Reactivity of 4,5-Dichlorophthalic Anhydride towards Thiosemicarbazide and Amines: Synthesis, Spectroscopic Analysis, and DFT Study
Abstract
:1. Introduction
2. Results and Discussion
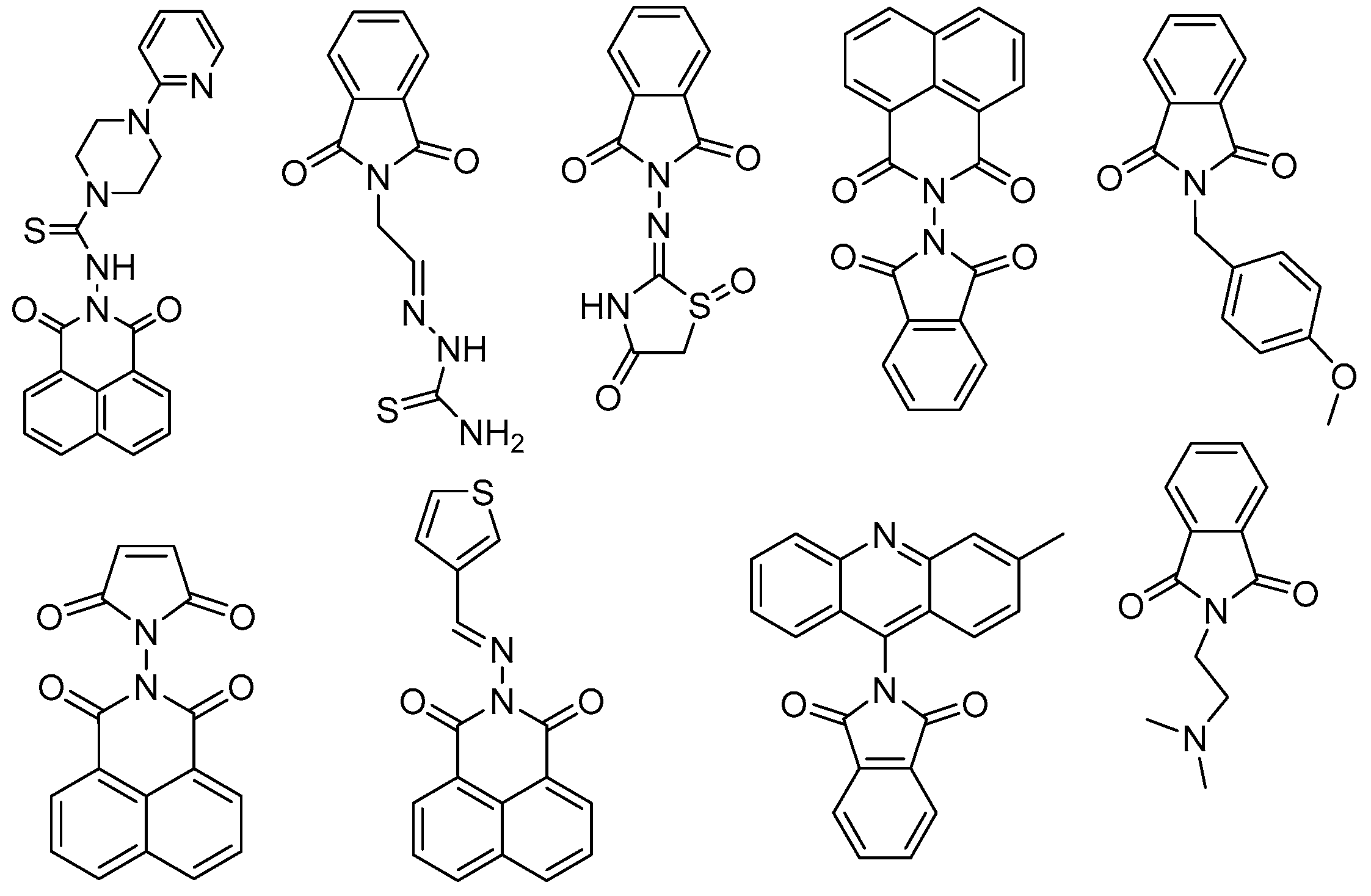
2.1. Chemistry
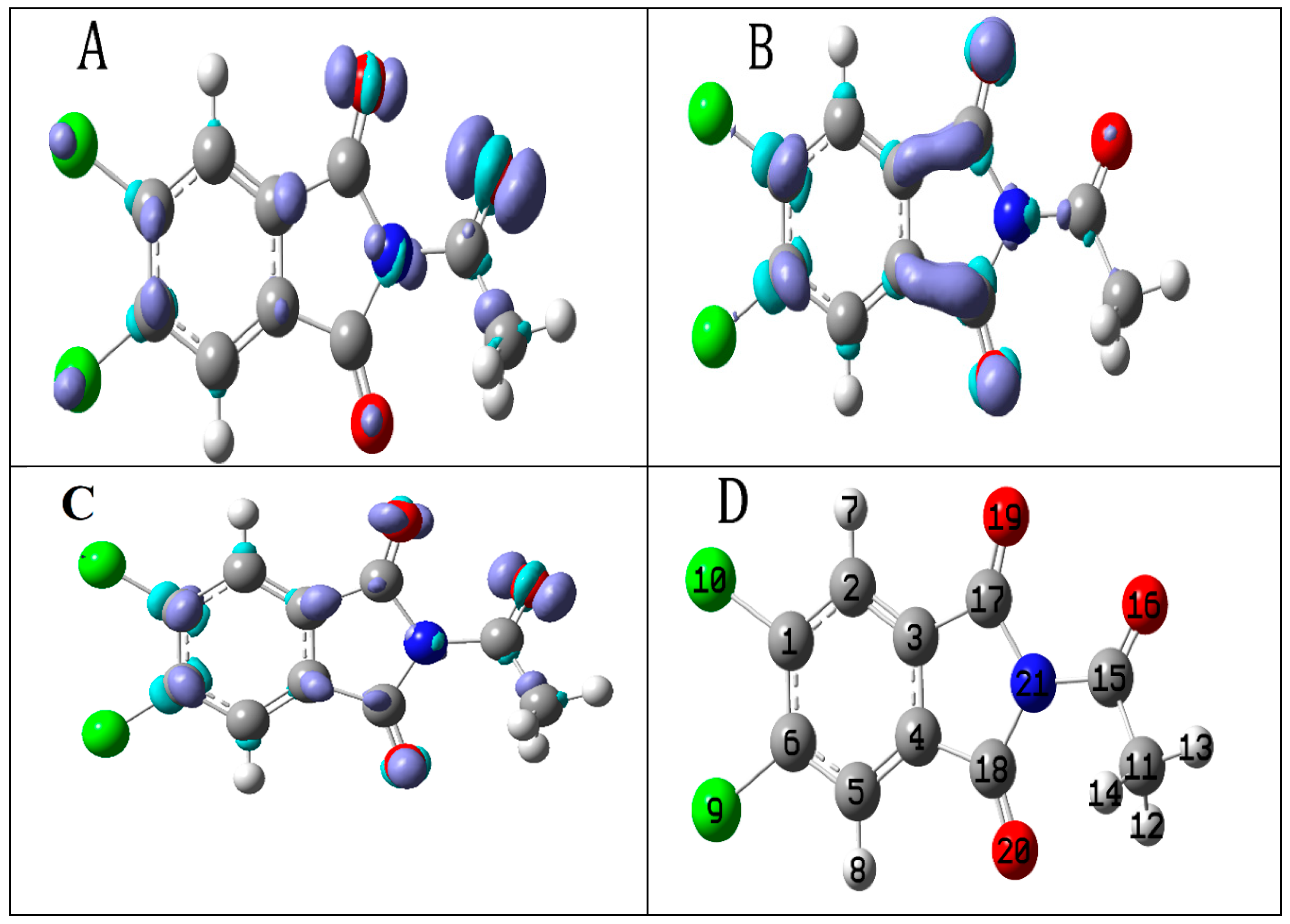
2.2. Computational Study
2.2.1. Reactivity Descriptors
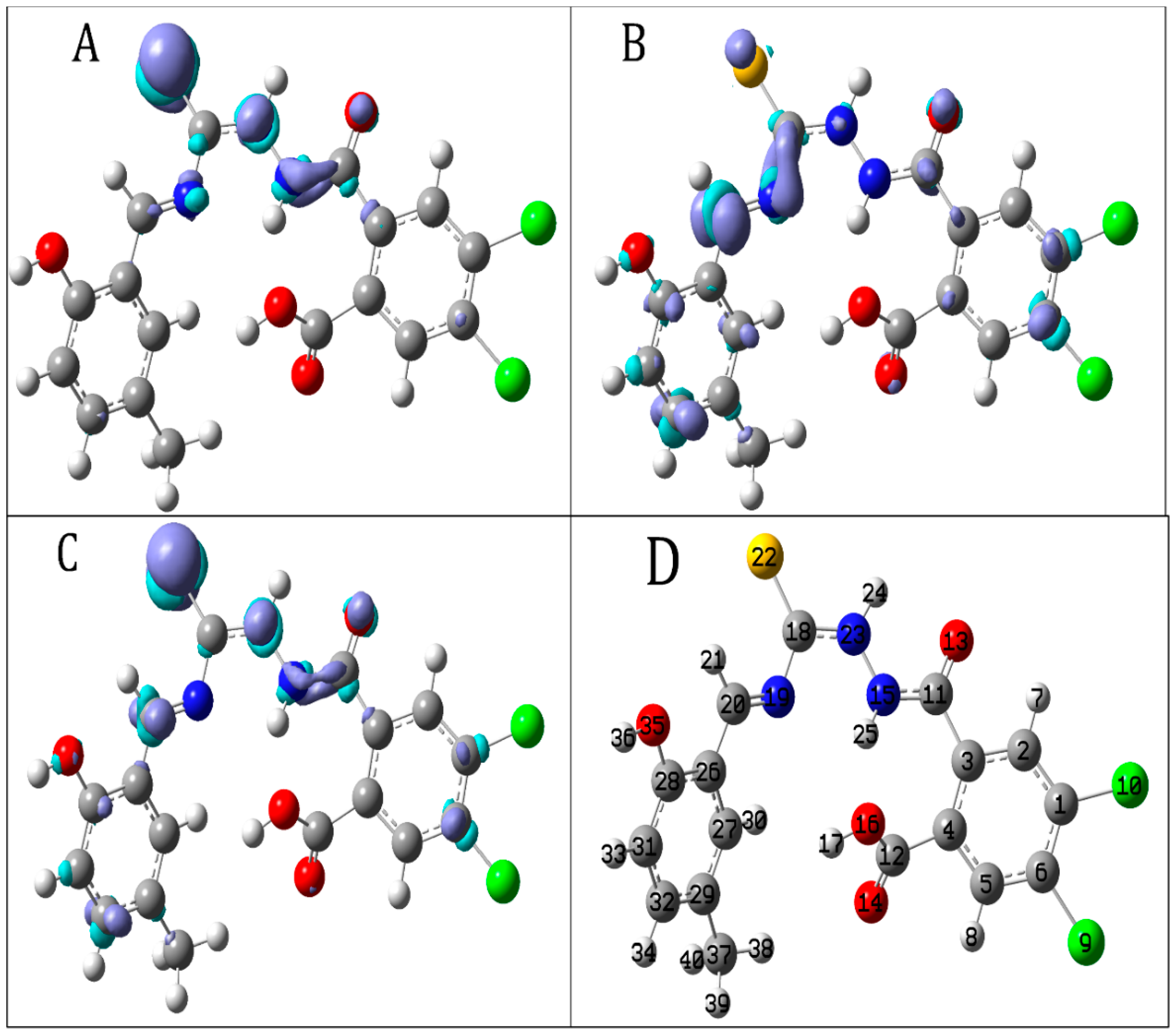
2.2.2. Local Reactivity Descriptors
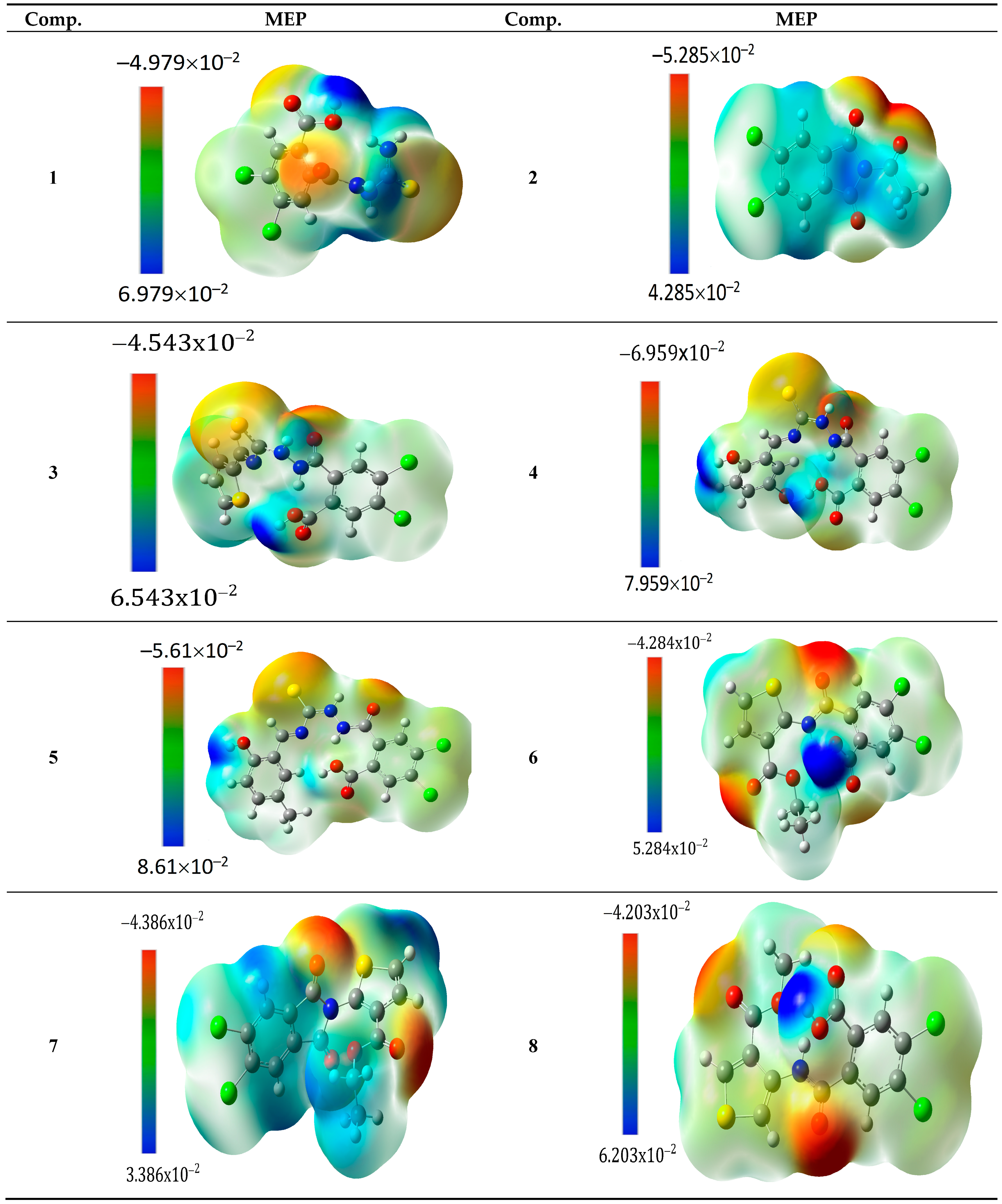
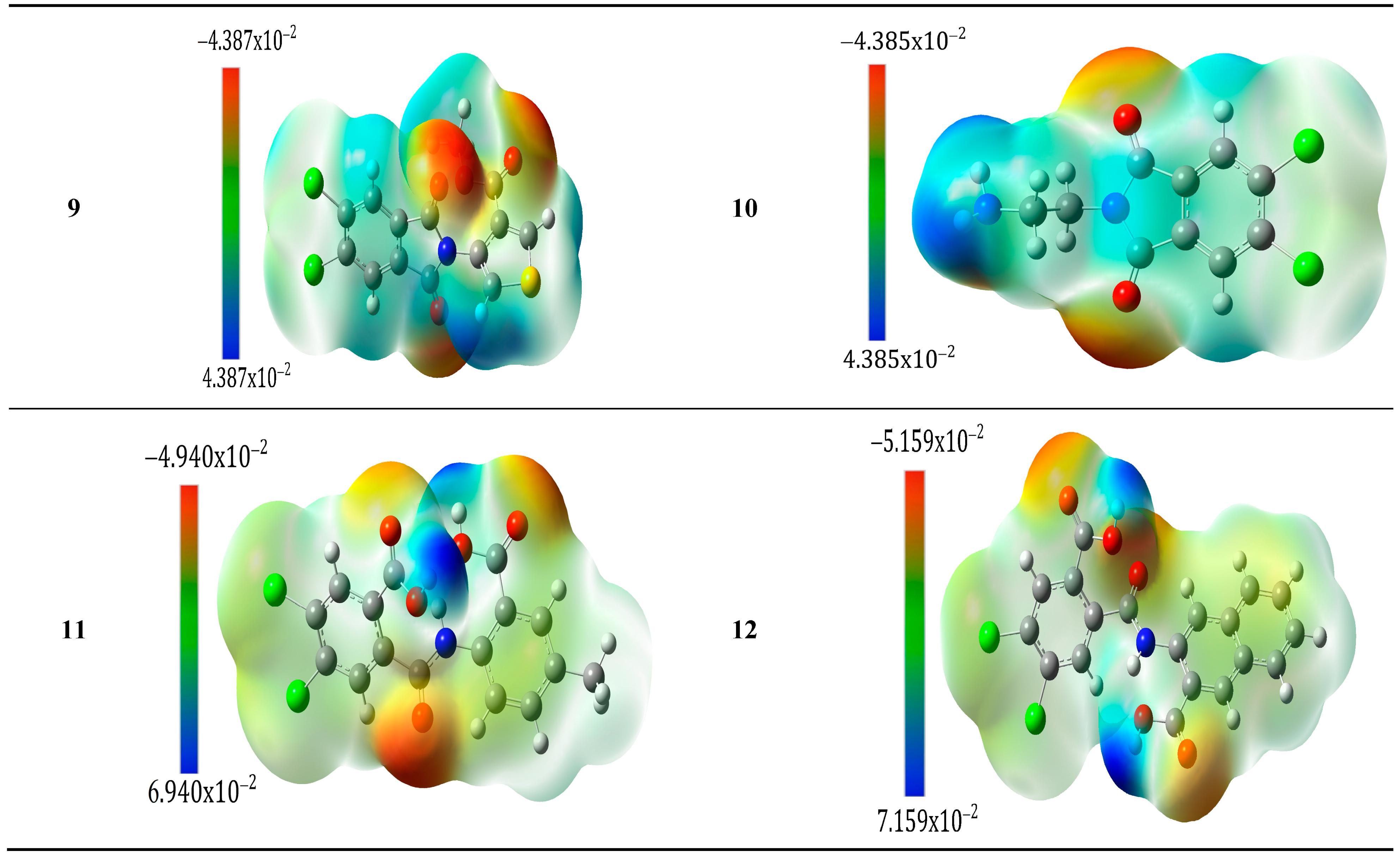
2.2.3. Molecular Electrostatic Potential (MEP)
2.2.4. Vibrational Analysis
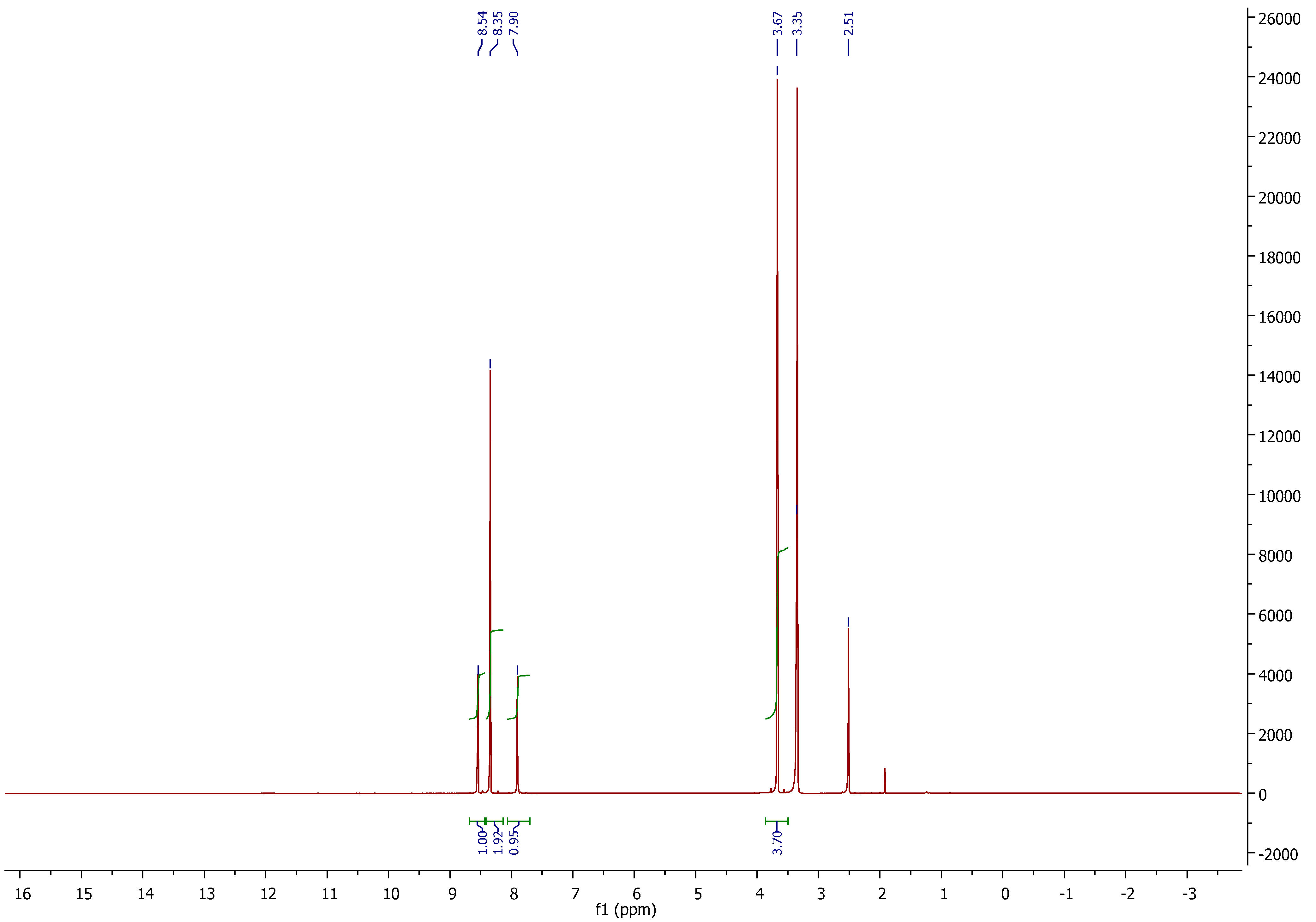
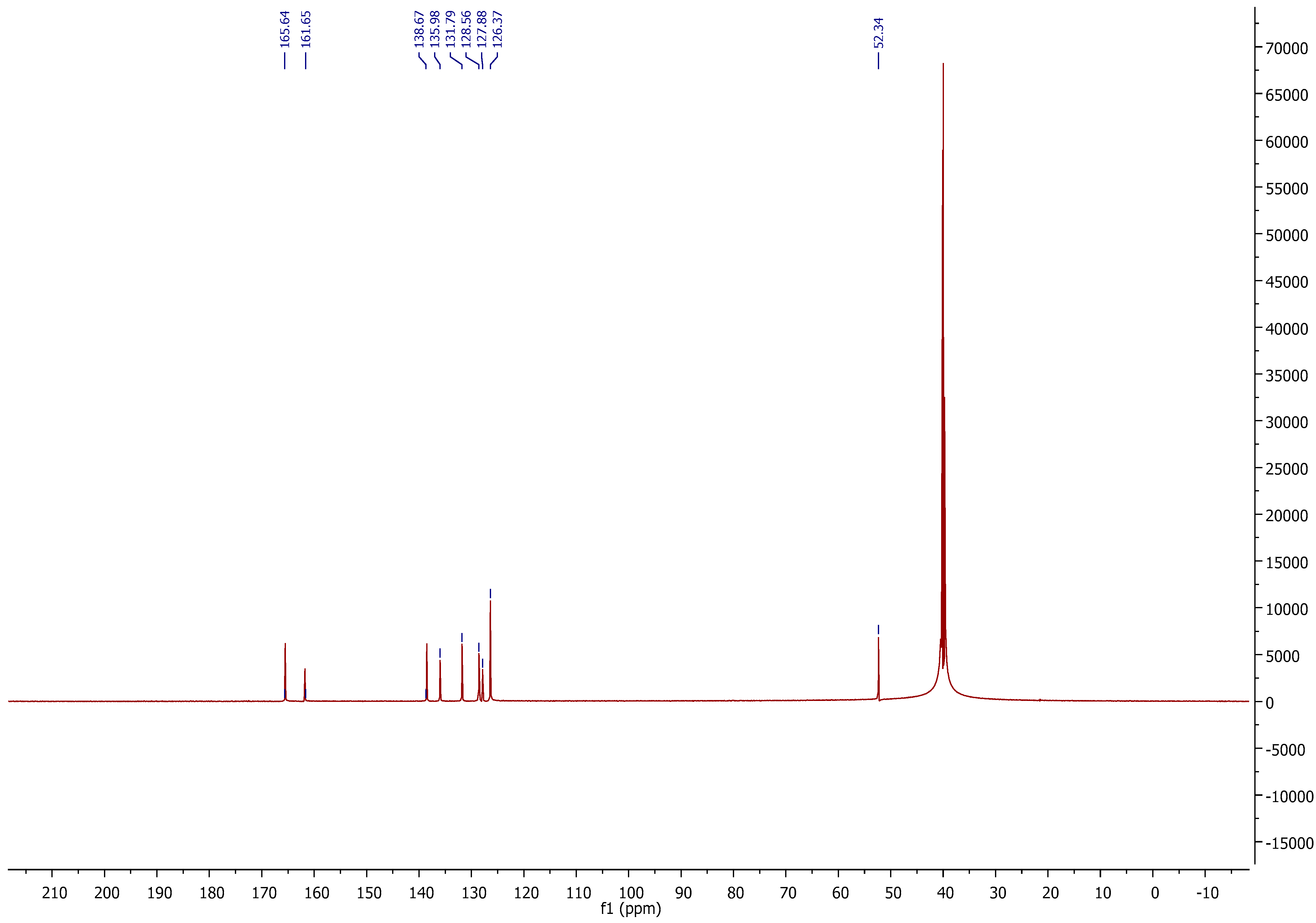
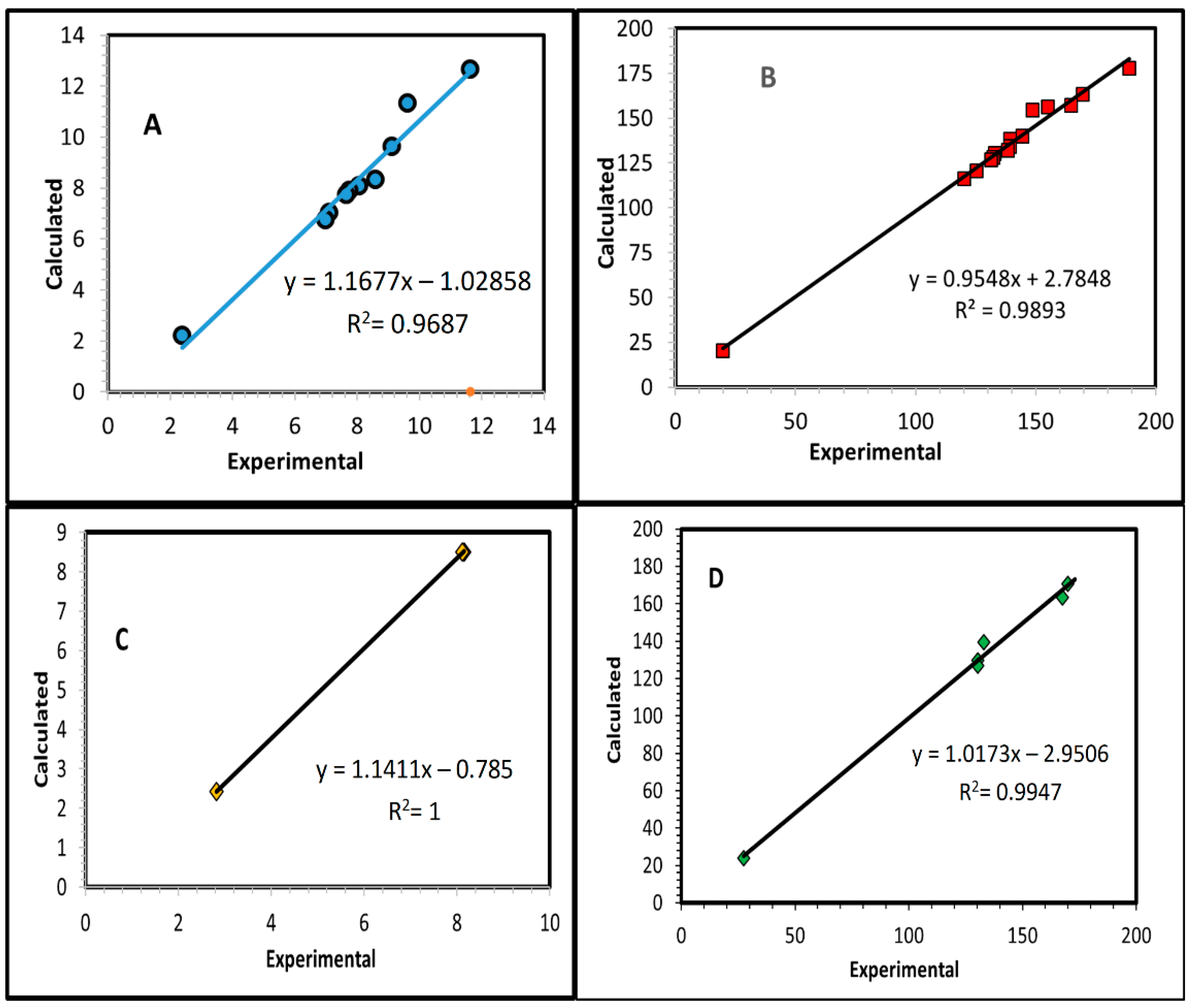
2.2.5. NMR Spectroscopy
3. Conclusions
4. Materials and Methods
4.1. General Information
4.1.1. 2-(2-Carbamothioylhydrazine-1-carbonyl)-4,5-dichlorobenzoic Acid (1)
4.1.2. 2-Acetyl-5,6-dichloroisoindoline-1,3-dione (2)
4.1.3. General Procedure for Synthesis of (3−5)
(E)-4,5-Dichloro-2-(2-((thiophen-2ylmethylene)carbamothioyl)hydrazine-1-carbonyl) benzoic Acid (3)
(E)-2-(2-((5-Bromo-2-hydroxybenzylidene) carbamothioyl)hydrazine-1-carb onyl)-4,5-dichlorobenzoic Acid (4)
(E)-4,5-Dichloro-2-(2-((2-hydroxy-5-methylbenzylidene)carbamothioyl)hydrazine-1-carbonyl)benzoic Acid (5)
4.1.4. General Procedure for Preparation of (6−9)
4,5-Dichloro-2-((3-(ethoxycarbonyl)thiophen-2-yl)carbamoyl)benzoic Acid (6)
Ethyl 2-(5,6-dichloro-1,3-dioxoisoindolin-2-yl)thiophene-3-carboxylate (7)
4,5-Dichloro-2-((4-(methoxycarbonyl)thiophen-3-yl)carbamoyl) benzoic Acid (8)
Methyl 4-(5,6-dichloro-1,3-dioxoisoindolin-2-yl)thiophene-3-carboxylate (9)
