Synthesis of Cyclic Fragrances via Transformations of Alkenes, Alkynes and Enynes: Strategies and Recent Progress
Abstract
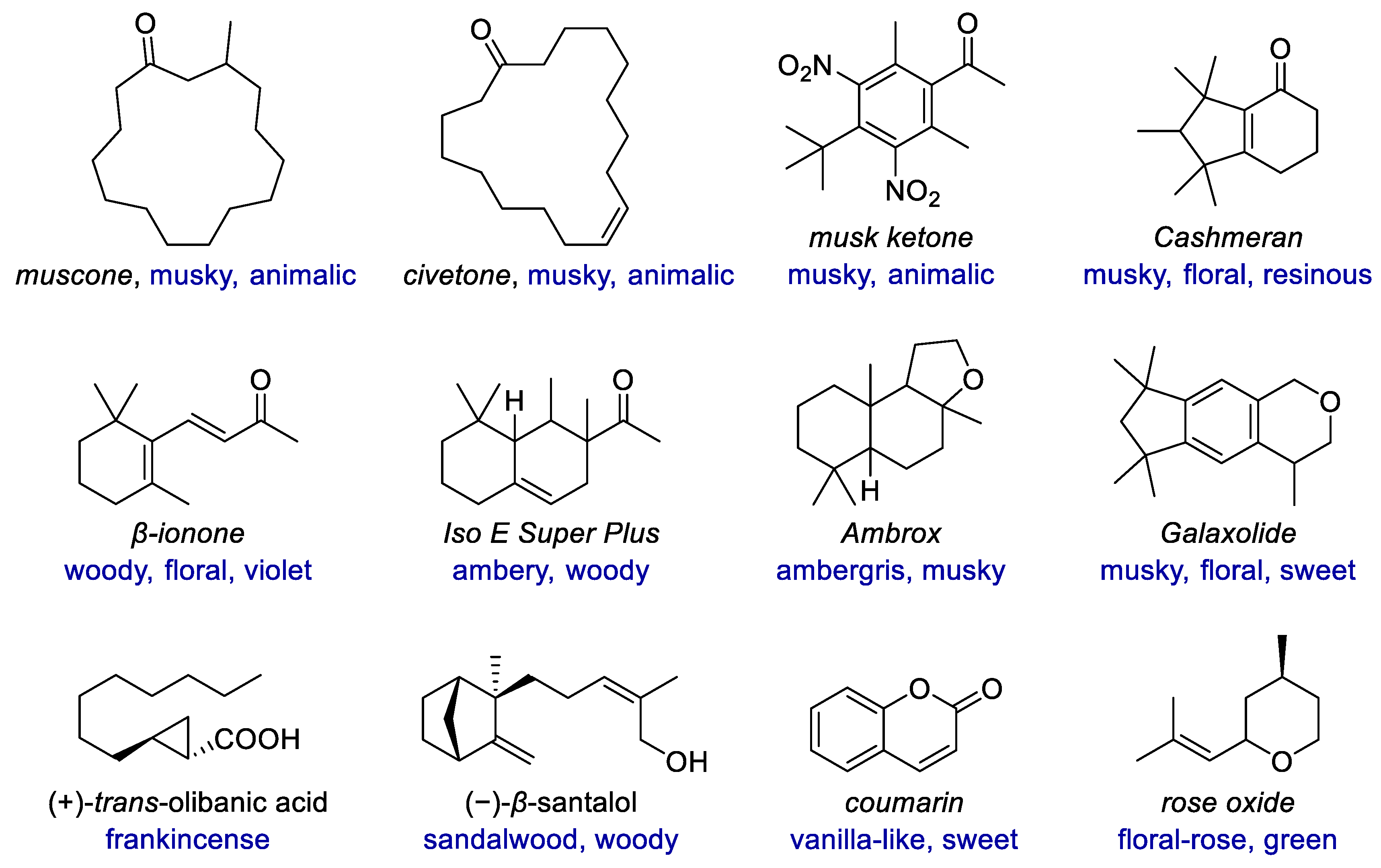
:1. Introduction
2. Background of the Olfactory System and Fragrance Chemistry
3. Synthesis of Fragrances via Cycloaddition or Formal Cycloaddition
4. Synthesis of Fragrances via Ring-Closing Metathesis
5. Synthesis of Fragrances via Rearrangement or Isomerization
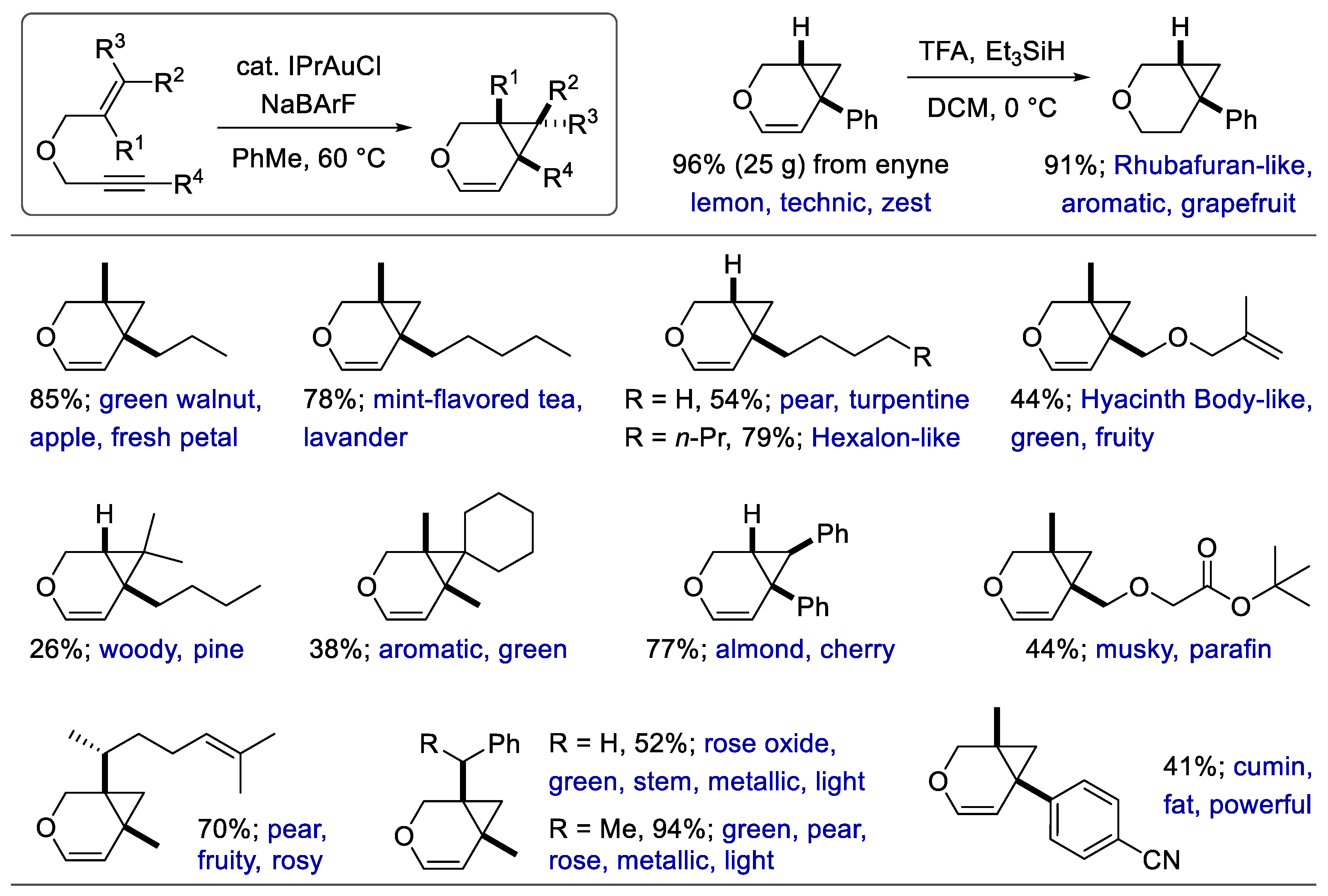
6. Synthesis of Heterocyclic Fragrances via Addition-Type Reactions
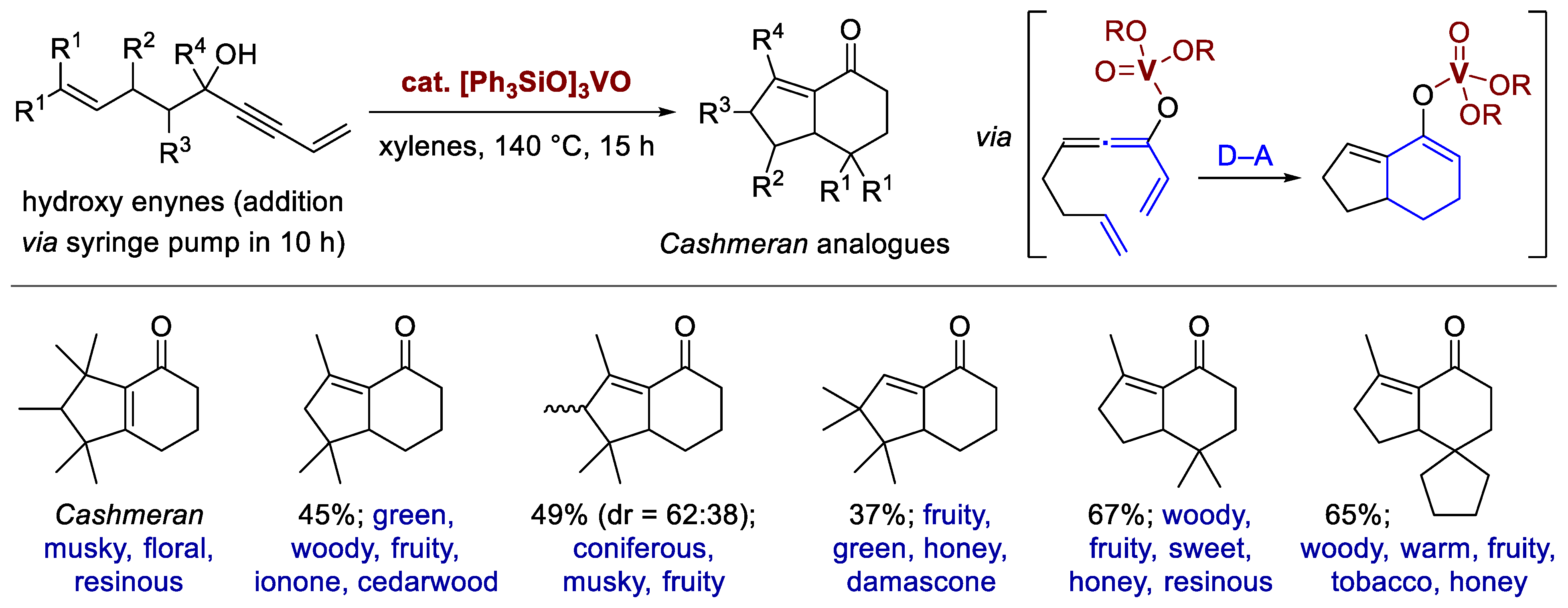
7. Synthesis of Fragrances via the Cascade Cyclization of Enynes
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schilling, B.; Kaiser, R.; Natsch, A.; Gautschi, M. Investigation of Odors in the Fragrance Industry. Chemoecology 2010, 20, 135–147. [Google Scholar] [CrossRef]
- Armanino, N.; Charpentier, J.; Flachsmann, F.; Goeke, A.; Liniger, M.; Kraft, P. What’s Hot, What’s Not: The Trends of the Past 20 Years in the Chemistry of Odorants. Angew. Chem. Int. Ed. 2020, 59, 16310–16344. [Google Scholar] [CrossRef] [PubMed]
- Kliszcz, A.; Danel, A.; Puła, J.; Barabasz-Krasny, B.; Możdżeń, K. Fleeting Beauty—The World of Plant Fragrances and Their Application. Molecules 2021, 26, 2473. [Google Scholar] [CrossRef] [PubMed]
- Sharmeen, J.B.; Mahomoodally, F.M.; Zengin, G.; Maggi, F. Essential Oils as Natural Sources of Fragrance Compounds for Cosmetics and Cosmeceuticals. Molecules 2021, 26, 666. [Google Scholar] [CrossRef] [PubMed]
- Firestein, S. How the olfactory system makes sense of scents. Nature 2001, 413, 211–218. [Google Scholar] [CrossRef]
- Sell, C.S. On the unpredictability of odor. Angew. Chem. Int. Ed. 2006, 45, 6254–6261. [Google Scholar] [CrossRef]
- Bushdid, C.; Magnasco, M.O.; Vosshall, L.B.; Keller, A. Humans Can Discriminate More than 1 Trillion Olfactory Stimuli. Science 2014, 343, 1370–1372. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, M.A.; Barrault, L.; Rodriguez, O.; Carvalho, C.C.; Rodrigues, A.E. Perfumery Radar 2.0: A Step toward Fragrance Design and Classification. Ind. Eng. Chem. Res. 2014, 53, 8890–8912. [Google Scholar] [CrossRef]
- Keller, A.; Gerkin, R.C.; Guan, Y.; Dhurandhar, A.; Turu, G.; Szalai, B.; Mainland, J.D.; Ihara, Y.; Yu, C.W.; Wolfinger, R.; et al. Predicting human olfactory perception from chemical features of odor molecules. Science 2017, 355, 820–826. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez, E.D.; Dhurandhar, A.; Keller, A.; Meyer, P.; Cecchi, G.A. Predicting natural language descriptions of mono- molecular odorants. Nat. Commun. 2018, 9, 4979. [Google Scholar] [CrossRef]
- Genva, M.; Kenne Kemene, T.; Deleu, M.; Lins, L.; Fauconnier, M.L. Is It Possible to Predict the Odor of a Molecule on the Basis of its Structure? Int. J. Mol. Sci. 2019, 20, 3018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravia, A.; Snitz, K.; Honigstein, D.; Finkel, M.; Zirler, R.; Perl, O.; Secundo, L.; Laudamiel, C.; Harel, D.; Sobel, N. A measure of smell enables the creation of olfactory metamers. Nature 2020, 588, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Kraft, P.; Di Cristofaro, V.; Jordi, S. From Cassyrane to Cashmeran—The Molecular Parameters of Odorants. Chem. Biodivers. 2014, 11, 1567–1596. [Google Scholar] [CrossRef] [PubMed]
- Pluskal, T.; Weng, J.K. Natural product modulators of human sensations and mood: Molecular mechanisms and therapeutic potential. Chem. Soc. Rev. 2018, 47, 1592–1637. [Google Scholar] [CrossRef]
- Block, E. Molecular Basis of Mammalian Odor Discrimination: A Status Report. J. Agric. Food Chem. 2018, 66, 13346–13366. [Google Scholar] [CrossRef]
- Bierling, A.L.; Croy, I.; Hummel, T.; Cuniberti, G.; Croy, A. Olfactory Perception in Relation to the Physicochemical Odor Space. Brain Sci. 2021, 11, 563. [Google Scholar] [CrossRef]
- Sharma, A.; Saha, B.K.; Kumar, R.; Varadwaj, P.K. OlfactionBase: A repository to explore odors, odorants, olfactory receptors and odorant–receptor interactions. Nucleic Acids Res. 2022, 50, D678–D686. [Google Scholar] [CrossRef]
- Burger, P.; Plainfosse, H.; Brochet, X.; Chemat, F.; Fernandez, X. Extraction of Natural Fragrance Ingredients: History Overview and Future Trends. Chem. Biodivers. 2019, 16, e1900424. [Google Scholar] [CrossRef]
- Baldovini, N.; Chaintreau, A. Identification of key odorants in complex mixtures occurring in nature. Nat. Prod. Rep. 2020, 37, 1589–1626. [Google Scholar] [CrossRef]
- Chapuis, C.; Jacoby, D. Catalysis in the preparation of fragrances and flavours. Appl. Catal. A 2001, 221, 93–117. [Google Scholar] [CrossRef]
- Brenna, E.; Fuganti, C.; Serra, S. Enantioselective perception of chiral odorants. Tetrahedron Asymmetry 2003, 14, 1–42. [Google Scholar] [CrossRef]
- Sell, C.S. Scent through the Looking Glass. Chem. Biodivers. 2004, 1, 1899–1920. [Google Scholar] [CrossRef] [PubMed]
- Saudan, L.A. Hydrogenation Processes in the Synthesis of Perfumery Ingredients. Acc. Chem. Res. 2007, 40, 1309–1319. [Google Scholar] [CrossRef] [PubMed]
