Targeted Therapy of B7 Family Checkpoints as an Innovative Approach to Overcome Cancer Therapy Resistance: A Review from Chemotherapy to Immunotherapy
Abstract
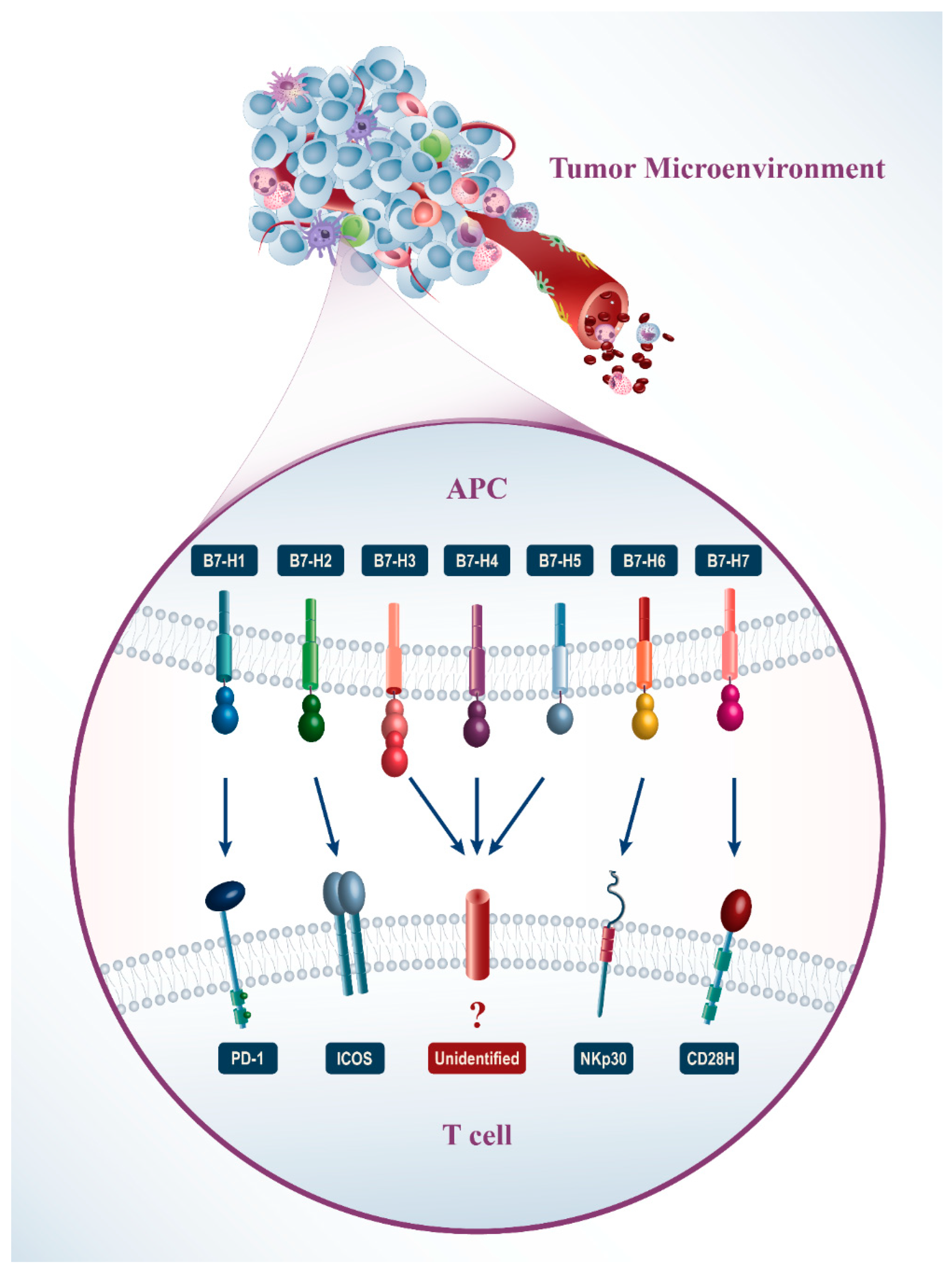
:1. Introduction
2. Different Characters of B7 Family Molecules
2.1. Specification of the B7 Family
2.2. The Expression of the B7 Family on Tumors
2.3. Clinical Importance of B7 Homolog Expression
2.3.1. B7-H1
2.3.2. B7-H2
2.3.3. B7-H3
2.3.4. B7-H4
2.3.5. B7-H5
2.3.6. B7-H6
2.3.7. B7-H7
3. Immune Checkpoints and Chemoresistance
3.1. Role of B7-H1 in Chemoresistance
3.1.1. B7-H1 and Breast Cancer
3.1.2. B7-H1 and Lymphoma
3.1.3. B7-H1 and Myeloma
3.1.4. B7-H1 and Triple-Negative Breast Cancer
3.1.5. B7-H1 and Head and Neck Cell Carcinoma
3.2. Role of B7-H3 in Chemoresistance
3.2.1. B7-H3 and Breast Cancer
3.2.2. B7-H3 and Mantle Cell Lymphoma
3.2.3. B7-H3 and Acute Monocytic Leukemia
3.2.4. B7-H3 and Neuroblastoma
3.2.5. B7-H3 and Melanoma
3.2.6. B7-H3 and Ovarian Cancer
3.3. Role of B7-H4 in Chemoresistance
3.3.1. B7-H4 and Melanoma
3.3.2. B7-H4 and Breast Cancer
3.4. Role of B7-H6 in Chemoresistance
3.4.1. B7-H6 and High-Risk Neuroblastoma
3.4.2. B7-H6 and Non-Hodgkin’s B-Cell Lymphoma
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the Global Cancer Incidence and Mortality in 2018: GLOBOCAN Sources and Methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, S.; Anthony, M.; Chowdhury, N.; Kathrotia, R.; Mishra, M.; Naithani, M.; Sindhwani, G.; Singh, N. Molecular Characterization of Lung Carcinomas: A Study on Diagnostic, Predictive, and Prognostic Markers Using Immunohistochemical Analysis at a Tertiary Care Center in Uttarakhand, India. J. Carcinog. 2021, 20, 17. [Google Scholar] [CrossRef] [PubMed]
- Faghfuri, E.; Pourfarzi, F.; Faghfouri, A.H.; Abdoli Shadbad, M.; Hajiasgharzadeh, K.; Baradaran, B. Recent Developments of RNA-Based Vaccines in Cancer Immunotherapy. Expert Opin. Biol. Ther. 2021, 21, 201–218. [Google Scholar] [CrossRef]
- Chukwueke, U.N.; Wen, P.Y. Use of the Response Assessment in Neuro-Oncology (RANO) Criteria in Clinical Trials and Clinical Practice. CNS Oncol. 2019, 8, CNS28. [Google Scholar] [CrossRef] [Green Version]
- Javadrashid, D.; Baghbanzadeh, A.; Derakhshani, A.; Leone, P.; Silvestris, N.; Racanelli, V.; Solimando, A.G.; Baradaran, B. Pancreatic Cancer Signaling Pathways, Genetic Alterations, and Tumor Microenvironment: The Barriers Affecting the Method of Treatment. Biomedicines 2021, 9, 373. [Google Scholar] [CrossRef]
- Ochsenbein, A.F. Principles of Tumor Immunosurveillance and Implications for Immunotherapy. Cancer Gene Ther. 2002, 9, 1043–1055. [Google Scholar] [CrossRef] [Green Version]
- Derakhshani, A.; Hashemzadeh, S.; Asadzadeh, Z.; Shadbad, M.A.; Rasibonab, F.; Safarpour, H.; Jafarlou, V.; Solimando, A.G.; Racanelli, V.; Singh, P.K.; et al. Cytotoxic T-Lymphocyte Antigen-4 in Colorectal Cancer: Another Therapeutic Side of Capecitabine. Cancers 2021, 13, 2414. [Google Scholar] [CrossRef]
- Chen, L.; Flies, D.B. Molecular Mechanisms of T Cell Co-Stimulation and Co-Inhibition. Nat. Rev. Immunol. 2013, 13, 227–242. [Google Scholar] [CrossRef]
- Chapoval, A.I.; Chapoval, S.P.; Shcherbakova, N.S.; Shcherbakov, D.N. Immune Checkpoints of the B7 Family. Part 1. General Characteristics and First Representatives: B7-1, B7-2, B7-H1, B7-H2, and B7-DC. Russ. J. Bioorganic Chem. 2019, 45, 225–240. [Google Scholar] [CrossRef]
- Kooshkaki, O.; Derakhshani, A.; Safarpour, H.; Najafi, S.; Vahedi, P.; Brunetti, O.; Torabi, M.; Lotfinejad, P.; Paradiso, A.V.; Racanelli, V.; et al. The Latest Findings of PD-1/PD-L1 Inhibitor Application in Gynecologic Cancers. Int. J. Mol. Sci. 2020, 21, 5034. [Google Scholar] [CrossRef] [PubMed]