2-(2-aminoethyl)-5,6-dichloroisoi ndoline-1,3-dione (10)
2-((2-Aminoethyl)carbamoyl)-4,5-dichlorobenzoic Acid (10-minor)
4.1.5. General Procedure for Preparation of 11 and 12
2-((2-Carboxy-4-methylphenyl)carbamoyl)-4,5-dichlorobenzoic Acid (11)
3-(2-Carboxy-4,5-dichlorobenzamido)-2-naphthoic Acid (12)
4.2. Computational Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Petersson, M.J.; Marchal, C.; Loughlin, W.A.; Jenkins, I.D.; Healy, P.C.; Almesåker, A. Unexpected regiospecific reactivity of a substituted phthalic anhydride. Tetrahedron 2007, 63, 1395–1401. [Google Scholar] [CrossRef]
- Fatima, A.; Khanum, G.; Sharma, A.; Garima, K.; Savita, S.; Verma, I.; Siddiqui, N.; Javed, S. Computational, Spectroscopic, Hirshfeld surface, Electronic state and Molecular docking studies on Phthalic anhydride. J. Mol. Struct. 2021, 1249, 131571. [Google Scholar] [CrossRef]
- Pessoa, C.; Ferreira, P.M.P.; Lotufo, L.V.C.; de Moraes, M.O.; Cavalcanti, S.M.T.; Coêlho, L.C.D.; Hernandes, M.Z.; Leite, A.C.L.; De Simone, C.A.; Costa, V.M.A. Inside Cover: Discovery of Phthalimides as Immunomodulatory and Antitumor Drug Prototypes (ChemMedChem 4/2010). ChemMedChem Chem. Enabling Drug Discov. 2010, 5, 486. [Google Scholar] [CrossRef]
- Sun, Y.-N.; Wang, C.-L.; Zhang, N.; Wang, Z.; Liu, Z.-L.; Liu, J.-L. Synthesis of tetrahydro-β-carbolines from phthalic anhydrides and tryptamine. Chin. Chem. Lett. 2014, 25, 1503–1506. [Google Scholar] [CrossRef]
- Noirbent, G.; Dumur, F. Recent advances on naphthalic anhydrides and 1, 8-naphthalimide-based photoinitiators of polymerization. Eur. Polym. J. 2020, 132, 109702. [Google Scholar] [CrossRef]