- Coulombel, L.; Grau, F.; Weiwer, M.; Favier, I.; Chaminade, X.; Heumann, A.; Bayon, J.C.; Aguirre, P.A.; Duñach, E. Lewis Super-Acid Catalyzed Cyclizations: A New Route to Fragrance Compounds. Chem. Biodivers. 2008, 5, 1070–1082. [Google Scholar] [CrossRef]
- Ciappa, A.; Bovo, S.; Bertoldini, M.; Scrivanti, A.; Matteoli, U. Homogeneous Asymmetric Catalysis in Fragrance Chemistry. Chem. Biodivers. 2008, 5, 1058–1069. [Google Scholar] [CrossRef]
- Brenna, E.; Fuganti, C.; Gatti, F.G.; Serra, S. Biocatalytic Methods for the Synthesis of Enantioenriched Odor Active Compounds. Chem. Rev. 2011, 111, 4036–4072. [Google Scholar] [CrossRef]
- Brenna, M.E.; Fuganti, C. Recent advances in the synthesis of fragrances. Curr. Org. Chem. 2011, 15, 987–1005. [Google Scholar] [CrossRef]
- Lemiere, G.; Dunach, E. Catalytic Activation of Olefins by Metal Triflates and Triflimides: Application to Fragrance Chemistry. Chem. Eur. J. 2013, 19, 3270–3280. [Google Scholar] [CrossRef]
- Narula, A.P.S. The Search for New Amber Ingredients. Chem. Biodivers. 2014, 11, 1629–1638. [Google Scholar] [CrossRef]
- Gusevskaya, E.V.; Jiménez-Pinto, J.; Börner, A. Hydroformylation in the Realm of Scents. ChemCatChem. 2014, 6, 382–411. [Google Scholar] [CrossRef]
- Schröder, F. Present and Future of Cyclopropanations in Fragrance Chemistry. Chem. Biodivers. 2014, 11, 1734–1751. [Google Scholar] [CrossRef] [PubMed]
- Doro, F.; Akeroyd, N.; Schiet, F.; Narula, A. The Prins Reaction in the Fragrance Industry: 100th Anniversary (1919–2019). Angew. Chem. Int. Ed. 2019, 58, 7174–7179. [Google Scholar] [CrossRef] [PubMed]
- Stepanyuk, A.; Kirschning, A. Synthetic Terpenoids in the World of Fragrances: Iso E Super® is the Showcase. Beilstein J. Org. Chem. 2019, 15, 2590–2602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- David, O.R.P. A chemical history of polycyclic musks. Chem. Eur. J. 2020, 26, 7537–7555. [Google Scholar] [CrossRef]
- Sytniczuk, A.; Milewski, M.; Kajetanowicz, A.; Grela, K. Preparation of macrocyclic musks via olefin metathesis: Comparison with classical syntheses and recent advances. Russ. Chem. Rev. 2020, 89, 469. [Google Scholar] [CrossRef]
- Firda, P.B.D.; Bahruji, H.; Bakar, M.B. Review on heterogeneous catalysts for the synthesis of perfumery chemicals via isomerization, acetalization and hydrogenation. Flavour Fragr. J. 2021, 36, 509–525. [Google Scholar] [CrossRef]
- Buck, L.; Axel, R. A Novel Multigene Family May Encode Odorant Receptors: A Molecular basis for Odor Recognition. Cell 1991, 65, 175–187. [Google Scholar] [CrossRef]
- Mori, K.; Nagao, H.; Yoshihara, Y. The olfactory bulb: Coding and processing of odor molecule information. Science 1999, 286, 711–715. [Google Scholar] [CrossRef] [Green Version]
- Malnic, B.; Hirono, J.; Sato, T.; Buck, L.B. Combinatorial Receptor Codes for Odors. Cell 1999, 96, 713–723. [Google Scholar] [CrossRef] [Green Version]
- Araneda, R.C.; Kini, A.D.; Firestein, S. The molecular receptive range of an odorant receptor. Nat. Neurosci. 2000, 3, 1248–1255. [Google Scholar] [CrossRef] [Green Version]
- Mombaerts, P. Genes and ligands for odorant, vomeronasal and taste receptors. Nat. Rev. Neurosci. 2004, 5, 263–278. [Google Scholar] [CrossRef] [PubMed]
- Triller, A.; Boulden, E.A.; Churchill, A.; Hatt, H.; Englund, J.; Spehr, M.; Sell, C.S. Odorant-receptor interactions and odor percept: A chemical perspective. Chem. Biodivers. 2008, 5, 862–886. [Google Scholar] [CrossRef] [PubMed]
- Mainland, J.D.; Li, Y.R.; Zhou, T.; Liu, W.L.L.; Matsunami, H. Human olfactory receptor responses to odorants. Sci. Data 2015, 2, 150002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behrens, M.; Briand, L.; de March, C.A.; Matsunami, H.; Yamashita, A.; Meyerhof, W.; Weyand, S. Structure-Function Relationships of Olfactory and Taste Receptors. Chem. Senses. 2018, 43, 81–87. [Google Scholar] [CrossRef]
- Rugard, M.; Jaylet, T.; Taboureau, O.; Tromelin, A.; Audouze, K. Smell compounds classification using UMAP to increase knowledge of odors and molecular structures linkages. PLoS ONE. 2021, 16, e0252486. [Google Scholar] [CrossRef]
- Si, G.; Kanwal, J.K.; Hu, Y.; Tabone, C.J.; Baron, J.; Berck, M.; Vignoud, G.; Samuel, A.D. Structured odorant response patterns across a complete olfactory receptor neuron population. Neuron 2019, 101, 950–962. [Google Scholar] [CrossRef] [Green Version]
- Manzini, I.; Schild, D.; Di Natale, C. Principles of odor coding in vertebrates and artificial chemosensory systems. Physiol Rev. 2022, 102, 61–154. [Google Scholar] [CrossRef]
- Cong, X.; Ren, W.; Pacalon, J.; Xu, R.; Xu, L.; Li, X.; de March, C.A.; Matsunami, H.; Yu, H.; Yu, Y.; et al. Large-Scale G Protein-Coupled Olfactory Receptor–Ligand Pairing. ACS Cent. Sci. 2022, 8, 379–387. [Google Scholar] [CrossRef]
- Shirasu, M.; Yoshikawa, K.; Takai, Y.; Nakashima, A.; Takeuchi, H.; Sakano, H.; Touhara, K. Olfactory Receptor and Neural Pathway Responsible for Highly Selective Sensing of Musk Odors. Neuron 2014, 81, 165–178. [Google Scholar] [CrossRef] [Green Version]
- Sato-Akuhara, N.; Horio, N.; Kato-Namba, A.; Yoshikawa, K.; Niimura, Y.; Ihara, S.; Shirasu, M.; Touhara, K. Ligand Specificity and Evolution of Mammalian Musk Odor Receptors: Effect of Single Receptor Deletion on Odor Detection. J. Neurosci. 2016, 36, 4482–4491. [Google Scholar] [CrossRef] [Green Version]