- Chapoval, A.I.; Chapoval, S.P.; Shcherbakova, N.S.; Shcherbakov, D.N. Immune Checkpoints of the B7 Family. Part 2. Representatives of the B7 Family B7-H3, B7-H4, B7-H5, B7-H6, B7-H7, and ILDR2 and Their Receptors. Russ. J. Bioorg. Chem. 2019, 45, 321–334. [Google Scholar] [CrossRef]
- Liu, D.; Jenkins, R.W.; Sullivan, R.J. Mechanisms of Resistance to Immune Checkpoint Blockade. Am. J. Clin. Dermatol. 2019, 20, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Emens, L.A. Breast Cancer Immunotherapy: Facts and Hopes. Clin. Cancer Res. 2018, 24, 511–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, W.; Chen, L. Inhibitory B7-Family Molecules in the Tumour Microenvironment. Nat. Rev. Immunol. 2008, 8, 467–477. [Google Scholar] [CrossRef]
- Zhou, Z.-H.; Ji, C.-D.; Zhu, J.; Xiao, H.-L.; Zhao, H.-B.; Cui, Y.-H.; Bian, X.-W. The Prognostic Value and Pathobiological Significance of Glasgow Microenvironment Score in Gastric Cancer. J. Cancer Res. Clin. Oncol. 2017, 143, 883–894. [Google Scholar] [CrossRef]
- Wang, J.; Chong, K.K.; Nakamura, Y.; Nguyen, L.; Huang, S.K.; Kuo, C.; Zhang, W.; Yu, H.; Morton, D.L.; Hoon, D.S.B. B7-H3 Associated with Tumor Progression and Epigenetic Regulatory Activity in Cutaneous Melanoma. J. Invest. Dermatol. 2013, 133, 2050–2058. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Luther, N.; Ibrahim, G.M.; Hawkins, C.; Vibhakar, R.; Handler, M.H.; Souweidane, M.M. B7-H3, a Potential Therapeutic Target, Is Expressed in Diffuse Intrinsic Pontine Glioma. J. Neurooncol. 2013, 111, 257–264. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.-H.; Zhang, G.-B.; Wang, J.-M.; Hu, H.-C. B7-H3 and CD133 Expression in Non-Small Cell Lung Cancer and Correlation with Clinicopathologic Factors and Prognosis. Saudi Med. J. 2010, 31, 980–986. [Google Scholar]
- Yamato, I.; Sho, M.; Nomi, T.; Akahori, T.; Shimada, K.; Hotta, K.; Kanehiro, H.; Konishi, N.; Yagita, H.; Nakajima, Y. Clinical Importance of B7-H3 Expression in Human Pancreatic Cancer. Br. J. Cancer 2009, 101, 1709–1716. [Google Scholar] [CrossRef]
- Crispen, P.L.; Sheinin, Y.; Roth, T.J.; Lohse, C.M.; Kuntz, S.M.; Frigola, X.; Thompson, R.H.; Boorjian, S.A.; Dong, H.; Leibovich, B.C.; et al. Tumor Cell and Tumor Vasculature Expression of B7-H3 Predict Survival in Clear Cell Renal Cell Carcinoma. Clin. Cancer Res. 2008, 14, 5150–5157. [Google Scholar] [CrossRef] [Green Version]
- Ingebrigtsen, V.A.; Boye, K.; Nesland, J.M.; Nesbakken, A.; Flatmark, K.; Fodstad, Ø. B7-H3 Expression in Colorectal Cancer: Associations with Clinicopathological Parameters and Patient Outcome. BMC Cancer 2014, 14, 602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zang, X.; Sullivan, P.S.; Soslow, R.A.; Waitz, R.; Reuter, V.E.; Wilton, A.; Thaler, H.T.; Arul, M.; Slovin, S.F.; Wei, J.; et al. Tumor Associated Endothelial Expression of B7-H3 Predicts Survival in Ovarian Carcinomas. Mod. Pathol. 2010, 23, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Arigami, T.; Narita, N.; Mizuno, R.; Nguyen, L.; Ye, X.; Chung, A.; Giuliano, A.E.; Hoon, D.S.B. B7–H3 Ligand Expression by Primary Breast Cancer and Associated With Regional Nodal Metastasis. Ann. Surg. 2010, 252, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-P.; Jiang, J.-T.; Tan, M.; Zhu, Y.-B.; Ji, M.; Xu, K.-F.; Zhao, J.-M.; Zhang, G.-B.; Zhang, X.-G. Relationship between Co-Stimulatory Molecule B7-H3 Expression and Gastric Carcinoma Histology and Prognosis. World J. Gastroenterol. 2006, 12, 457. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.-H.; Zhu, G.; Sica, G.L.; Strome, S.E.; Cheville, J.C.; Lau, J.S.; Zhu, Y.; Flies, D.B.; Tamada, K.; Chen, L. Genomic Organization and Expression Analysis of B7-H4, an Immune Inhibitory Molecule of the B7 Family. J. Immunol. 2003, 171, 4650–4654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Wu, H.; Lu, D.; Li, G.; Sun, C.; Song, H.; Li, J.; Zhai, T.; Huang, L.; Hou, C.; et al. The Costimulatory Molecule B7-H4 Promote Tumor Progression and Cell Proliferation through Translocating into Nucleus. Oncogene 2013, 32, 5347–5358. [Google Scholar] [CrossRef] [Green Version]