- Rzycka-Korzec, R.; Malarz, K.; Gawecki, R.; Mrozek-Wilczkiewicz, A.; Małecki, J.G.; Schab-Balcerzak, E.; Korzec, M.; Polanski, J. Effect of the complex-formation ability of thiosemicarbazones containing (aza) benzene or 3-nitro-1, 8-naphthalimide unit towards Cu (II) and Fe (III) ions on their anticancer activity. J. Photochem. Photobiol. A Chem. 2021, 415, 113314. [Google Scholar] [CrossRef]
- Nekvinda, J.; Różycka, D.; Rykowski, S.; Wyszko, E.; Fedoruk-Wyszomirska, A.; Gurda, D.; Orlicka-Płocka, M.; Giel-Pietraszuk, M.; Kiliszek, A.; Rypniewski, W. Synthesis of naphthalimide-carborane and metallacarborane conjugates: Anticancer activity, DNA binding ability. Bioorg. Chem. 2020, 94, 103432. [Google Scholar] [CrossRef]
- Al-Salahi, R.; Alswaidan, I.; Ghabbour, H.A.; Ezzeldin, E.; Elaasser, M.; Marzouk, M. Docking and antiherpetic activity of 2-aminobenzo [de]-isoquinoline-1, 3-diones. Molecules 2015, 20, 5099–5111. [Google Scholar] [CrossRef] [Green Version]
- Al-Salahi, R.; Marzouk, M. Synthesis of novel 2-amino-benzo[de]isoquinolin-1,3-dione derivatives. Asian J. Chem. 2014, 26, 2166–2172. [Google Scholar] [CrossRef]
- Homsi, A.; Kasideh, A. Synthesis of some N-phthalimide amino acids derivatives and Evaluation their Biological Activity. Synthesis (Stuttg) 2015, 8, 1817–1825. [Google Scholar]
- Al-Salahi, R.; Alswaidan, I.; Marzouk, M. Cytotoxicity evaluation of a new set of 2-aminobenzo[de]iso-quinoline-1,3-diones. Int. J. Mol. Sci. 2014, 15, 22483–22491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arya, S.; Kumar, A.; Kumar, N.; Roy, P.; Sondhi, S.M. Synthesis and anticancer activity evaluation of some acridine derivatives. Med. Chem. Res. 2015, 24, 1942–1951. [Google Scholar] [CrossRef]
