. Lett. 2012, 5, 721–724. [Google Scholar] [CrossRef]
- Liu, J.Q.; Yang, Y.F.; Li, X.Y.; Liu, E.Q.; Li, Z.R.; Zhou, L.; Li, Y.; Qiu, M.H. Cytotoxicity of naturally occurring rhamnofolane diterpenes from Jatropha curcas. Phytochemistry 2013, 96, 265–272. [Google Scholar] [CrossRef]
- Igbinosa, O.O.; Igbinosa, I.H.; Chigor, V.N.; Uzunuigbe, O.E.; Oyedemi, S.O.; Odjadjare, E.E.; Okoh, A.I.; Igbinosa, E.O. Polyphenolic contents and antioxidant potential of stem bark extracts from Jatropha curcas (Linn). Int. J. Mol. Sci. 2011, 12, 2958–2971. [Google Scholar] [CrossRef]
- Mujumdar, A.M.; Misar, A.V. Anti-inflammatory activity of Jatropha curcas roots in mice and rats. J. Ethnopharmacol. 2004, 90, 11–15. [Google Scholar] [CrossRef]
- Matsuse, I.; Lim, Y.; Hattori, M.; Correa, M.; Gupta, M.P. A search for anti-viral properties in Panamanian medicinal plants.: The effects on HIV and its essential enzymes. J. Ethnopharmacol. 1998, 64, 15–22. [Google Scholar] [CrossRef]
- Katagi, A.; Sui, L.; Kamitori, K.; Suzuki, T.; Katayama, T.; Hossain, A.; Noguchi, C.; Dong, Y.; Yamaguchi, F.; Tokuda, M. Inhibitory effect of isoamericanol A from Jatropha curcas seeds on the growth of MCF-7 human breast cancer cell line by G2/M cell cycle arrest. Heliyon 2016, 2, e00055. [Google Scholar] [CrossRef]
- Osoniyi, O.; Onajobi, F. Coagulant and anticoagulant activities in Jatropha curcas latex. J. Ethnopharmacol. 2003, 89, 101–105. [Google Scholar] [CrossRef]
- Mujumdar, A.; Misar, A.; Salaskar, M.; Upadhye, A.S. Antidiarrhoeal effect of isolated fraction (JC) of Jatropha curcas roots in mice. J. Nat. Rem. 2001, 1, 89–93. [Google Scholar]
- Liu, S.; Sporer, F.; Wink, M.; Jourdane, J.; Henning, R.; Li, Y.; Ruppel, A. Anthraquinones in Rheum palmatum and Rumex dentatus (Polygonaceae), and phorbol esters in Jatropha curcas (Euphorbiaceae) with molluscicidal activity against the schistosome vector snails Oncomelania, Biomphalaria, and Bulinus. Trop. Med. Int. Health 1997, 2, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shi, H.M.; Sun, Z.; Ling, X.M.; Tu, P.F. Enantiomer separation of the four diastereomers of guaiacyl glycerol from hydnocarpus annamensis by capillary electrophoresis with HP-β-CD as a chiral selector. J. Chromatogr. Sci. 2007, 45, 605–609. [Google Scholar] [CrossRef][Green Version]
- Li, X.H.; Feng, J.T.; Shi, Y.P. Triterpenoids from Saussurea ussuriensis. Can. J. Chem. 2008, 86, 281–284. [Google Scholar] [CrossRef]
- Whiting, D.A. Ligans and neolignans. Nat. Prod. Rep. 1985, 2, 191–211. [Google Scholar] [CrossRef]
- Kingsbury, C. Conformational preferences in diastereomers. V. Hydrogen-bonding systems. J. Org. Chem. 1970, 35, 1319–1323. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, C.J.; Chen, J.; Zhao, Q.Q.; Li, Y.; Gao, K. Thiophene acetylenes and furanosesquiterpenes from Xanthopappus subacaulis and their antibacterial activities. Phytochemistry 2014, 106, 134–140. [Google Scholar] [CrossRef]