- Jovancevic, N.; Khalfaoui, S.; Weinrich, M.; Weidinger, D.; Simon, A.; Kalbe, B.; Kernt, M.; Kampik, A.; Gisselmann, G.; Gelis, L.; et al. Odorant receptor 51E2 agonist β-ionone regulates RPE cell migration and proliferation. Front. Physiol. 2017, 8, 888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aloum, L.; Alefishat, E.; Adem, A.; Petroianu, G. Ionone is more than a violet’s fragrance: A review. Molecules 2020, 25, 5822. [Google Scholar] [CrossRef] [PubMed]
- Funel, J.A.; Abele, S. Industrial Applications of the Diels-Alder Reaction. Angew. Chem. Int. Ed. 2013, 52, 3822–3863. [Google Scholar] [CrossRef] [PubMed]
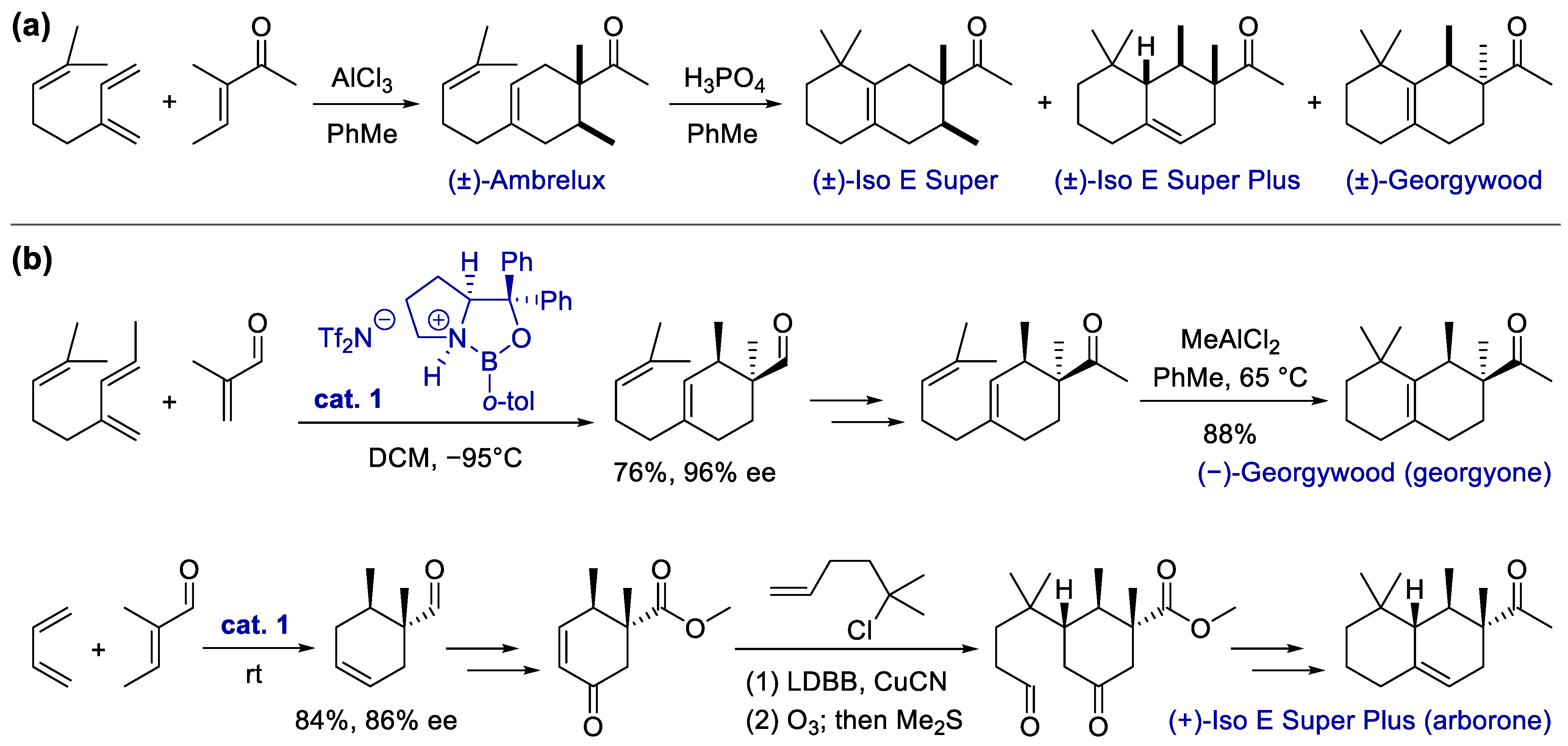
- Hong, S.; Corey, E.J. Enantioselective Syntheses of Georgyone, Arborone, and Structural Relatives. Relevance to the Molecular-Level Understanding of Olfaction. J. Am. Chem. Soc. 2006, 128, 1346–1352. [Google Scholar] [CrossRef] [PubMed]
- Fráter, G.; Müller, U.; Schröder, F. Synthesis and olfactory properties of (−)-(1R,2S)-Georgywood. Tetrahedron Asymmetry 2004, 15, 3967–3972. [Google Scholar] [CrossRef]
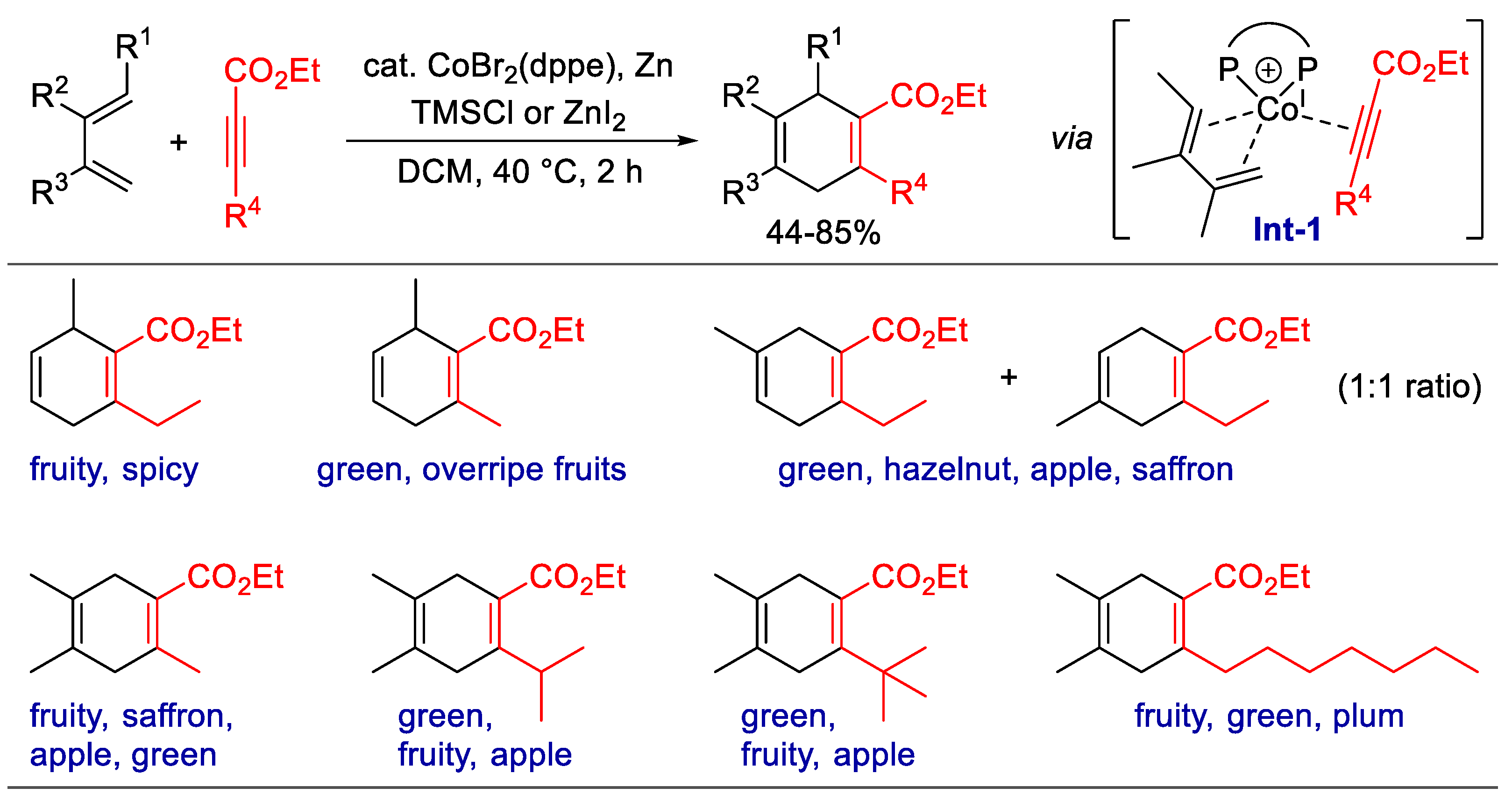
- Charpentier, J.; Voirol, F.; Flachsmann, F.; Tanner, S.; Aeberli, N.; Brunner, G.; Goeke, A. Synthesis of 1, 4-Cyclohexadiene Carboxylates through a Formal [2+4]-Cycloaddition of Propiolates under Cobalt Catalysis. Helv. Chim. Acta. 2020, 103, e2000175. [Google Scholar] [CrossRef]
- Bao, R.L.Y.; Yin, J.; Shi, L.; Zheng, L. Rh(I)-Catalyzed enantioselective and scalable [4+2] cycloaddition of 1,3-dienes with dialkyl acetylenedicarboxylates. Org. Biomol. Chem. 2020, 18, 2956–2961. [Google Scholar] [CrossRef]
- Schreyer, L.; Properzi, R.; List, B. IDPi Catalysis. Angew. Chem. Int. Ed. 2019, 58, 12761–12777. [Google Scholar] [CrossRef]
- Liu, L.; Kim, H.; Xie, Y.; Fares, C.; Kaib, P.S.J.; Goddard, R.; List, B. Catalytic Asymmetric [4+2]-Cycloaddition of Dienes with Aldehydes. J. Am. Chem. Soc. 2017, 139, 13656–13659. [Google Scholar] [CrossRef] [Green Version]
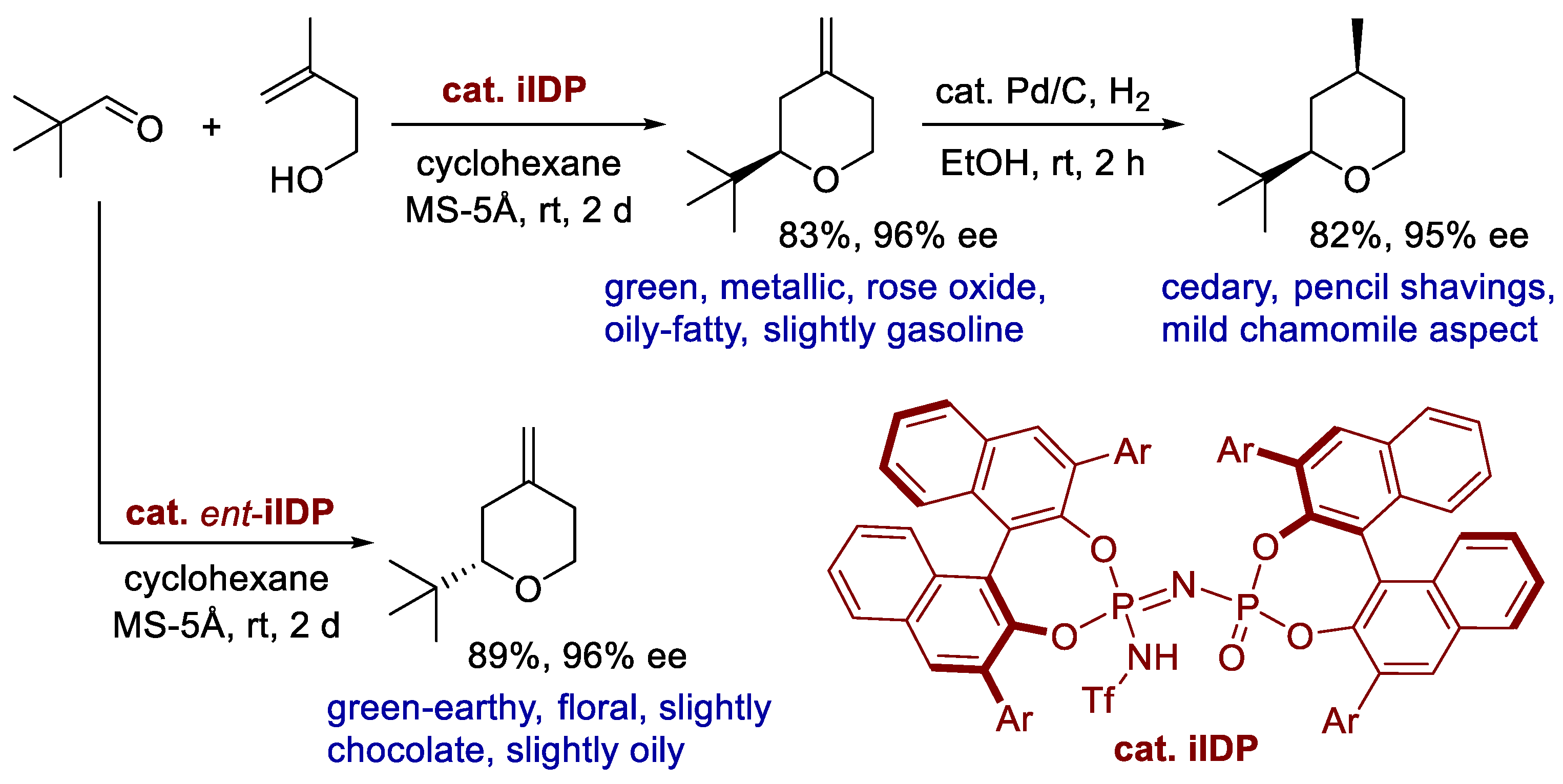
- Ouyang, J.; Bae, H.; Jordi, S.; Dao, Q.M.; Dossenbach, S.; Dehn, S.; Lingnau, J.B.; De, C.K.; Kraft, P.; List, B. The smelling principle of vetiver oil, unveiled by chemical synthesis. Angew. Chem. Int. Ed. 2021, 60, 5666–5672. [Google Scholar] [CrossRef]
- Gatzenmeier, T.; Kaib, P.S.J.; Lingnau, J.B.; Goddard, R.; List, B. The Catalytic Asymmetric Mukaiyama–Michael Reaction of Silyl Ketene Acetals with α,β-Unsaturated Methyl Esters. Angew. Chem. Int. Ed. 2018, 57, 2464–2468. [Google Scholar] [CrossRef] [PubMed]
- Dorrich, S.; Bauer, J.B.; Lorenzen, S.; Mahler, C.; Schweeberg, S.; Burschka, C.; Baus, J.A.; Tacke, R.; Kraft, P. Disila-galaxolide and derivatives: Synthesis and olfactory characterization of silicon-containing derivatives of the musk odorant galaxolide. Chem. Eur. J. 2013, 19, 11396–11408. [Google Scholar] [CrossRef] [PubMed]
- Metz, S.; Nätscher, J.B.; Burschka, C.; Götz, K.; Kaupp, M.; Kraft, P.; Tacke, R. Disila-Phantolide and Derivatives: Synthesis and Olfactory Characterization of Silicon-Containing Derivatives of the Musk Odorant Phantolide. Organometallics 2009, 28, 4700–4712. [Google Scholar] [CrossRef]
- Yang, F.; Jin, Y.; Wang, C. Nickel-Catalyzed Asymmetric Intramolecular Reductive Heck Reaction of Unactivated Alkenes. Org. Lett. 2019, 21, 6989–6994. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.-X.; Jia, Y.-X. Aromatic π-Components for Enantioselective Heck Reactions and Heck/Anion-Capture Domino Sequences. Acc. Chem. Res. 2022, 55, 734–745. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, Q.; Li, P.; Qu, Z.; Sun, S.; Ma, Y.; Su, D.; Zong, Y.; Zhang, J. Six-Membered Silacycle Odorants: Synthesis and Olfactory Characterization of Si Analogues of Artemone, β-Dynascone, and Herbac. Eur. J. Inorg. Chem. 2014, 2014, 3435–3440. [Google Scholar] [CrossRef]
- Liu, J.; Zou, Y.; Fan, W.; Mao, J.; Chai, G.; Li, P.; Qu, Z.; Zong, Y.; Zhang, J.; Kraft, P. Synthesis and Olfactory Properties of Silicon-Containing Analogs of Rosamusk, Romandolide, and Applelide: Insights into the Structural Parameters of Linear Alicyclic Musks. Eur. J. Org. Chem. 2016, 2016, 976–982. [Google Scholar] [CrossRef]
- Brunner, G.; Elmer, S.; Schröder, F. Transition-Metal-Catalyzed Cyclopropanation of Nonactivated Alkenes in Dibromomethane with Triisobutylaluminum. Eur. J. Org. Chem. 2011, 2011, 4623–4633. [Google Scholar] [CrossRef]
- Cerutti-Delasalle, C.; Mehiri, M.; Cagliero, C.; Rubiolo, P.; Bicchi, C.; Meierhenrich, U.J.; Baldovini, N. The (+)-cis- and (+)-trans-olibanic acids: Key odorants of frankincense. Angew. Chem. Int. Ed. 2016, 55, 13719–13723. [Google Scholar] [CrossRef]
- Ebner, C.; Carreira, E.M. Cyclopropanation Strategies in Recent Total Syntheses. Chem. Rev. 2017, 117, 11651–11679. [Google Scholar] [CrossRef]
- Knight, A.M.; Kan, S.B.J.; Lewis, R.D.; Brandenberg, O.F.; Chen, K.; Arnold, F.H. Diverse engineered heme proteins enable stereodivergent cyclopropanation of unactivated alkenes. ACS Cent. Sci. 2018, 4, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Gund, M.; Déry, M.; Amzallag, V.; Spino, C. Dialkoxycarbenes in (4+1) Cycloadditions: Application to the Synthesis of Carotol. Org. Lett. 2016, 18, 4280–4283. [Google Scholar] [CrossRef] [PubMed]