- Al-Salahi, R.; Marzouk, M.S. Some 2-Amino-benzo[de]isoquinolin-1,3-diones as Antimicrobial Agents. Asian J. Chem. 2014, 26, 8163–8165. [Google Scholar] [CrossRef]
- Abuelizz, H.A.; Awad, H.M.; Marzouk, M.; Nasr, F.A.; Bakheit, A.H.; Naglah, A.M.; Al-Shakliah, N.S.; Al-Salahi, R. Exploiting the 4-Hydrazinobenzoic acid moiety for the development of anticancer agents: Synthesis and biological profile. Bioorg. Chem. 2020, 102, 104098. [Google Scholar] [CrossRef] [PubMed]
- Al-Salahi, R.; Elsayed, E.A.; Rabab, A.; Wadaan, M.; Ezzeldin, E.; Marzouk, M. Cytotoxicity of new 5-hydrazono-[1,2,4] triazolo [1, 5-a] quinazolines (Part II). Lat. Am. J. Pharm 2016, 35, 66–73. [Google Scholar]
- Al-Salahi, R.; Al-Omar, M.; Marzouk, M.; Ng, S.W. N-[2,4-Dioxo-3-azatricyclo-[7.3.1.05,13]trideca-1(13),5,7,9,11- pentaen-3-yl]thiourea. Acta Crystallogr. Sect. E Struct. Rep. Online 2012, 68, o1811. [Google Scholar] [CrossRef]
- Sankhe, S.; Chindarkar, N. Synthesis, Characterization and Preliminary Biological Evaluation of New 3 and 4-nitro Isoindoline-1, 3-dione/phthalimide Analogues. J. Pharm. Res. Int. 2021, 33, 20–27. [Google Scholar] [CrossRef]
- Salih, B.D.; Dalaf, A.H.; Alheety, M.A.; Rashed, W.M.; Abdullah, I.Q. Biological activity and laser efficacy of new Co (II), Ni (II), Cu (II), Mn (II) and Zn (II) complexes with phthalic anhydride. Mater. Today Proc. 2021, 43, 869–874. [Google Scholar] [CrossRef]
- Silva, S.C.C.C.; Braz, E.M.A.; Brito, C.A.R.S.; Alves, M.M.M.; Carvalho, F.A.A.; Barreto, H.M.; Oliveira, A.L.; Silva, D.A.; Silva-Filho, E.C. Phthalic anhydride esterified chicha gum: Characterization and antibacterial activity. Carbohydr. Polym. 2021, 251, 117077. [Google Scholar] [CrossRef]