- Yao, Y.; Wang, X.; Jin, K.; Zhu, J.; Wang, Y.; Xiong, S.; Mao, Y.; Zhou, L. B7-H4 Is Preferentially Expressed in Non-Dividing Brain Tumor Cells and in a Subset of Brain Tumor Stem-like Cells. J. Neurooncol. 2008, 89, 121–129. [Google Scholar] [CrossRef]
- Peng, H.; Wu, W.; Yang, D.; Jing, R.; Li, J.; Zhou, F.; Jin, Y.; Wang, S.; Chu, Y. Role of B7-H4 SiRNA in Proliferation, Migration, and Invasion of LOVO Colorectal Carcinoma Cell Line. BioMed Res. Int. 2015, 2015, 1–10. [Google Scholar] [CrossRef]
- Byers, J.T.; Paniccia, A.; Kaplan, J.; Koenig, M.; Kahn, N.; Wilson, L.; Chen, L.; Schulick, R.D.; Edil, B.H.; Zhu, Y. Expression of the Novel Costimulatory Molecule B7-H5 in Pancreatic Cancer. Ann. Surg. Oncol. 2015, 22, 1574–1579. [Google Scholar] [CrossRef]
- Schlecker, E.; Fiegler, N.; Arnold, A.; Altevogt, P.; Rose-John, S.; Moldenhauer, G.; Sucker, A.; Paschen, A.; von Strandmann, E.P.; Textor, S.; et al. Metalloprotease-Mediated Tumor Cell Shedding of B7-H6, the Ligand of the Natural Killer Cell–Activating Receptor NKp30. Cancer Res. 2014, 74, 3429–3440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semeraro, M.; Rusakiewicz, S.; Minard-Colin, V.; Delahaye, N.F.; Enot, D.; Vély, F.; Marabelle, A.; Papoular, B.; Piperoglou, C.; Ponzoni, M.; et al. Clinical Impact of the NKp30/B7-H6 Axis in High-Risk Neuroblastoma Patients. Sci. Transl. Med. 2015, 7, 283ra55. [Google Scholar] [CrossRef] [PubMed]
- Che, F.; Xie, X.; Wang, L.; Su, Q.; Jia, F.; Ye, Y.; Zang, L.; Wang, J.; Li, H.; Quan, Y.; et al. B7-H6 Expression Is Induced by Lipopolysaccharide and Facilitates Cancer Invasion and Metastasis in Human Gliomas. Int. Immunopharmacol. 2018, 59, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jin, X.; Liu, J.; Zhao, K.; Xu, H.; Wen, J.; Jiang, L.; Zeng, X.; Li, J.; Chen, Q. The Prognostic Value of B7-H6 Protein Expression in Human Oral Squamous Cell Carcinoma. J. Oral Pathol. Med. 2017, 46, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Shen, J.; Wang, M.H.; Yi, T.; Yu, Y.; Zhu, Y.; Chen, B.; Chen, J.; Li, L.; Li, M. Comprehensive Molecular Profiling of the B7 Family of Immune-Regulatory Ligands in Breast Cancer. Oncoimmunology 2016, 5, e1207841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Xu, Y.; Chen, L.; Xu, B.; Wu, C.; Jiang, J. B7-H6 Expression Correlates with Cancer Progression and Patient’s Survival in Human Ovarian Cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 9428–9433. [Google Scholar]
- Janakiram, M.; Chinai, J.M.; Fineberg, S.; Fiser, A.; Montagna, C.; Medavarapu, R.; Castano, E.; Jeon, H.; Ohaegbulam, K.C.; Zhao, R.; et al. Expression, Clinical Significance, and Receptor Identification of the Newest B7 Family Member HHLA2 Protein. Clin. Cancer Res. 2015, 21, 2359–2366. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Bajorath, J.; Flies, D.B.; Dong, H.; Honjo, T.; Chen, L. Molecular Modeling and Functional Mapping of B7-H1 and B7-DC Uncouple Costimulatory Function from PD-1 Interaction. J. Exp. Med. 2003, 197, 1083–1091. [Google Scholar] [CrossRef]
- Dong, H.; Zhu, G.; Tamada, K.; Chen, L. B7-H1, a Third Member of the B7 Family, Co-Stimulates T-Cell Proliferation and Interleukin-10 Secretion. Nat. Med. 1999, 5, 1365–1369. [Google Scholar] [CrossRef]
- Baradaran, B.; Hajiasgharzadeh, K. Breast Cancer Among Young Women in Iran. Int. J. Women’s Heal. Reprod. Sci. 2019, 7, 140. [Google Scholar] [CrossRef]