- Kula, J.; Izydorczyk, K.; Czajkowska, A.; Bonikowski, R. Chemical composition of carrot umbel oils from Daucus carota L. ssp. sativus cultivated in Poland. Flavour Fragr. J. 2006, 21, 667–669. [Google Scholar] [CrossRef]
- Sieniawska, E.; Swiatek, Ł.; Rajtar, B.; Kozioł, E.; Polz-Dacewicz, M.; Skalicka-Wozniak, K. Carrot seed essential oil—Source of carotol and cytotoxicity study. Ind. Crops Prod. 2016, 92, 109–115. [Google Scholar] [CrossRef]
- Sytniczuk, A.; Dąbrowski, M.; Banach, Ł.; Urban, M.; Czarnocka-Śniadała, S.; Milewski, M.; Kajetanowicz, A.; Grela, K. At Long Last: Olefin Metathesis Macrocyclization at High Concentration. J. Am. Chem. Soc. 2018, 140, 8895–8901. [Google Scholar] [CrossRef]
- Rana, R.; Shreya; Upadhyay, R.; Maurya, S.K. Synthesis of Macrocyclic Lactones and Dilactones Using Olive Oil. ACS Omega 2021, 6, 25381–25388. [Google Scholar] [CrossRef]
- Koh, M.J.; Khan, R.K.M.; Torker, S.; Yu, M.; Mikus, M.S.; Hoveyda, A.H. High-value alcohols and higher-oxidation-state compounds by catalytic Z-selective cross-metathesis. Nature 2015, 517, 181–186. [Google Scholar] [CrossRef]
- Sytniczuk, A.; Leszczyńska, A.; Kajetanowicz, A.; Grela, K. Preparation of Musk-Smelling Macrocyclic Lactones from Biomass: Looking for the Optimal Substrate Combination. Chem. Sus. Chem. 2018, 11, 3157–3166. [Google Scholar] [CrossRef]
- Sytniczuk, A.; Forcher, G.; Grotjahn, D.B.; Grela, K. Sequential Alkene Isomerization and Ring-Closing Metathesis in Production of Macrocyclic Musks from Biomass. Chem. Eur. J. 2018, 24, 10403–10408. [Google Scholar] [CrossRef]
- Monfette, S.; Eyholzer, M.; Roberge, D.M.; Fogg, D.E. Getting Ring-Closing Metathesis off the Bench: Reaction-Reactor Matching Transforms Metathesis Efficiency in the Assembly of Large Rings. Chem. Eur. J. 2010, 16, 11720–11725. [Google Scholar] [CrossRef]
- Gambacorta, G.; Sharley, J.S.; Baxendale, I.R. A comprehensive review of flow chemistry techniques tailored to the flavours and fragrances industries. Beilstein J. Org. Chem. 2021, 17, 1181–1312. [Google Scholar] [CrossRef] [PubMed]
- Morin, É.; Sosoe, J.; Raymond, M.; Amorelli, B.; Boden, R.M.; Collins, S.K. Synthesis of a Renewable Macrocyclic Musk: Evaluation of Batch, Microwave, and Continuous Flow Strategies. Org. Process. Res. Dev. 2019, 23, 283–287. [Google Scholar] [CrossRef]
- Wang, X.; Hong, P.; Kiss, A.A.; Wang, Q.; Li, L.; Wang, H.; Qiu, T. From batch to continuous sustainable production of 3-methyl-3-penten-2-one for synthetic ketone fragrances. ACS Sustain. Chem. Eng. 2020, 8, 17201–17214. [Google Scholar] [CrossRef]
- Morvan, J.; McBride, T.; Curbet, I.; Colombel-Rouen, S.; Roisnel, T.; Crévisy, C.; Browne, D.L.; Mauduit, M. Continuous Flow Z-stereoselective Olefin Metathesis: Development and Applications in the Synthesis of Pheromones and Macrocyclic Odorant Molecules. Angew. Chem. Int. Ed. 2021, 60, 19685–19690. [Google Scholar] [CrossRef]
- Callejo, R.; Corr, M.J.; Yang, M.; Wang, M.; Cordes, D.B.; Slawin, A.M.; O’Hagan, D. Fluorinated Musk Fragrances: The CF2 Group as a Conformational Bias Influencing the Odour of Civetone and (R)-Muscone. Chem. Eur. J. 2016, 22, 8137–8151. [Google Scholar] [CrossRef] [Green Version]
- Corr, M.J.; Cormanich, R.A.; von Hahmann, C.N.; Bühl, M.; Cordes, D.B.; Slawin, A.M.Z.; O’Hagan, D. Fluorine in Fragrances: Exploring the Difluoromethylene (CF2) Group as a Conformational Constraint in Macrocyclic Musk Lactones. Org. Biomol. Chem. 2016, 14, 211–219. [Google Scholar] [CrossRef] [Green Version]
- Lowe, P.T.; O’Hagan, D. A Role for Fluorine in Flavours, Fragrances and Pheromones. J. Fluorine Chem. 2020, 230, 109420. [Google Scholar] [CrossRef]
- Dörrich, S.; Ulmer, A.; Mahler, C.; Burschka, C.; Baus, J.A.; Tacke, R.; Chai, A.; Ding, C.; Zou, Y.; Brunner, G.; et al. Sila-α-galbanone and Analogues: Synthesis and Olfactory Characterization of Silicon-Containing Derivatives of the Galbanum Odorant α-Galbanone. Eur. J. Inorg. Chem. 2014, 2014, 4394–4407. [Google Scholar] [CrossRef]
- Lovchik, M.A.; Kraft, P. Synthesis and Olfactory Properties of a 6’-Silasubstituted “Spiro[4.5]-delta-Damascone”. Chem. Eur. J. 2017, 23, 4590–4596. [Google Scholar] [CrossRef]
- Fürstner, A.; Guth, O.; Rumbo, A.; Seidel, G. Ring Closing Alkyne Metathesis. Comparative Investigation of Two Different Catalyst Systems and Application to the Stereoselective Synthesis of Olfactory Lactones, Azamacrolides, and the Macrocyclic Perimeter of the Marine Alkaloid Nakadomarin A. J. Am. Chem. Soc. 1999, 121, 11108–11113. [Google Scholar] [CrossRef] [Green Version]
- Fürstner, A.; Siedel, G. Ring closing alkyne metathesis: Stereoselective synthesis of civetone. J. Organomet. Chem. 2000, 606, 75–78. [Google Scholar] [CrossRef]
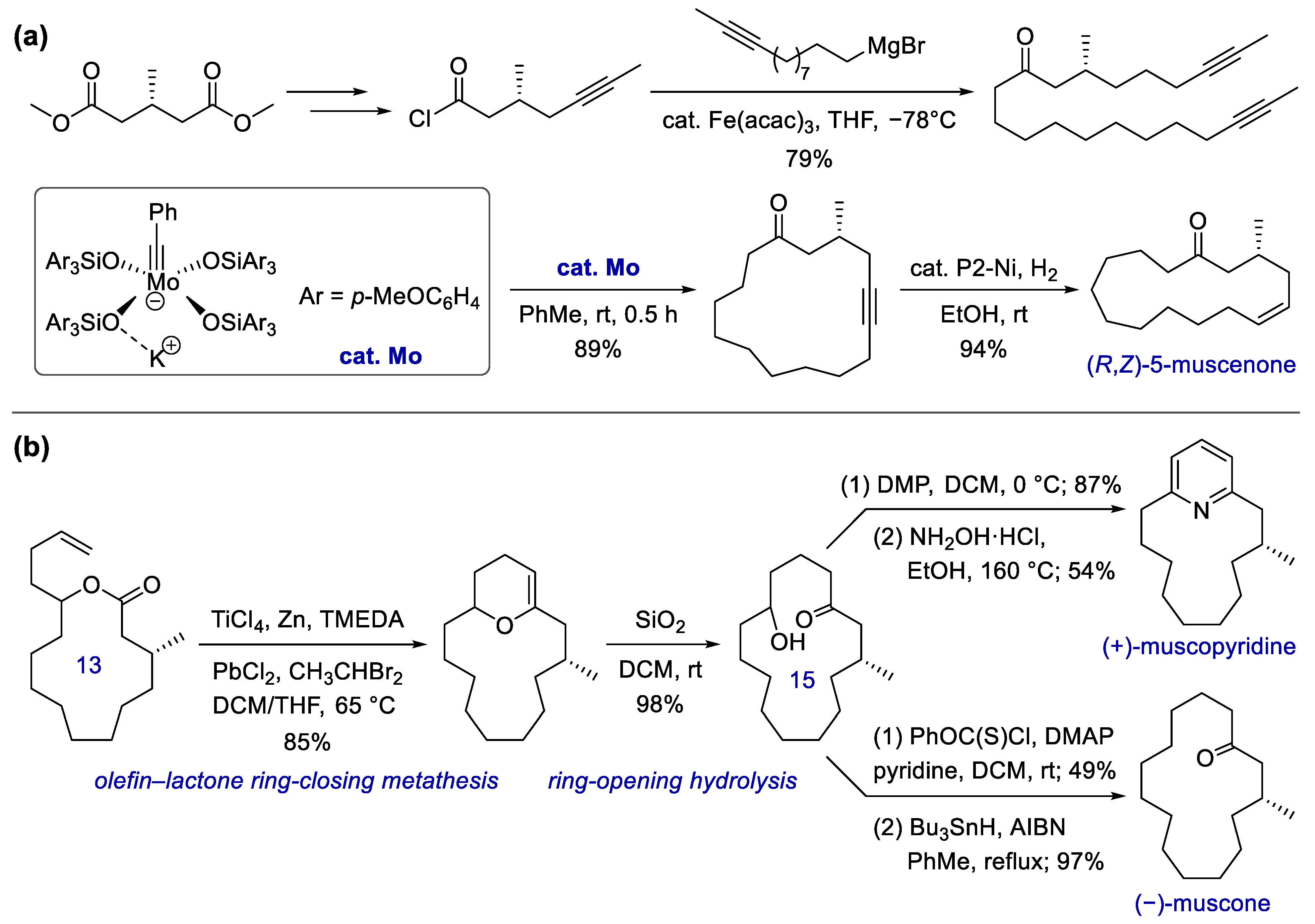
- Lehr, K.; Fürstner, A. An efficient route to the musk odorant (R,Z)-5-muscenone via base-metal-catalysis. Tetrahedron 2012, 68, 7695–7700. [Google Scholar] [CrossRef]