- Jelali, H.; Mansour, L.; Deniau, E.; Sauthier, M.; Hamdi, N. An Efficient Synthesis of Phthalimides and Their Biological Activities. Polycycl. Aromat. Compd. 2020, 42, 1806–1813. [Google Scholar] [CrossRef]
- Harb, Z. Theoretical Determination of NMR Parameters of Metabolites and Proteins. Ph.D. Thesis, Université Claude Bernard-Lyon I, Lyon, Farance, 2011. [Google Scholar]
- Demir Kanmazalp, S.; Başaran, E.; Karaküçük-Iyidoğan, A.; Oruç-Emre, E.E.; Şen, F.; Dege, N. Synthesis, characterization, spectroscopy, X-ray structure and gaussian hybrid computational investigation of (−)-(S)-1-[2-(benzenesulfonamido)-3-phenylpropanoyl]-4-[(4-methyl) phenyl] thiosemicarbazide. Phosphorus. Sulfur. Silicon Relat. Elem. 2018, 193, 675–684. [Google Scholar] [CrossRef]
- Becke, A.D. A new mixing of Hartree–Fock and local density-functional theories. J. Chem. Phys. 1993, 98, 1372–1377. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09 Revis; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Govindasamy, P.; Gunasekaran, S. Experimental and theoretical studies of (FT-IR, FT-Raman, UV–Visible and DFT) 4-(6-methoxynaphthalen-2-yl) butan-2-one. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 149, 800–811. [Google Scholar] [CrossRef]
- Jamroz, M.H. Vibrational Energy Distribution Analysis VEDA 4, Warsaw, 2004–2010. Spectrochim Acta A 2013, 114, 220–230. [Google Scholar]
- Abuelizz, H.A.; Soliman, S.M.; Ghabbour, H.A.; Marzouk, M.; Abdellatif, M.M.; Al-Salahi, R. DFT Calculation, Hirshfeld Analysis and X-ray Crystal Structure of Some Synthesized N-alkylated (S-alkylated)-[1,2,4] triazolo [1,5-a] quinazolines. Crystals 2021, 11, 1195. [Google Scholar] [CrossRef]