- Li, Y.; Wei, Y.L.; Xu, J.J.; Liang, Y.L.; Li, Y.P. Two new lignan derivatives from Jatropha curcas. Chem. Nat. Compd. 2019, 55, 614–617. [Google Scholar] [CrossRef]
- Tammela, P.; Alvesalo, J.; Riihimäki, L.; Airenne, S.; Leinonen, M.; Hurskainen, P.; Enkvist, K.; Vuorela, P. Development and validation of a time-resolved fluorometric immunoassay for screening of antichlamydial activity using a genus-specific europium-conjugated antibody. Anal Biochem. 2004, 333, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Bannwarth, C.; Caldeweyher, E.; Ehlert, S.; Hansen, A.; Pracht, P.; Seibert, J.; Spicher, S.; Grimme, S. Extended tight-binding quantum chemistry methods. WIREs Comput. Mol. Sci. 2021, 11, e1493. [Google Scholar] [CrossRef]
- Grimme, S. Exploration of Chemical Compound, Conformer, and Reaction Space with Meta-Dynamics Simulations Based on Tight-Binding Quantum Chemical Calculations. J. Chem. Theory Comput. 2019, 155, 2847–2862. [Google Scholar] [CrossRef]
- Pracht, P.; Grimme, S. Calculation of absolute molecular entropies and heat capacities made simple. Chem. Sci. 2021, 12, 6551–6568. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Bannwarth, C.; Shushkov, P. A Robust and Accurate Tight-Binding Quantum Chemical Method for Structures, Vibrational Frequencies, and Noncovalent Interactions of Large Molecular Systems Parametrized for All spd-Block Elements (Z = 1–86Co). J. Chem. Theory Comput. 2017, 13, 1989–2009. [Google Scholar] [CrossRef]
- Bannwarth, C.; Ehlert, S.; Grimme, S. GFN2-xTB—An Accurate and Broadly Parametrized Self-Consistent Tight-Binding Quantum Chemical Method with Multipole Electrostatics and Density-Dependent Dispersion Contributions. J. Chem. Theory Comput. 2019, 15, 1652–1671. [Google Scholar] [CrossRef]
- Pracht, P.; Caldeweyher, E.; Ehlert, S.; Grimme, S. A Robust Non-Self-Consistent Tight-Binding Quantum Chemistry Method for large Molecules. ChemRxiv 2019, 1–19. [Google Scholar] [CrossRef]
- Neese, F. The ORCA program system. Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system, version 4.0. Comput. Mol. Sci. 2017, 8, e1327. [Google Scholar] [CrossRef]
- Brandenburg, J.G.; Bannwarth, C.; Hansen, A.; Grimme, S. B97-3c: A revised low-cost variant of the B97-D density functional method. J. Chem. Phys. 2018, 148, 064104. [Google Scholar] [CrossRef]
- Lu, T. Molclus Program, Version 1992. Available online: http://www.keinsci.com/research/molclus.html (accessed on 10 May 2022).
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01. Gaussian Inc.: Wallingford, CT, USA, 2016. Available online: https://gaussian.com/citation/ (accessed on 10 May 2022).