- Mathys, M.; Kraft, P. Synthesis by Ring-Closing Alkyne Metathesis with Selective Hydrogenation, and Olfactory Comparison of (7E)- and (7Z)-Cyclohexadec-7-enone (Aurelione®). Chem. Biodivers. 2014, 11, 1597–1607. [Google Scholar] [CrossRef]
- Iyer, K.; Rainier, J.D. Olefinic Ester and Diene Ring-Closing Metathesis Using a Reduced Titanium Alkylidene. J. Am. Chem. Soc. 2007, 129, 12604–12605. [Google Scholar] [CrossRef] [PubMed]
- Rohanna, J.C.; Rainier, J.D. Olefinic-Lactone Cyclizations to Macrocycles. Org. Lett. 2009, 11, 493–495. [Google Scholar] [CrossRef] [Green Version]
- Masato, I.; Sachiko, K.; Takao, I. Cp*Ru(PN) complex-catalyzed isomerization of allylic alcohols and its application to the asymmetric synthesis of muscone. J. Am. Chem. Soc. 2005, 127, 6172–6173. [Google Scholar] [CrossRef]
- Diaf, I.; Joffrin, A.; Lemière, G.; Duñach, E. Synthesis and odour evaluation of allenic derivatives. Flavour Fragr. J. 2015, 30, 478–484. [Google Scholar] [CrossRef]
- Lempenauer, L.; Appleson, T.; Lemière, G.; Duñach, E. Synthesis and olfactory evaluation of allylic α-quaternary thioether ketones. Flavour Fragr. J. 2019, 34, 36–42. [Google Scholar] [CrossRef] [Green Version]
- Brunzel, T.; Heppekausen, J.; Panten, J.; Köckritz, A. Selective Wacker type oxidation of a macrocyclic diene to the corresponding monounsaturated ketone used as fragrance. RSC Adv. 2019, 9, 27865–27873. [Google Scholar] [CrossRef] [Green Version]
- Kambiré, D.A.; Boti, J.B.; Ouattara, Z.A.; Yapi, A.T.; Bighelli, A.; Tomi, F.; Casanova, J. Leaf essential oil from Ivoirian Isolona dewevrei (Annonaceae): Chemical composition and structure elucidation of four new natural sesquiterpenes. Flavour Fragr. J. 2021, 36, 22–33. [Google Scholar] [CrossRef]
- Liu, Y.; Yeung, Y.Y. Synthesis of Macrocyclic Ketones through Catalyst-Free Electrophilic Halogen-Mediated Semipinacol Rearrangement: Application to the Total Synthesis of (±)-Muscone. Org. Lett. 2017, 19, 1422–1425. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Mouhib, H.; Stahl, W.; Goeke, A.; Wang, Q.; Kraft, P. Efficient macrocyclization by a novel oxy-oxonia-Cope reaction: Synthesis and olfactory properties of new macrocyclic musks. Chem. Eur. J. 2012, 18, 7010–7015. [Google Scholar] [CrossRef] [PubMed]
- Birkbeck, A.A. The synthesis of fragrant natural products from Santalum album L.: (+)-(Z)-α-santalol and (–)-(Z)-β-santalol. Chimia 2017, 71, 823–835. [Google Scholar] [CrossRef]
- Fehr, C.; Magpantay, I.; Arpagaus, J.; Marquet, X.; Vuagnoux, M. Enantioselective Synthesis of (-)-β-Santalol by a Copper-Catalyzed Enynol Cyclization–Fragmentation Reaction. Angew. Chem. Int. Ed. 2009, 48, 7221–7223. [Google Scholar] [CrossRef] [PubMed]
- Ahrendt, K.A.; Borths, C.J.; MacMillan, D.W.C. New Strategies for Organic Synthesis: The First Highly Enantioselective Organocatalytic Diels-Alder Reaction. J. Am. Chem. Soc. 2000, 122, 4243–4244. [Google Scholar] [CrossRef]
- Hayashi, Y.; Samanta, S.; Gotoh, H.; Ishikawa, H. Asymmetric Diels–Alder Reactions of α,β-Unsaturated Aldehydes Catalyzed by a Diarylprolinol Silyl Ether Salt in the Presence of Water. Angew. Chem. Int. Ed. 2008, 47, 6634–6637. [Google Scholar] [CrossRef] [PubMed]
- Fehr, C.; Magpantay, I.; Vuagnoux, M.; Dupau, P. The Synthesis of (Z)-Trisubstituted Allylic Alcohols by the Selective 1,4-Hydrogenation of Dienol Esters: Improved Synthesis of (-)-β-Santalol. Chem. Eur. J. 2011, 17, 1257–1260. [Google Scholar] [CrossRef] [PubMed]
- Chapuis, C.; Skuy, D.; de Saint Laumer, J.Y.; Brauchli, R. endo/exo Stereoselectivity in Diels−Alder Reactions of α,β-Dialkylated Conjugated Enals to Cyclic 1,3-Dienes: Intermediates in the Synthesis of (−)-β-Santalol and Its Analogs. Chem. Biodivers. 2014, 11, 1470–1516. [Google Scholar] [CrossRef]
- Fehr, C.; Vuagnoux, M.; Buzas, A.; Arpagaus, J.; Sommer, H. Gold- and Copper-Catalyzed Cycloisomerizations towards the Synthesis of Thujopsanone-Like Compounds. Chem. Eur. J. 2011, 17, 6214–6220. [Google Scholar] [CrossRef]
- Scarso, A.; Colladon, M.; Sgarbossa, P.; Santo, C.; Michelin, R.A.; Strukul, G. Highly Active and Selective Platinum(II)-Catalyzed Isomerization of Allylbenzenes: Efficient Access to (E)-Anethole and Other Fragrances via Unusual Agostic Intermediates. Organometallics 2010, 29, 1487–1497. [Google Scholar] [CrossRef]
- Kapat, A.; Sperger, T.; Guven, S.; Schoenebeck, F. E-Olefins through Intramolecular Radical Relocation. Science 2019, 363, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, Y.; Ohashi, T.; Kawaguchi, K.; Tanaka, N.; Katsuta, R.; Yajima, A.; Nukada, T.; Ishigami, K. Synthesis and odour evaluation of double-bond isomers of DAMASCENOLIDE, 4-(4-methylpent-3-en-1-yl)-2(5H)-furanone, which has a citrus-like odour. Flavour Fragr. J. 2020, 35, 341–349. [Google Scholar] [CrossRef]
- Derrer, S.; Flachsmann, F.; Plessis, C.; Stang, M. Applied Photochemistry—Light Controlled Perfume Release. Chimia 2007, 61, 665–669. [Google Scholar] [CrossRef]
- Flachsmann, F.; Gautschi, M.; Bachmann, J.P.; Brunner, G. Enzyme-triggered and self-cleaving fragrant alcohol precursors. Chem. Biodivers. 2008, 5, 1115–1136. [Google Scholar] [CrossRef]