- Abuelizz, H.A.; Taie, H.A.A.; Bakheit, A.H.; Marzouk, M.; Abdellatif, M.M.; Al-Salahi, R. Biological Evaluation of 4-(1H-triazol-1-yl) benzoic Acid Hybrids as Antioxidant Agents: In Vitro Screening and DFT Study. Appl. Sci. 2021, 11, 11642. [Google Scholar] [CrossRef]
- Abuelizz, H.A.; Taie, H.A.A.; Bakheit, A.H.; Mostafa, G.A.E.; Marzouk, M.; Rashid, H.; Al-Salahi, R. Investigation of 4-Hydrazinobenzoic Acid Derivatives for Their Antioxidant Activity: In Vitro Screening and DFT Study. ACS Omega 2021, 6, 31993–32004. [Google Scholar] [CrossRef]
- Perdew, J.P.; Parr, R.G.; Levy, M.; Balduz, J.L., Jr. Density-functional theory for fractional particle number: Derivative discontinuities of the energy. Phys. Rev. Lett. 1982, 49, 1691. [Google Scholar] [CrossRef]
- Parr, R.G.; Szentpály, L.V.; Liu, S. Electrophilicity index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Janak, J.F. Proof that∂ E∂ n i= ε in density-functional theory. Phys. Rev. B 1978, 18, 7165. [Google Scholar] [CrossRef]
- Pearson, R.G. Absolute electronegativity and hardness correlated with molecular orbital theory. Proc. Natl. Acad. Sci. USA 1986, 83, 8440–8441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukui, K. The role of frontier orbitals in chemical reactions (Nobel Lecture). Angew. Chemie Int. Ed. Engl. 1982, 21, 801–809. [Google Scholar] [CrossRef]

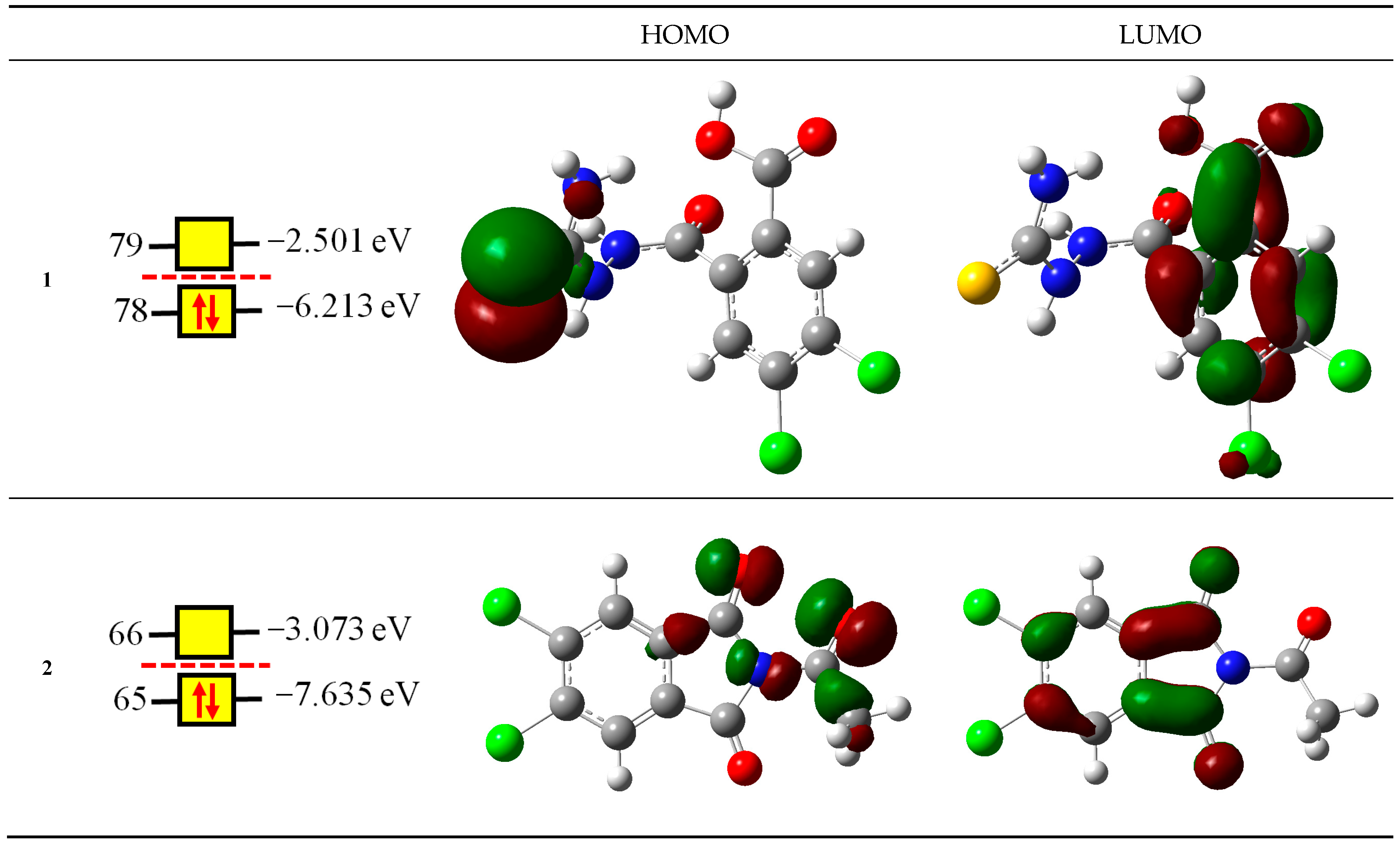
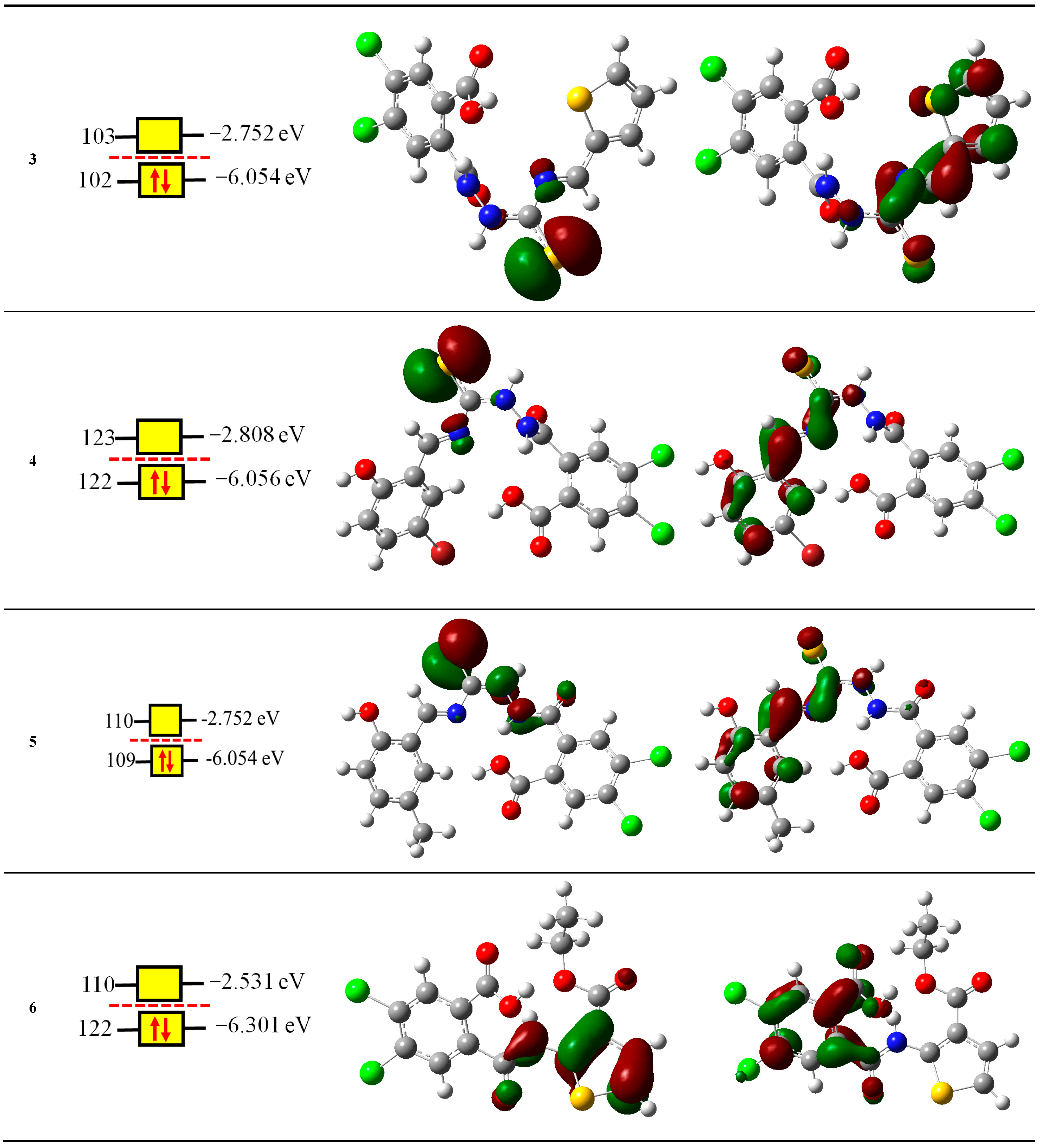
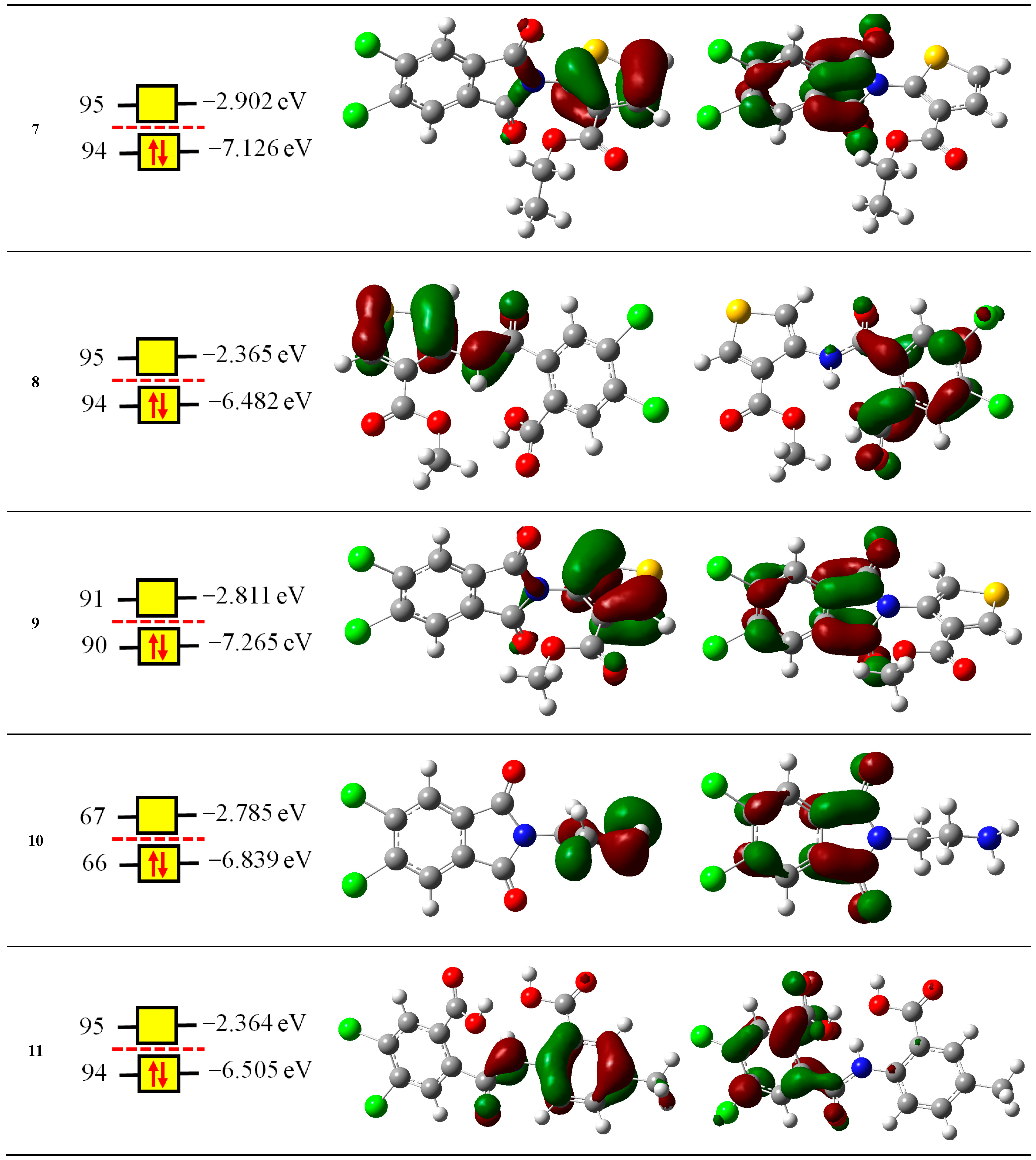
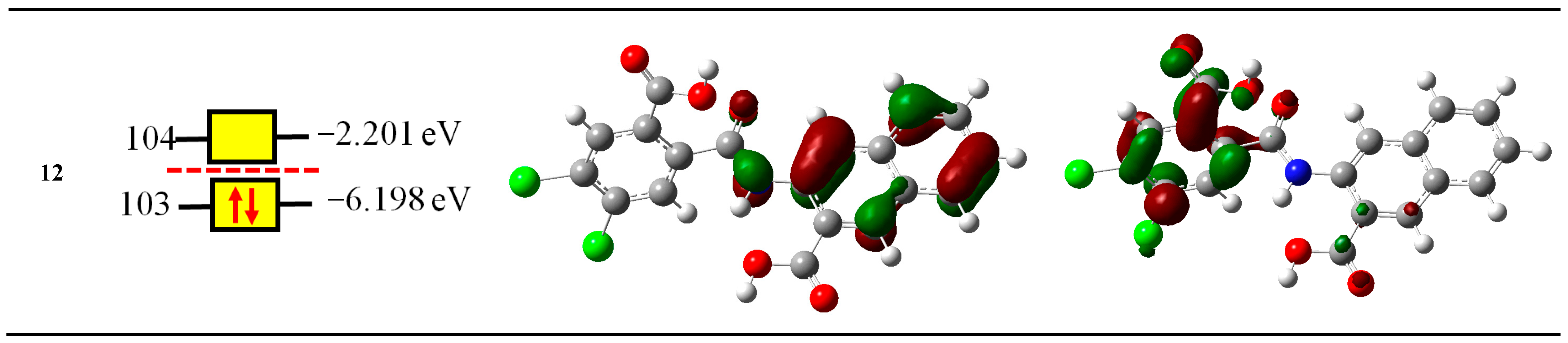