- Schwartz, J.-C.D.; Zhang, X.; Nathenson, S.G.; Almo, S.C. Structural Mechanisms of Costimulation. Nat. Immunol. 2002, 3, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Ling, V.; Wu, P.W.; Spaulding, V.; Kieleczawa, J.; Luxenberg, D.; Carreno, B.M.; Collins, M. Duplication of Primate and Rodent B7-H3 Immunoglobulin V-and C-like Domains: Divergent History of Functional Redundancy and Exon Loss. Genomics 2003, 82, 365–377. [Google Scholar] [CrossRef]
- Borriello, F.; Freeman, G.J.; Edelhoff, S.; Disteche, C.M.; Nadler, L.M.; Sharpe, A.H. Characterization of the Murine B7-1 Genomic Locus Reveals an Additional Exon Encoding an Alternative Cytoplasmic Domain and a Chromosomal Location of Chromosome 16, Band B5. J. Immunol. 1994, 153, 5038–5048. [Google Scholar] [PubMed]
- Borriello, F.; Oliveros, J.; Freeman, G.J.; Nadler, L.M.; Sharpe, A.H. Differential Expression of Alternate MB7-2 Transcripts. J. Immunol. 1995, 155, 5490–5497. [Google Scholar] [PubMed]
- Guo, Y.; Wu, Y.; Zhao, M.; Kong, X.P.; Liu, Y. Mutational Analysis and an Alternatively Spliced Product of B7 Defines Its CD28/CTLA4-Binding Site on Immunoglobulin C-like Domain. J. Exp. Med. 1995, 181, 1345–1355. [Google Scholar] [CrossRef] [Green Version]
- Ling, V.; Wu, P.W.; Miyashiro, J.S.; Marusic, S.; Finnerty, H.F.; Collins, M. Differential Expression of Inducible Costimulator-Ligand Splice Variants: Lymphoid Regulation of Mouse GL50-B and Human GL50 Molecules. J. Immunol. 2001, 166, 7300–7308. [Google Scholar] [CrossRef] [Green Version]
- Mages, H.W.; Hutloff, A.; Heuck, C.; Büchner, K.; Himmelbauer, H.; Oliveri, F.; Kroczek, R.A. Molecular Cloning and Characterization of Murine ICOS and Identification of B7h as ICOS Ligand. Eur. J. Immunol. 2000, 30, 1040–1047. [Google Scholar] [CrossRef]
- Hutloff, A.; Dittrich, A.M.; Beier, K.C.; Eljaschewitsch, B.; Kraft, R.; Anagnostopoulos, I.; Kroczek, R.A. ICOS Is an Inducible T-Cell Co-Stimulator Structurally and Functionally Related to CD28. Nature 1999, 397, 263–266. [Google Scholar] [CrossRef]
- Sullivan, B.A.; Tsuji, W.; Kivitz, A.; Peng, J.; Arnold, G.E.; Boedigheimer, M.J.; Chiu, K.; Green, C.L.; Kaliyaperumal, A.; Wang, C.; et al. Inducible T-Cell Co-Stimulator Ligand (ICOSL) Blockade Leads to Selective Inhibition of Anti-KLH IgG Responses in Subjects with Systemic Lupus Erythematosus. Lupus Sci. Med. 2016, 3, e000146. [Google Scholar] [CrossRef] [Green Version]
- Chapoval, A.I.; Ni, J.; Lau, J.S.; Wilcox, R.A.; Flies, D.B.; Liu, D.; Dong, H.; Sica, G.L.; Zhu, G.; Tamada, K.; et al. B7-H3: A Costimulatory Molecule for T Cell Activation and IFN-γ Production. Nat. Immunol. 2001, 2, 269–274. [Google Scholar] [CrossRef]
- Loos, M.; Hedderich, D.M.; Ottenhausen, M.; Giese, N.A.; Laschinger, M.; Esposito, I.; Kleeff, J.; Friess, H. Expression of the Costimulatory Molecule B7-H3 Is Associated with Prolonged Survival in Human Pancreatic Cancer. BMC Cancer 2009, 9, 463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.; Yu, S.; Li, H.; Liu, C.; Li, J.; Lin, W.; Gao, A.; Wang, L.; Gao, W.; Sun, Y. ILT4 Drives B7-H3 Expression via PI3K/AKT/MTOR Signalling and ILT4/B7-H3 Co-Expression Correlates with Poor Prognosis in Non-Small Cell Lung Cancer. FEBS Lett. 2015, 589, 2248–2256. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Zhang, G.; Zhang, X.; Lv, G.; Wei, X.; Yuan, H.; Hou, J. Overexpression of B7-H3 in CD14+ Monocytes Is Associated with Renal Cell Carcinoma Progression. Med. Oncol. 2014, 31, 349. [Google Scholar] [CrossRef] [PubMed]