- Herrmann, A. Using photolabile protecting groups for the controlled release of bioactive volatiles. Photochem. Photobiol. Sci. 2012, 11, 446–459. [Google Scholar] [CrossRef]
- Liu, M.; Han, J.; Guo, Z.; Xiao, Z.; Zhu, W.H. Advances in preparation and controlled release of profragrances under ambient conditions. Sci. Sin. Chim. 2019, 49, 581–590. [Google Scholar] [CrossRef]
- Liu, X.; Rong, X.; Liu, S.; Lan, Y.; Liu, Q. Cobalt-Catalyzed Desymmetric Isomerization of Exocyclic Olefins. J. Am. Chem. Soc. 2021, 143, 20633–20639. [Google Scholar] [CrossRef]
- Kvittingen, L.; Sjursnes, B.J.; Schmid, R. Limonene in Citrus: A String of Unchecked Literature Citings? J. Chem. Educ. 2021, 98, 3600–3607. [Google Scholar] [CrossRef]
- Endo, K.; Hamada, D.; Yakeishi, S.; Shibata, T. Effect of multinuclear copper/aluminum complexes in highly asymmetric conjugate addition of trimethylaluminum to acyclic enones. Angew. Chem. Int. Ed. 2013, 52, 606–610. [Google Scholar] [CrossRef]
- Kraft, P.; Jordi, S.; Denizot, N.; Felker, I. On the Dienone Motif of Musks: Synthesis and Olfactory Properties of Partially and Fully Hydrogenated Dienone Musks. Eur. J. Org. Chem. 2014, 2014, 554–563. [Google Scholar] [CrossRef]
- Felker, I.; Pupo, G.; Kraft, P.; List, B. Design and Enantioselective Synthesis of Cashmeran Odorants by Using “Enol Catalysis”. Angew. Chem. Int. Ed. 2015, 54, 1960–1964. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, M.; Kuroyanagi, S.; Ito, T.; Morita, H.; Tanaka, Y.; Toyooka, N. Synthesis and odor properties of Phantolide analogues. Tetrahedron 2017, 73, 2089–2099. [Google Scholar] [CrossRef]
- Wiesenfeldt, M.P.; Knecht, T.; Schlepphorst, C.; Glorius, F. Silylarene Hydrogenation—A Strategic Approach Enabling Direct Access to Versatile Silylated Saturated Carbo- and Heterocycles. Angew. Chem. Int. Ed. 2018, 57, 8297–8300. [Google Scholar] [CrossRef] [PubMed]
- Touge, T.; Sakaguchi, K.; Tamaki, N.; Nara, H.; Yokozawa, T.; Matsumura, K.; Kayaki, Y. Multiple Absolute Stereocontrol in Cascade Lactone Formation via Dynamic Kinetic Resolution Driven by the Asymmetric Transfer Hydrogenation of Keto Acids with Oxo-Tethered Ruthenium Catalysts. J. Am. Chem. Soc. 2019, 141, 16354–16361. [Google Scholar] [CrossRef] [PubMed]
- Saget, R.; Jaunky, P.; Plessis, C.; Duñach, E. Synthesis and odour perception of a series of highly substituted cyclohexane derivatives. Flavour Fragr. J. 2022, 37, 63–71. [Google Scholar] [CrossRef]
- Förster, B.; Bertermann, R.; Kraft, P.; Tacke, R. Sila-rhubafuran and Derivatives: Synthesis and Olfactory Characterization of Novel Silicon-Containing Odorants. Organometallics 2013, 33, 338–346. [Google Scholar] [CrossRef]
- Rodrigues, L.; Majik, M.S.; Tilve, S.G.; Wahidulla, S. Synthesis of (-)-elemoxide, a commercially important fragrance compound. Tetrahedron Lett. 2018, 59, 3413–3415. [Google Scholar] [CrossRef]
- Ondet, P.; Filippi, J.J.; Lemière, G.; Duñach, E. Stereocontrolled Cascade Cyclisation of Campholenic Enol Ether Derivatives: En Route to Vetiver-Scented Spiroxides. Eur. J. Org. Chem. 2018, 2018, 980–989. [Google Scholar] [CrossRef]
- Ondet, P.; Lempenauer, L.; Duñach, E.; Lemière, G. Bismuth(III) triflate-catalysed tandem cyclisations towards complex polycyclic ethers. Org. Chem. Front. 2016, 3, 999–1003. [Google Scholar] [CrossRef]
- Ondet, P.; Plessis, C.; Lemière, G.; Duñach, E. Synthesis and olfactory evaluation of spiro tricyclic diether structures. Flavour Fragr. J. 2017, 32, 119–125. [Google Scholar] [CrossRef]
- Delolo, F.G.; Oliveira, K.C.B.; dos Santos, E.N.; Gusevskaya, E.V. Hydroformylation of biomass-based hydroxyolefins in eco-friendly solvents: New fragrances from myrtenol and nopol. Mol. Catal. 2019, 462, 1–9. [Google Scholar] [CrossRef]
- de Camargo Faria, A.; Oliveira, K.C.B.; Monteiro, A.C.; dos Santos, E.N.; Gusevskaya, E.V. New scents using eco-friendly solvents: Oxo synthesis of aldehydes from caryophyllane sesquiterpenes. Catal. Today 2020, 344, 24–31. [Google Scholar] [CrossRef]
- Costa, V.V.; da Silva Rocha, K.A.; Mesquita, R.A.; Kozhevnikova, E.F.; Kozhevnikov, I.V.; Gusevskaya, E.V. Heteropoly Acid Catalysts for the Synthesis of Fragrance Compounds from Biorenewables: Cycloaddition of Crotonaldehyde to Limonene, α-Pinene, and β-Pinene. ChemCatChem. 2013, 5, 3022–3026. [Google Scholar] [CrossRef]
- Vieira, C.G.; de Freitas, M.C.; dos Santos, E.N.; Gusevskaya, E.V. Synthesis of fragrance ingredients by tandem hydroformylation-cyclisation of limonene catalysed by rhodium complexes and pyridiunium p-toluenesulphonate. ChemCatChem. 2012, 4, 795–801. [Google Scholar] [CrossRef]
- Vieira, C.G.; de Freitas, M.C.; de Oliveira, K.C.B.; de Camargo Faria, A.; dos Santos, E.N.; Gusevskaya, E.V. Synthesis of fragrance compounds from renewable resources: The aqueous biphasic hydroformylation of acyclic terpenes. Catal. Sci. Technol. 2015, 5, 960–966. [Google Scholar] [CrossRef]
- dos Santos Costa, M.; de Camargo Faria, A.; Gusevskaya, E.V. New scents from bio-renewable cis-jasmone by aerobic palladium catalyzed oxidations. Appl. Catal. A 2019, 584, 117171. [Google Scholar] [CrossRef]