| Compounds | NOMO | IP eV | EA eV | χ eV | μ eV | η eV | S eV−1 | ω eV | N eV |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 78 | 8.3026 | 0.8264 | 4.5645 | −4.5645 | 7.4761 | 0.1338 | 1.3934 | 2.9079 |
| 2 | 65 | 9.3286 | 1.3546 | 5.3416 | −5.3416 | 7.9740 | 0.1254 | 1.7891 | 1.4862 |
| 3 | 102 | 7.8832 | 1.3558 | 4.6195 | −4.6195 | 6.5274 | 0.1532 | 1.6346 | 3.0676 |
| 4 | 122 | 7.8169 | 1.4795 | 4.6482 | −4.6482 | 6.3373 | 0.1578 | 1.7046 | 3.0646 |
| 5 | 109 | 7.5565 | 1.5188 | 4.5376 | −4.5376 | 6.0377 | 0.1656 | 1.7051 | 3.1522 |
| 6 | 99 | 7.9733 | 1.0535 | 4.5134 | −4.5134 | 6.9198 | 0.1445 | 1.4719 | 2.82 |
| 7 | 94 | 8.7139 | 1.2813 | 4.9976 | −4.9976 | 7.4326 | 0.1345 | 1.6801 | 1.9955 |
| 8 | 95 | 8.1561 | 0.8204 | 4.4882 | −4.4882 | 7.3357 | 0.1363 | 1.373 | 2.6394 |
| 9 | 90 | 8.7966 | 1.1712 | 4.9839 | −4.9839 | 7.6254 | 0.1311 | 1.6287 | 1.8566 |
| 10 | 66 | 8.7233 | 1.0662 | 4.8947 | −4.8947 | 7.657 | 0.1306 | 1.5645 | 2.2821 |
| 11 | 94 | 8.094 | 0.95 | 4.522 | −4.522 | 7.144 | 1.4312 | 2.6159 | 8.094 |
| 12 | 103 | 7.7296 | 0.9403 | 4.335 | −4.335 | 6.7893 | 0.1473 | 1.3839 | 2.9233 |
| Calculated Wavenumber (cm−1) B3LYP/6–311G(d, p) | Experimental | Vibrational Band Assignment (% PED) | |
|---|---|---|---|
| Un scaled | Scaled | υ(IR) | |
| 470 | 451 | 434.98 | (64) τHCCC |
| 579 | 556 | 521.94 | (10) βCCC+CCO |
| 615 | 591 | 587.74 | (59) βCCCl+CCO+CNC |
| 632 | 607 | 617.25 | (30) βCCC |
| 766 | 736 | 733.31 | (29) βCCC |
| 794 | 762 | 770.25 | (10) βOCC |
| 875 | 840 | 802.19 | (43) βCCC+CCCl+CCO |
| 932 | 895 | 896.47 | (13) βHCH |
| 964 | 926 | 977.47 | (66) βHCH |