- Zang, X.; Thompson, R.H.; Al-Ahmadie, H.A.; Serio, A.M.; Reuter, V.E.; Eastham, J.A.; Scardino, P.T.; Sharma, P.; Allison, J.P. B7-H3 and B7x Are Highly Expressed in Human Prostate Cancer and Associated with Disease Spread and Poor Outcome. Proc. Natl. Acad. Sci. USA 2007, 104, 19458–19463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.; Chen, L.; Zhang, G.; Jiang, J.; Zhu, M.; Tan, Y.; Wang, H.; Lu, B.; Zhang, X. Clinical Significance and Regulation of the Costimulatory Molecule B7-H3 in Human Colorectal Carcinoma. Cancer Immunol. Immunother. 2010, 59, 1163–1171. [Google Scholar] [CrossRef]
- Loo, D.; Alderson, R.F.; Chen, F.Z.; Huang, L.; Zhang, W.; Gorlatov, S.; Burke, S.; Ciccarone, V.; Li, H.; Yang, Y.; et al. Development of an Fc-Enhanced Anti–B7-H3 Monoclonal Antibody with Potent Antitumor Activity. Clin. Cancer Res. 2012, 18, 3834–3845. [Google Scholar] [CrossRef] [Green Version]
- Kramer, K.; Kushner, B.H.; Modak, S.; Pandit-Taskar, N.; Smith-Jones, P.; Zanzonico, P.; Humm, J.L.; Xu, H.; Wolden, S.L.; Souweidane, M.M.; et al. Compartmental Intrathecal Radioimmunotherapy: Results for Treatment for Metastatic CNS Neuroblastoma. J. Neurooncol. 2010, 97, 409–418. [Google Scholar] [CrossRef] [Green Version]
- Zhukovsky, E.A.; Morse, R.J.; Maus, M.V. Bispecific Antibodies and CARs: Generalized Immunotherapeutics Harnessing T Cell Redirection. Curr. Opin. Immunol. 2016, 40, 24–35. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Ma, P.; Zhao, C.; Xue, X.; Han, H.; Liu, C.; Tao, H.; Xiu, W.; Cai, J.; Zhang, M. B7-H3 as a Promising Target for Cytotoxicity T Cell in Human Cancer Therapy. Oncotarget 2016, 7, 29480–29491. [Google Scholar] [CrossRef] [Green Version]
- Gill, S.; June, C.H. Going Viral: Chimeric Antigen Receptor T-Cell Therapy for Hematological Malignancies. Immunol. Rev. 2015, 263, 68–89. [Google Scholar] [CrossRef]
- Sadelain, M. CAR Therapy: The CD19 Paradigm. J. Clin. Invest. 2015, 125, 3392–3400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.; Martin-Orozco, N.; Zheng, P.; Li, J.; Zhang, P.; Tan, H.; Park, H.J.; Jeong, M.; Chang, S.H.; Kim, B.-S.; et al. Inhibition of the B7-H3 Immune Checkpoint Limits Tumor Growth by Enhancing Cytotoxic Lymphocyte Function. Cell Res. 2017, 27, 1034–1045. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.E.; Darnell, J.E. STATs: Transcriptional Control and Biological Impact. Nat. Rev. Mol. Cell Biol. 2002, 3, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Schlessinger, K.; Levy, D.E. Malignant Transformation but Not Normal Cell Growth Depends on Signal Transducer and Activator of Transcription 3. Cancer Res. 2005, 65, 5828–5834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Tekle, C.; Chen, Y.-W.; Kristian, A.; Zhao, Y.; Zhou, M.; Liu, Z.; Ding, Y.; Wang, B.; Mælandsmo, G.M.; et al. B7-H3 Silencing Increases Paclitaxel Sensitivity by Abrogating Jak2/Stat3 Phosphorylation. Mol. Cancer Ther. 2011, 10, 960–971. [Google Scholar] [CrossRef] [Green Version]
- Gritsko, T.; Williams, A.; Turkson, J.; Kaneko, S.; Bowman, T.; Huang, M.; Nam, S.; Eweis, I.; Diaz, N.; Sullivan, D.; et al. Persistent Activation of Stat3 Signaling Induces Survivin Gene Expression and Confers Resistance to Apoptosis in Human Breast Cancer Cells. Clin. Cancer Res. 2006, 12, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Barré, B.; Vigneron, A.; Perkins, N.; Roninson, I.B.; Gamelin, E.; Coqueret, O. The STAT3 Oncogene as a Predictive Marker of Drug Resistance. Trends Mol. Med. 2007, 13, 4–11. [Google Scholar] [CrossRef]
- Aoki, Y.; Feldman, G.M.; Tosato, G. Inhibition of STAT3 Signaling Induces Apoptosis and Decreases Survivin Expression in Primary Effusion Lymphoma. Blood 2003, 101, 1535–1542. [Google Scholar] [CrossRef]
- Henderson, I.C.; Berry, D.A.; Demetri, G.D.; Cirrincione, C.T.; Goldstein, L.J.; Martino, S.; Ingle, J.N.; Cooper, M.R.; Hayes, D.F.; Tkaczuk, K.H.; et al. Improved Outcomes From Adding Sequential Paclitaxel but Not From Escalating Doxorubicin Dose in an Adjuvant Chemotherapy Regimen for Patients With Node-Positive Primary Breast Cancer. J. Clin. Oncol. 2003, 21, 976–983. [Google Scholar] [CrossRef]