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378. [Google Scholar] [CrossRef] [PubMed]
- Bruhn, T.; Schaumlöffel, A.; Hemberger, Y.; Bringmann, G. SpecDis: Quantifying the Comparison of Calculated and Experimental Electronic Circular Dichroism Spectra. Chirality 2013, 25, 243–249. [Google Scholar] [CrossRef] [PubMed]

| 1 | 2 | ||||
|---|---|---|---|---|---|
| Position | δC | δH (J Hz) | δC | δH (J Hz) | HMBC |
| 1 | 130.0 s | 129.5 s | |||
| 2 | 110.0 d | 6.91 d (1.6) | 109.6 d | 6.93 d (1.5) | H-2/C-1,3,6,7 |
| 3 | 150.9 s | 150.7 s | |||
| 4 | 145.6 s | 145.7 s | |||
| 5 | 114.1 d | 6.87 d (8.2) | 114.1 d | 6.88 d (8.5) | H-5/C-1,3,6 |
| 6 | 121.1 d | 6.86 dd (8.2, 1.6) | 120.8 d | 6.84 dd (8.5, 1.5) | H-6/C-1,3,5 |
| 7 | 82.6 d | 4.39 d (8.8) | 83.2 d | 4.42 d (5.8) | H-7/C-1,2,6,8,9/OCH3 |
| 8 | 82.0 d | 4.46 m | 81.6 d | 4.50 ddd (6.1,5.8,4.0) | H-8/C-7,9,4′ |
| 9 | 63.8 t | 4.42 m | 63.8 t | 4.05 dd (11.8, 6.1) | H2-9/C-7,8,OAC |
| 4.42 m | 4.22 dd (11.8, 4.0) | ||||
| 1′ | 131.2 s | 130.9 s | |||
| 2′ | 110.2 d | 6.85 d (1.6) | 109.9 d | 6.86 d (1.5) | H-2′/C-1′,3′,4′,5′,6′,7′ |
| 3′ | 146.7 s | 146.8 s | |||
| 4′ | 148.1 s | 148.6 s | |||
| 5′ | 118.4 d | 6.67 d (8.3) | 117.8 d | 6.91 d (8.5) | H-5′/C-1′,3′,4′,6′ |
| 6′ | 119.9 d | 6.81 dd (8.3, 1.6) | 119.8 d | 6.88 dd (8.5, 1.5) | H-6′/C-2′,4′,7′ |
| 7′ | 134.2 d | 6.54 d (15.9) | 134.2 d | 6.58 d (15.7) | H-7′/C-1′,2′,6′, 9′ |
| 8′ | 121.9 d | 6.14 dt (15.9, 6.6) | 121.7 d | 6.16 dt (15.7, 6.6) | H-8′/C-1′, 9′ |
| 9′ | 65.3 t | 4.69 dd (6.6, 1.3) | 65.2 t | 4.71 d (6.6) | H2-9′/C-7′,8′,OAc |
| 4.69 dd (6.6, 1.3) | 4.71 d (6.6) | ||||
| 7-OCH3 | 57.3 q | 3.28 (s) | 57.1 q | 3.28 (s) | CH3/C-7 |
| 3-OCH3 | 55.9 q | 3.78 (s) | 55.8 q | 3.84 (s) | CH3/C-3 |
| 3′-OCH3 | 56.1 q | 3.84 (s) | 56.0 q | 3.87 (s) | CH3/C-3′ |
| 9-OAc | 21.0 q, 171.0 s | 2.01 (s) | 20.8 q, 170.7 s | 1.97 (s) | CH3/C=O |
| 9′-OAc | 21.2 q, 171.0 s | 2.09 (s) | 21.0 q, 170.9 s | 2.10 (s) | CH3/C=O |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.-L.; Huang, P.-Z.; Yang, H.-Y.; Feng, W.-J.; Li, Z.-C.; Gao, K. Jatrolignans C and D: New Neolignan Epimers from Jatropha curcas. Molecules 2022, 27, 3540. https://doi.org/10.3390/molecules27113540
He Y-L, Huang P-Z, Yang H-Y, Feng W-J, Li Z-C, Gao K. Jatrolignans C and D: New Neolignan Epimers from Jatropha curcas. Molecules. 2022; 27(11):3540. https://doi.org/10.3390/molecules27113540
Chicago/Turabian StyleHe, Yi-Lin, Pei-Zhi Huang, Hong-Ying Yang, Wei-Jiao Feng, Zhao-Cai Li, and Kun Gao. 2022. "Jatrolignans C and D: New Neolignan Epimers from Jatropha curcas" Molecules 27, no. 11: 3540. https://doi.org/10.3390/molecules27113540
APA StyleHe, Y.-L., Huang, P.-Z., Yang, H.-Y., Feng, W.-J., Li, Z.-C., & Gao, K. (2022). Jatrolignans C and D: New Neolignan Epimers from Jatropha curcas. Molecules, 27(11), 3540. https://doi.org/10.3390/molecules27113540