- Ribeiro, C.J.; Pereira, M.M.; Kozhevnikova, E.F.; Kozhevnikov, I.V.; Gusevskaya, E.V.; da Silva Rocha, K.A. Heteropoly acid catalysts in upgrading of biorenewables: Synthesis of para-menthenic fragrance compounds from α-pinene oxide. Catal. Today 2020, 344, 166–170. [Google Scholar] [CrossRef]
- de Oliveira, M.P.; Delolo, F.G.; Villarreal, J.A.; dos Santos, E.N.; Gusevskaya, E.V. Hydroformylation and one-pot hydroformylation/epoxy ring cleavage of limonene oxide: A sustainable access to biomass-based multi-functional fragrances. Appl. Catal. A 2021, 616, 118082. [Google Scholar] [CrossRef]
- Liu, L.; Kaib, P.S.; Tap, A.; List, B. A General Catalytic Asymmetric Prins Cyclization. J. Am. Chem. Soc. 2016, 138, 10822–10825. [Google Scholar] [CrossRef]
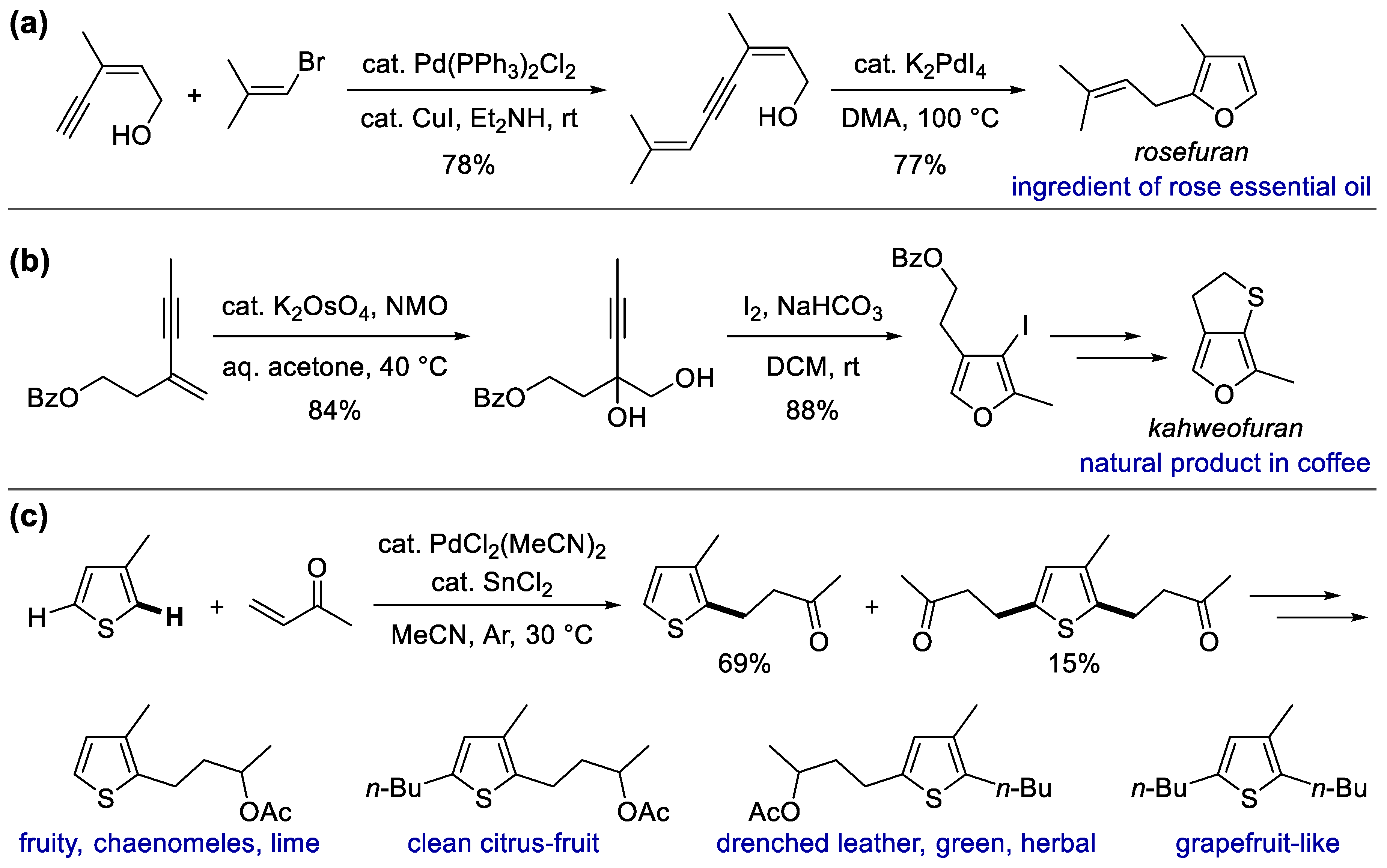
- Gabriele, B.; Salerno, G. A New and Efficient Synthesis of Rosefuran. A General Synthesis of Furans by Palladium-Catalysed Cycloisomerization of (Z)-2-En-4-yn-1-ols. Chem. Commun. 1997, 1083–1084. [Google Scholar] [CrossRef]
- Gabriele, B.; Salerno, G.; Lauria, E. A General and Facile Synthesis of Substituted Furans by Palladium-Catalyzed Cycloisomerization of (Z)-2-En-4-yn-1-ols. J. Org. Chem. 1999, 64, 7687–7692. [Google Scholar] [CrossRef]
- King, I.S.; Knight, D.W. The conundrum of odourless Kahweofuran, a roasting “flavour” of coffee. Tetrahedron 2021, 81, 131871. [Google Scholar] [CrossRef]
- Buechi, G.; Degen, P.; Gautschi, F.; Willhalm, B. Structure and synthesis of kahweofuran, a constituent of coffee aroma. J. Org. Chem. 1971, 36, 199–200. [Google Scholar] [CrossRef]
- Bonikowski, R.; Sikora, M.; Kula, J.; Kunicka, A. Thienyl analogues of acyclic monoterpene alcohols and their biological activity. J. Sci. Food Agric. 2009, 89, 2088–2095. [Google Scholar] [CrossRef]
- Kost, B.; Matysiak, S.; Kula, J.; Bonikowski, R. Synthesis of thiophene derivatives with long-lasting citrusy type odour. Flavour Fragr. J. 2018, 33, 279–284. [Google Scholar] [CrossRef]
- Das, D.; Pratihar, S.; Roy, S. Heterobimetallic Pd−Sn Catalysis: Michael Addition Reaction with C-, N-, O-, and S-Nucleophiles and in Situ Diagnostics. J. Org. Chem. 2013, 78, 2430–2442. [Google Scholar] [CrossRef]
- Schalk, M.; Pastore, L.; Mirata, M.A.; Khim, S.; Schouwey, M.; Deguerry, F.; Pineda, V.; Rocci, L.; Daviet, L. Toward a biosynthetic route to sclareol and amber odorants. J. Am. Chem. Soc. 2012, 134, 18900–18903. [Google Scholar] [CrossRef]
- Schempp, F.M.; Drummond, L.; Buchhaupt, M.; Schrader, J. Microbial cell factories for the production of terpenoid flavor and fragrance compounds. J. Agric. Food Chem. 2018, 66, 2247–2258. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, C.; Lindley, N.D. Metabolic engineering strategies for sustainable terpenoid flavor and fragrance synthesis. J. Agric. Food Chem. 2019, 68, 10252–10264. [Google Scholar] [CrossRef]
- Moser, S.; Leitner, E.; Plocek, T.J.; Vanhessche, K.; Pichler, H. Engineering of Saccharomyces cerevisiae for the production of (+)-ambrein. Yeast 2020, 37, 163–172. [Google Scholar] [CrossRef] [Green Version]
- Heath, R.S.; Ruscoe, R.E.; Turner, N.J. The beauty of biocatalysis: Sustainable synthesis of ingredients in cosmetics. Nat. Prod. Rep. 2022, 39, 335–388. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.S.; Gravestock, M.B.; McCarry, B.E. Acetylenic bond participation in biogenetic-like olefinic cyclizations. II. Synthesis of dl-progesterone. J. Am. Chem. Soc. 1971, 93, 4332–4334. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Pedrero, O.; Rodriguez, F. Cationic Cyclization Reactions with Alkyne Terminating Groups: A Useful Tool in Biomimetic Synthesis. Chem. Commun. 2022, 58, 1089–1099. [Google Scholar] [CrossRef] [PubMed]
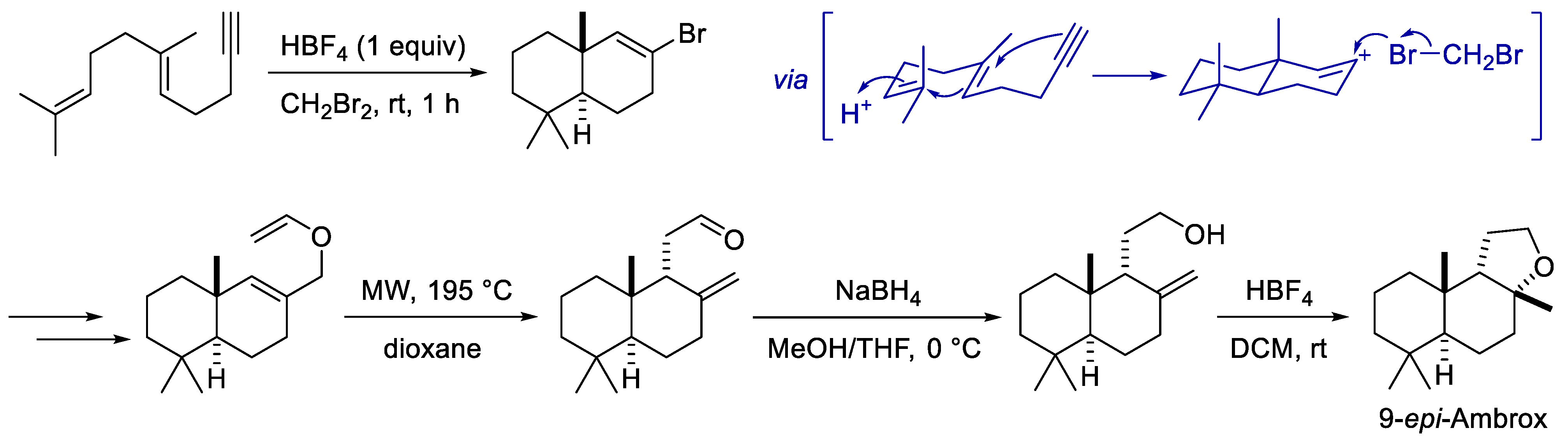
- Eichhorn, E.; Locher, E.; Guillemer, S.; Wahler, D.; Fourage, L.; Schilling, B. Biocatalytic Process for (−)-Ambrox Production Using Squalene Hopene Cyclase. Adv. Synth. Catal. 2018, 360, 2339–2351. [Google Scholar] [CrossRef]
- Fontaneda, R.; Alonso, P.; Fananas, F.J.; Rodriguez, F. Scalable Synthesis of the Amber Odorant 9-epi-Ambrox through a Biomimetic Cationic Cyclization/Nucleophilic Bromination Reaction. Org. Lett. 2016, 18, 4626–4629. [Google Scholar] [CrossRef]
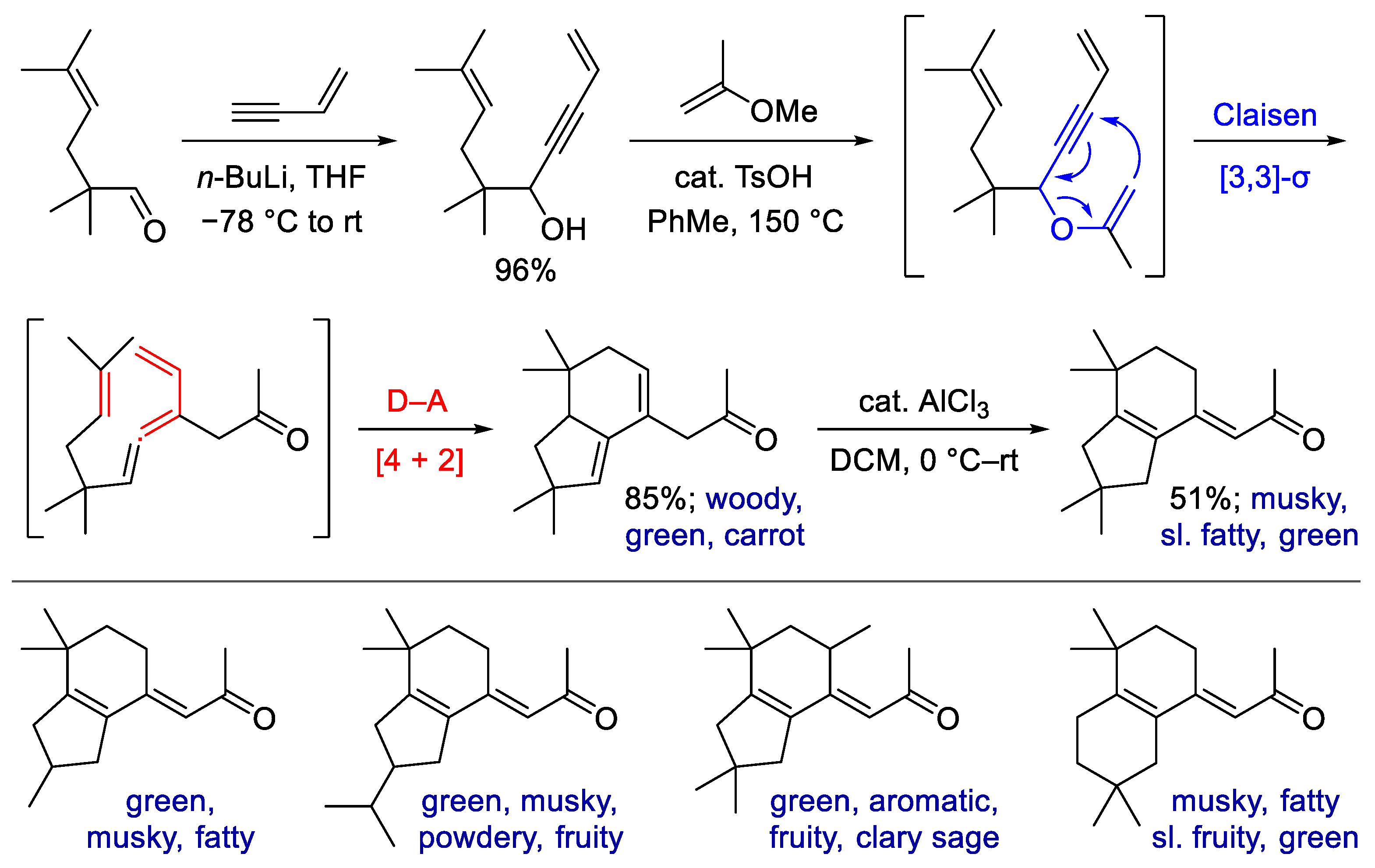
- Liu, J.; Hürlimann, V.; Emter, R.; Natsch, A.; Esposito, C.; Linker, S.M.; Zou, Y.; Zhou, L.; Wang, Q.; Riniker, S.; et al. A New Family of Rigid Dienone Musks Challenges the Perceptive Range of the Human Olfactory Receptor OR5AN1. Synlett 2020, 31, 972–976. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, L.; Zou, Y.; Wang, Q.; Goeke, A. Oxovanadium-catalysed domino reactions of hydroxy enynes for the construction of Cashmeran-like odorants. Org. Biomol. Chem. 2020, 18, 7832–7836. [Google Scholar] [CrossRef]
- Laher, R.; Marin, C.; Michelet, V. When Gold Meets Perfumes: Synthesis of Olfactive Compounds via Gold-Catalyzed Cycloisomerization Reactions. Org. Lett. 2020, 22, 4058–4062. [Google Scholar] [CrossRef]
- Laher, R.; Gentilini, E.; Marin, C.; Michelet, V. Oxygen-tethered 1, 6-enynes and [4.1. 0]-Bicyclic ether skeletons as hedonic materials for the fragrance industry. Synthesis 2021, 53, 4020–4029. [Google Scholar] [CrossRef]