- Gil, L.; Kazmierczak, M.; Kroll-Balcerzak, R.; Komarnicki, M. Bendamustine-Based Therapy as First-Line Treatment for Non-Hodgkin Lymphoma. Med. Oncol. 2014, 31, 944. [Google Scholar] [CrossRef] [Green Version]
- Sica, G.L.; Choi, I.-H.; Zhu, G.; Tamada, K.; Wang, S.-D.; Tamura, H.; Chapoval, A.I.; Flies, D.B.; Bajorath, J.; Chen, L. B7-H4, a Molecule of the B7 Family, Negatively Regulates T Cell Immunity. Immunity 2003, 18, 849–861. [Google Scholar] [CrossRef] [Green Version]
- Lines, J.L.; Pantazi, E.; Mak, J.; Sempere, L.F.; Wang, L.; O’Connell, S.; Ceeraz, S.; Suriawinata, A.A.; Yan, S.; Ernstoff, M.S.; et al. VISTA Is an Immune Checkpoint Molecule for Human T Cells. Cancer Res. 2014, 74, 1924–1932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ni, L.; Dong, C. New B7 Family Checkpoints in Human Cancers. Mol. Cancer Ther. 2017, 16, 1203–1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, K.A.; Wang, L.; Noelle, R.J.; Green, W.R. Selective Involvement of the Checkpoint Regulator VISTA in Suppression of B-Cell, but Not T-Cell, Responsiveness by Monocytic Myeloid-Derived Suppressor Cells from Mice Infected with an Immunodeficiency-Causing Retrovirus. J. Virol. 2015, 89, 9693–9698. [Google Scholar] [CrossRef] [Green Version]
- Le Mercier, I.; Chen, W.; Lines, J.L.; Day, M.; Li, J.; Sergent, P.; Noelle, R.J.; Wang, L. VISTA Regulates the Development of Protective Antitumor Immunity. Cancer Res. 2014, 74, 1933–1944. [Google Scholar] [CrossRef] [Green Version]
- Cherif, B.; Triki, H.; Charfi, S.; Bouzidi, L.; Kridis, W.B.; Khanfir, A.; Chaabane, K.; Sellami-Boudawara, T.; Rebai, A. Immune Checkpoint Molecules B7-H6 and PD-L1 Co-Pattern the Tumor Inflammatory Microenvironment in Human Breast Cancer. Sci. Rep. 2021, 11, 7550. [Google Scholar] [CrossRef]
- Jiang, T.; Wu, W.; Zhang, H.; Zhang, X.; Zhang, D.; Wang, Q.; Huang, L.; Wang, Y.; Hang, C. High Expression of B7-H6 in Human Glioma Tissues Promotes Tumor Progression. Oncotarget 2017, 8, 37435–37447. [Google Scholar] [CrossRef]
- Kaifu, T.; Escalière, B.; Gastinel, L.N.; Vivier, E.; Baratin, M. B7-H6/NKp30 Interaction: A Mechanism of Alerting NK Cells against Tumors. Cell. Mol. Life Sci. 2011, 68, 3531–3539. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, M.-R.; Sentman, C.L. An NKp30-Based Chimeric Antigen Receptor Promotes T Cell Effector Functions and Antitumor Efficacy In Vivo. J. Immunol. 2012, 189, 2290–2299. [Google Scholar] [CrossRef]
- Zhu, Y.; Yao, S.; Iliopoulou, B.P.; Han, X.; Augustine, M.M.; Xu, H.; Phennicie, R.T.; Flies, S.J.; Broadwater, M.; Ruff, W.; et al. B7-H5 Costimulates Human T Cells via CD28H. Nat. Commun. 2013, 4, 2043. [Google Scholar] [CrossRef] [Green Version]
- Sowmya, S.; Rao, R.; Prasad, K. Development of Clinico-Histopathological Predictive Model for the Assessment of Metastatic Risk of Oral Squamous Cell Carcinoma. J. Carcinog. 2020, 19, 2. [Google Scholar] [CrossRef] [PubMed]
- Brasseur, K.; Gévry, N.; Asselin, E. Chemoresistance and Targeted Therapies in Ovarian and Endometrial Cancers. Oncotarget 2017, 8, 4008–4042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, C.; Shervington, A. Chemoresistance in Gliomas. Mol. Cell. Biochem. 2008, 312, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Siddeek, R.T.; Gupta, A.; Gupta, S.; Goyal, B.; Gupta, A.; Agrawal, S.; Roshan, R.; Kumar, U.; Kumar, N.; Gupta, M.; et al. Evaluation of Platelet Distribution Width as Novel Biomarker in Gall Bladder Cancer. J. Carcinog. 2020, 19, 5. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, M.; Tamura, H.; Sunakawa, M.; Kondo-Onodera, A.; Okuyama, N.; Hamada, Y.; Moriya, K.; Choi, I.; Tamada, K.; Inokuchi, K. Myeloma Drug Resistance Induced by Binding of Myeloma B7-H1 (PD-L1) to PD-1. Cancer Immunol. Res. 2016, 4, 779–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Wang, J.; Li, C.; Ke, X.-Y. Contribution of PD-L1 to Oncogenesis of Lymphoma and Its RNAi-Based Targeting Therapy. Leuk. Lymphoma 2012, 53, 2015–2023. [Google Scholar] [CrossRef]