| 1105 | 1061 | 1041.79 | (11) βHCC |
| 1148 | 1102 | 1096.95 | (12) βCCCl+CCC+CCO |
| 1278 | 1228 | 1211.72 | (16) ʋNC |
| 1334 | 1281 | 1256.44 | (35) ʋCC+NC |
| 1395 | 1340 | 1309.31 | (54) ʋCC+NC |
| 1463 | 1405 | 1373.9 | (44) ʋC=C+NC |
| 1483 | 1424 | 1427.28 | (70) ʋCC |
| 1634 | 1569 | 1606.07 | (11) ʋCC |
| 1814 | 1742 | 1727.62 | (76) ʋOC |
| 1870 | 1796 | 1796.96 | (76) ʋOC |
| 3060 | 2938 | 2934.53 | (95) ʋCH3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abuelizz, H.A.; Bakheit, A.H.; Marzouk, M.; Abdellatif, M.M.; Al-Salahi, R. Reactivity of 4,5-Dichlorophthalic Anhydride towards Thiosemicarbazide and Amines: Synthesis, Spectroscopic Analysis, and DFT Study. Molecules 2022, 27, 3550. https://doi.org/10.3390/molecules27113550
Abuelizz HA, Bakheit AH, Marzouk M, Abdellatif MM, Al-Salahi R. Reactivity of 4,5-Dichlorophthalic Anhydride towards Thiosemicarbazide and Amines: Synthesis, Spectroscopic Analysis, and DFT Study. Molecules. 2022; 27(11):3550. https://doi.org/10.3390/molecules27113550
Chicago/Turabian StyleAbuelizz, Hatem A., Ahmed H. Bakheit, Mohamed Marzouk, Mohamed M. Abdellatif, and Rashad Al-Salahi. 2022. "Reactivity of 4,5-Dichlorophthalic Anhydride towards Thiosemicarbazide and Amines: Synthesis, Spectroscopic Analysis, and DFT Study" Molecules 27, no. 11: 3550. https://doi.org/10.3390/molecules27113550
APA StyleAbuelizz, H. A., Bakheit, A. H., Marzouk, M., Abdellatif, M. M., & Al-Salahi, R. (2022). Reactivity of 4,5-Dichlorophthalic Anhydride towards Thiosemicarbazide and Amines: Synthesis, Spectroscopic Analysis, and DFT Study. Molecules, 27(11), 3550. https://doi.org/10.3390/molecules27113550