- Wu, X.; Li, Y.; Liu, X.; Chen, C.; Harrington, S.M.; Cao, S.; Xie, T.; Pham, T.; Mansfield, A.S.; Yan, Y.; et al. Targeting B7-H1 (PD-L1) Sensitizes Cancer Cells to Chemotherapy. Heliyon 2018, 4, e01039. [Google Scholar] [CrossRef] [Green Version]
- Shen, B.; Huang, D.; Ramsey, A.J.; Ig-Izevbekhai, K.; Zhang, K.; Lajud, S.A.; O’Malley, B.W.; Li, D. PD-L1 and MRN Synergy in Platinum-Based Chemoresistance of Head and Neck Squamous Cell Carcinoma. Br. J. Cancer 2020, 122, 640–647. [Google Scholar] [CrossRef]
- Flem-Karlsen, K.; Tekle, C.; Øyjord, T.; Flørenes, V.A.; Mælandsmo, G.M.; Fodstad, Ø.; Nunes-Xavier, C.E. P38 MAPK Activation through B7-H3-Mediated DUSP10 Repression Promotes Chemoresistance. Sci. Rep. 2019, 9, 5839. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Zhao, Y. B7-H3 Induces Ovarian Cancer Drugs Resistance Through An PI3K/AKT/BCL-2 Signaling Pathway. Cancer Manag. Res. 2019, 11, 10205–10214. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Yang, C.; Liu, X.; Wang, L.; Kang, F. B7-H4 Overexpression Contributes to Poor Prognosis and Drug-Resistance in Triple-Negative Breast Cancer. Cancer Cell Int. 2018, 18, 100. [Google Scholar] [CrossRef] [PubMed]
- Ghebeh, H.; Lehe, C.; Barhoush, E.; Al-Romaih, K.; Tulbah, A.; Al-Alwan, M.; Hendrayani, S.-F.; Manogaran, P.; Alaiya, A.; Al-Tweigeri, T.; et al. Doxorubicin Downregulates Cell Surface B7-H1 Expression and Upregulates Its Nuclear Expression in Breast Cancer Cells: Role of B7-H1 as an Anti-Apoptotic Molecule. Breast Cancer Res. 2010, 12, R48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, A.; Gupta, S.; Rajput, D.; Durgapal, P.; Chennatt, J.; Kishore, S.; Rao, S.; Dhar, P.; Gupta, M.; Kant, R. Expression and Clinicopathological Correlation of Ki-67 in Gallbladder Carcinoma. J. Carcinog. 2021, 20, 11. [Google Scholar] [CrossRef] [PubMed]
- Mortenson, M.M.; Galante, J.G.; Gilad, O.; Schlieman, M.G.; Virudachalam, S.; Kung, H.-J.; Bold, R.J. BCL-2 Functions as an Activator of the AKT Signaling Pathway in Pancreatic Cancer. J. Cell. Biochem. 2007, 102, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- Suhara, T.; Kim, H.-S.; Kirshenbaum, L.A.; Walsh, K. Suppression of Akt Signaling Induces Fas Ligand Expression: Involvement of Caspase and Jun Kinase Activation in Akt-Mediated Fas Ligand Regulation. Mol. Cell. Biol. 2002, 22, 680–691. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Wang, Y.; Wang, J.; Dong, F.; Zhu, M.; Wan, W.; Li, H.; Wu, F.; Yan, X.; Ke, X. B7-H3 Silencing Inhibits Tumor Progression of Mantle Cell Lymphoma and Enhances Chemosensitivity. Int. J. Oncol. 2015, 46, 2562–2572. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Wang, J.; Wang, Y.; Dong, F.; Zhu, M.; Wan, W.; Li, H.; Wu, F.; Yan, X.; Ke, X. B7-H3 Silencing by RNAi Inhibits Tumor Progression and Enhances Chemosensitivity in U937 Cells. Onco Targets Ther. 2015, 8, 1721. [Google Scholar] [CrossRef] [Green Version]
- Esu, E.; Effa, E.E.; Opie, O.N.; Uwaoma, A.; Meremikwu, M.M. Artemether for Severe Malaria. Cochrane Database Syst. Rev. 2014, 6, CD010678. [Google Scholar] [CrossRef] [Green Version]
- Alcântara, D.D.F.Á.; Ribeiro, H.F.; dos Cardoso, P.C.S.; Araújo, T.M.T.; Burbano, R.R.; Guimarães, A.C.; Khayat, A.S.; de Oliveira Bahia, M. In Vitro Evaluation of the Cytotoxic and Genotoxic Effects of Artemether, an Antimalarial Drug, in a Gastric Cancer Cell Line (PG100). J. Appl. Toxicol. 2013, 33, 151–156. [Google Scholar] [CrossRef]
- Wang, Y.-B.; Hu, Y.; Li, Z.; Wang, P.; Xue, Y.-X.; Yao, Y.-L.; Yu, B.; Liu, Y.-H. Artemether Combined with ShRNA Interference of Vascular Cell Adhesion Molecule-1 Significantly Inhibited the Malignant Biological Behavior of Human Glioma Cells. PLoS ONE 2013, 8, e60834. [Google Scholar] [CrossRef]
- Tan, W.-Q.; Chen, G.; Ye, M.; Jia, B. Artemether Regulates Chemosensitivity to Doxorubicin via Regulation of B7-H3 in Human Neuroblastoma Cells. Med. Sci. Monit. 2017, 23, 4252–4259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, T.; Xu, F.; Sheng, Y.; Zhang, W.; Chen, Y. A Targeted Proteomics Approach to the Quantitative Analysis of ERK/Bcl-2-Mediated Anti-Apoptosis and Multi-Drug Resistance in Breast Cancer. Anal. Bioanal. Chem. 2016, 408, 7491–7503. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.-L.; Sasano, H.; Gao, H. Bcl-2 Family in Non-Small Cell Lung Cancer: Its Prognostic and Therapeutic Implications. Pathol. Int. 2017, 67, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Park, G.B.; Song, H.; Kim, Y.-S.; Sung, M.; Ryu, J.W.; Lee, H.-K.; Cho, D.-H.; Kim, D.; Lee, W.J.; Hur, D.Y. Cell Cycle Arrest Induced by Engagement of B7-H4 on Epstein-Barr Virus-Positive B-Cell Lymphoma Cell Lines. Immunology 2009, 128, 360–368. [Google Scholar] [CrossRef]
- Wu, F.; Wang, J.; Ke, X. Knockdown of B7-H6 Inhibits Tumor Progression and Enhances Chemosensitivity in B-Cell Non-Hodgkin Lymphoma. Int. J. Oncol. 2016, 48, 1561–1570. [Google Scholar] [CrossRef] [Green Version]
| B7 Family Member | Alternative Names | Ligand | Type of Response |
|---|---|---|---|
| B7-1 | CD80 | CTLA-4, CD28 | Positive |
| B7-2 | CD86 | CTLA-4, CD28 | Positive |
| B7-H1 | PD-L1, CD274 | PD-1 | Negative |
| B7-DC | PDCD1LG2, PD-L2, CD273 | PD-1 | Negative |
| B7-H2 | B7RP1, ICOS-L, CD275 | ICOS | Negative |
| B7-H3 | CD276 | ? | Positive/Negative |
| B7-H4 | B7x, B7S1, Vtcn1 | ? | ? |
| B7-H5 | VISTA, Platelet receptor, Gi24, SISP1 | ? | Negative |
| B7-H6 | NCR3LG1 | NKp30 | Negative |
| B7-H7 | HHLA2 | TMIGD2 | Negative/? |
| ILDR2 | ? | ? | Negative/? |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amir Taghavi, B.; Alizadeh, N.; Saeedi, H.; Karim Ahangar, N.; Derakhshani, A.; Hajiasgharzadeh, K.; Silvestris, N.; Baradaran, B.; Brunetti, O. Targeted Therapy of B7 Family Checkpoints as an Innovative Approach to Overcome Cancer Therapy Resistance: A Review from Chemotherapy to Immunotherapy. Molecules 2022, 27, 3545. https://doi.org/10.3390/molecules27113545
Amir Taghavi B, Alizadeh N, Saeedi H, Karim Ahangar N, Derakhshani A, Hajiasgharzadeh K, Silvestris N, Baradaran B, Brunetti O. Targeted Therapy of B7 Family Checkpoints as an Innovative Approach to Overcome Cancer Therapy Resistance: A Review from Chemotherapy to Immunotherapy. Molecules. 2022; 27(11):3545. https://doi.org/10.3390/molecules27113545
Chicago/Turabian StyleAmir Taghavi, Bita, Nazila Alizadeh, Hossein Saeedi, Noora Karim Ahangar, Afshin Derakhshani, Khalil Hajiasgharzadeh, Nicola Silvestris, Behzad Baradaran, and Oronzo Brunetti. 2022. "Targeted Therapy of B7 Family Checkpoints as an Innovative Approach to Overcome Cancer Therapy Resistance: A Review from Chemotherapy to Immunotherapy" Molecules 27, no. 11: 3545. https://doi.org/10.3390/molecules27113545
APA StyleAmir Taghavi, B., Alizadeh, N., Saeedi, H., Karim Ahangar, N., Derakhshani, A., Hajiasgharzadeh, K., Silvestris, N., Baradaran, B., & Brunetti, O. (2022). Targeted Therapy of B7 Family Checkpoints as an Innovative Approach to Overcome Cancer Therapy Resistance: A Review from Chemotherapy to Immunotherapy. Molecules, 27(11), 3545. https://doi.org/10.3390/molecules27113545