- Harwood, S.J.; Palkowitz, M.D.; Gannett, C.N.; Perez, P.; Yao, Z.; Sun, L.; Abruña, H.D.; Anderson, S.L.; Baran, P.S. Modular terpene synthesis enabled by mild electrochemical couplings. Science 2022, 375, 745–752. [Google Scholar] [CrossRef]
- Noble, A.; McCarver, S.J.; MacMillan, D.W.C. Merging photoredox and nickel catalysis: Decarboxylative cross-coupling of carboxylic acids with vinyl halides. J. Am. Chem. Soc. 2015, 137, 624–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manning, J.; Tavanti, M.; Porter, J.L.; Kress, N.; De Visser, S.P.; Turner, N.J.; Flitsch, S.L. Regio- and Enantio-selective Chemo-Enzymatic C–H-Lactonization of Decanoic Acid to (S)-δ-Decalactone. Angew. Chem. Int. Ed. 2019, 58, 5668–5671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, L.; Hua, R. Recent advances in construction of polycyclic natural product scaffolds via one-pot reactions involving alkyne annulation. Front. Chem. 2020, 8, 580355. [Google Scholar] [CrossRef] [PubMed]
- Sombret, J.; Quintaine, J.; Biremond, T.; Barnes, Q.; de Saint-Laumer, J.-Y.; Saudan, L. High trans-2-Decalones by Photoredox Catalyzed β-Isomerization. Helv. Chim. Acta. 2022, 105, e202100180. [Google Scholar] [CrossRef]
- Bolding, K.A.; Franks, K.M. Recurrent Cortical Circuits Implement Concentration-Invariant Odor Coding. Science. 2018, 361, eaat6904. [Google Scholar] [CrossRef]
- Zhang, L.; Mao, H.; Liu, L.; Du, J.; Gani, R. A machine learning based computer-aided molecular design/screening methodology for fragrance molecules. Comput. Chem. Eng. 2018, 115, 295–308. [Google Scholar] [CrossRef]
- Radhakrishnapany, K.T.; Wong, C.Y.; Tan, F.K.; Chong, J.W.; Tan, R.R.; Aviso, K.B.; Janairo, J.I.B.; Chemmangattuvalappil, N.G. Design of fragrant molecules through the incorporation of rough sets into computer-aided molecular design. Mol. Syst. Des. Eng. 2020, 5, 1391–1416. [Google Scholar] [CrossRef]
- Chacko, R.; Jain, D.; Patwardhan, M.; Puri, A.; Karande, S.; Rai, B. Data based predictive models for odor perception. Sci. Rep. 2020, 10, 17136. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, R.; Ranjta, S.; Varadwaj, P.K. SMILES to Smell: Decoding the Structure–Odor Relationship of Chemical Compounds Using the Deep Neural Network Approach. J. Chem. Inf. Model. 2021, 61, 676–688. [Google Scholar] [CrossRef]
- Zhang, L.; Mao, H.; Zhuang, Y.; Wang, L.; Liu, L.; Dong, Y.; Du, J.; Xie, W.; Yuan, Z. Odor prediction and aroma mixture design using machine learning model and molecular surface charge density profiles. Chem. Eng. Sci. 2021, 245, 116947. [Google Scholar] [CrossRef]
- Mahmoud, S.; Irwin, B.; Chekmarev, D.; Vyas, S.; Kattas, J.; Whitehead, T.; Mansley, T.; Bikker, J.; Conduit, G.; Segall, M. Imputation of sensory properties using deep learning. J. Comput.-Aided Mol. Des. 2021, 35, 1125–1140. [Google Scholar] [CrossRef] [PubMed]
- Santana, V.V.; Martins, M.A.; Loureiro, J.M.; Ribeiro, A.M.; Rodrigues, A.E.; Nogueira, I.B. Optimal fragrances formulation using a deep learning neural network architecture: A novel systematic approach. Comput. Chem. Eng. 2021, 150, 107344. [Google Scholar] [CrossRef]
- Ooi, Y.J.; Aung, K.N.G.; Chong, J.W.; Tan, R.R.; Aviso, K.B.; Chemmangattuvalappil, N.G. Design of fragrance molecules using computer-aided molecular design with machine learning. Comput. Chem. Eng. 2022, 157, 107585. [Google Scholar] [CrossRef]
- Boethling, R.S. Incorporating Environmental Attributes into Musk Design. Green Chem. 2011, 13, 3386–3396. [Google Scholar] [CrossRef]
- Avonto, C.; Wang, Y.H.; Chittiboyina, A.G.; Vukmanovic, S.; Khan, I.A. In Chemico Assessment of Potential Sensitizers: Stability and Direct Peptide Reactivity of 24 Fragrance Ingredients. J. Appl. Toxicol. 2019, 39, 398–408. [Google Scholar] [CrossRef]
- Date, M.S.; O’Brien, D.; Botelho, D.; Schultz, T.W.; Liebler, D.C.; Penning, T.M.; Salvito, D.T. Clustering a Chemical Inventory for Safety Assessment of Fragrance Ingredients: Identifying Read-Across Analogs to Address Data Gaps. Chem. Res. Toxicol. 2020, 33, 1709–1718. [Google Scholar] [CrossRef]
- Suresh, A.; Velusamy, S.; Ayyasamy, S.; Rathinasamy, M. Techniques for Essential Oil Extraction from Kaffir Lime and its Application in Health Care Products—A Review. Flavour Fragr. J. 2021, 36, 5–21. [Google Scholar] [CrossRef]
- Zeni, V.; Benelli, G.; Campolo, O.; Giunti, G.; Palmeri, V.; Maggi, F.; Rizzo, R.; Lo Verde, G.; Lucchi, A.; Canale, A. Toxics or Lures? Biological and Behavioral Effects of Plant Essential Oils on Tephritidae Fruit Flies. Molecules 2021, 26, 5898. [Google Scholar] [CrossRef]
- Lunz, K.; Stappen, I. Back to the roots—an overview of the chemical composition and bioactivity of selected root-essential oils. Molecules 2021, 26, 3155. [Google Scholar] [CrossRef]
- Liu, K.; Xie, L.; Deng, M.; Zhang, X.; Luo, J.; Li, X. Zoology, chemical composition, pharmacology, quality control and future perspective of Musk (Moschus): A review. Chin. Med. 2021, 16, 46. [Google Scholar] [CrossRef]




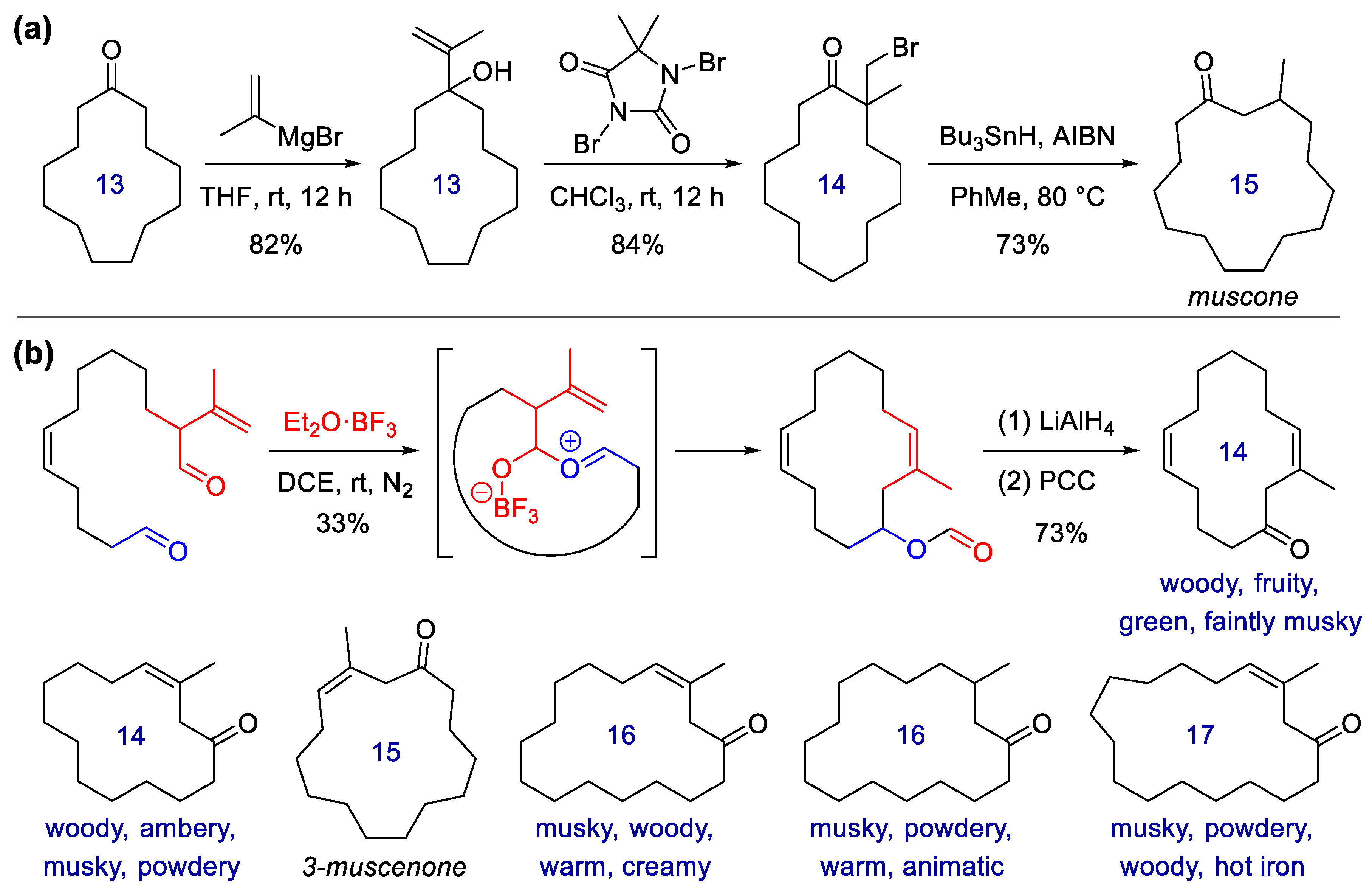
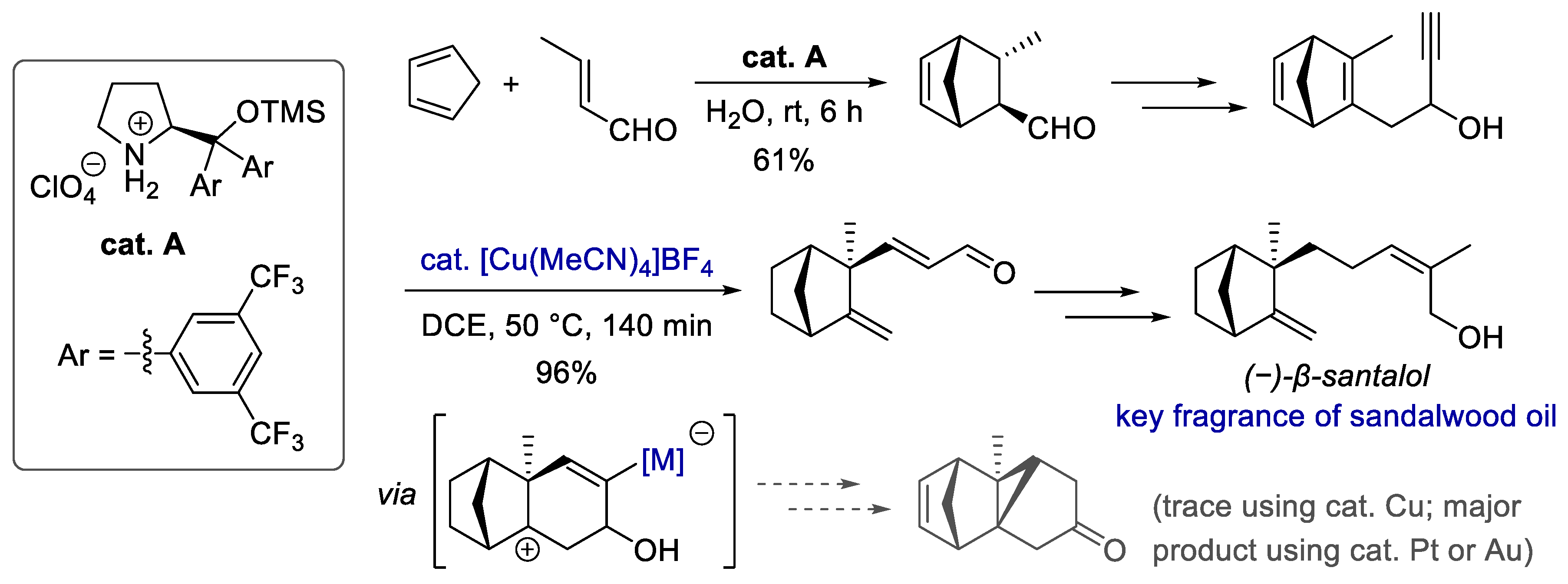
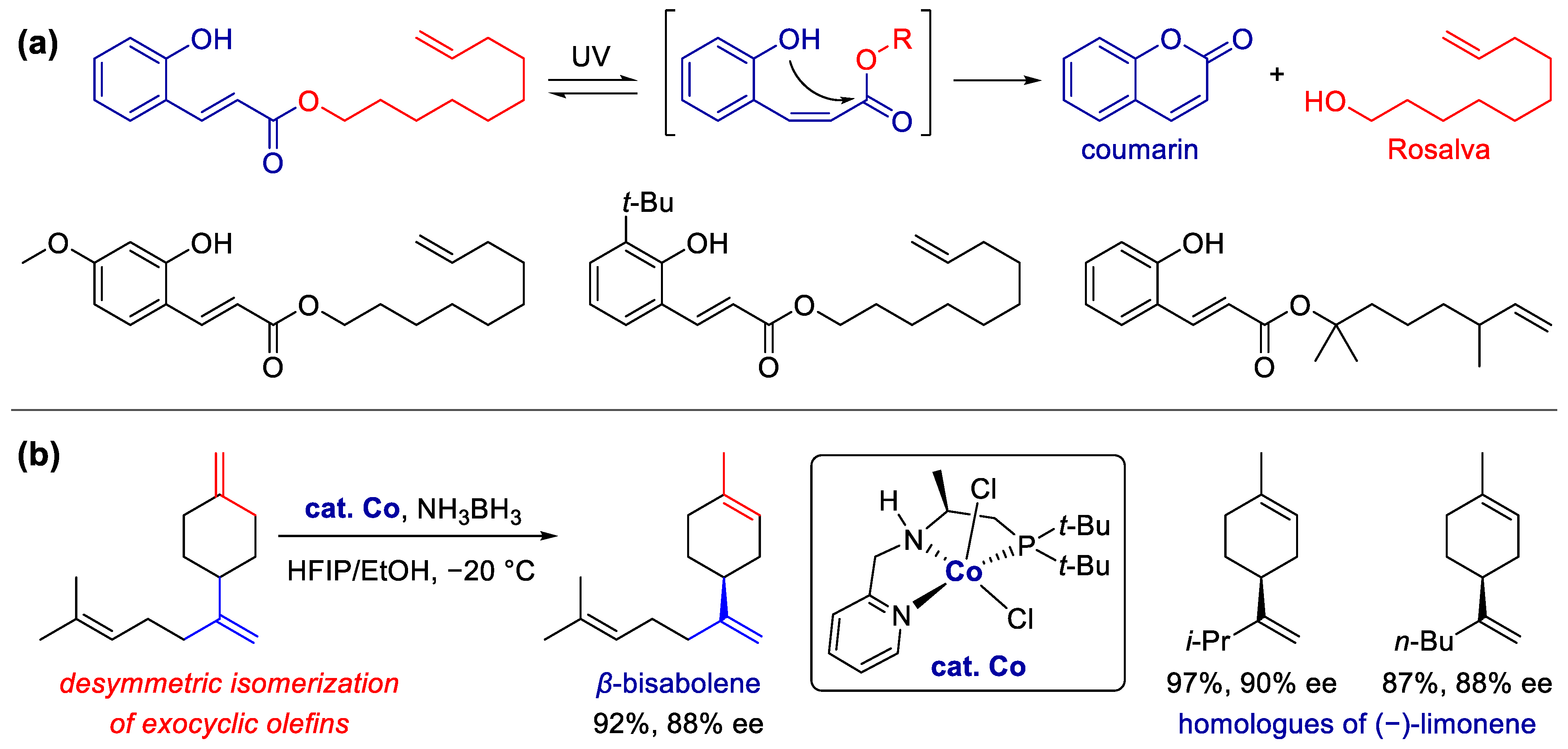

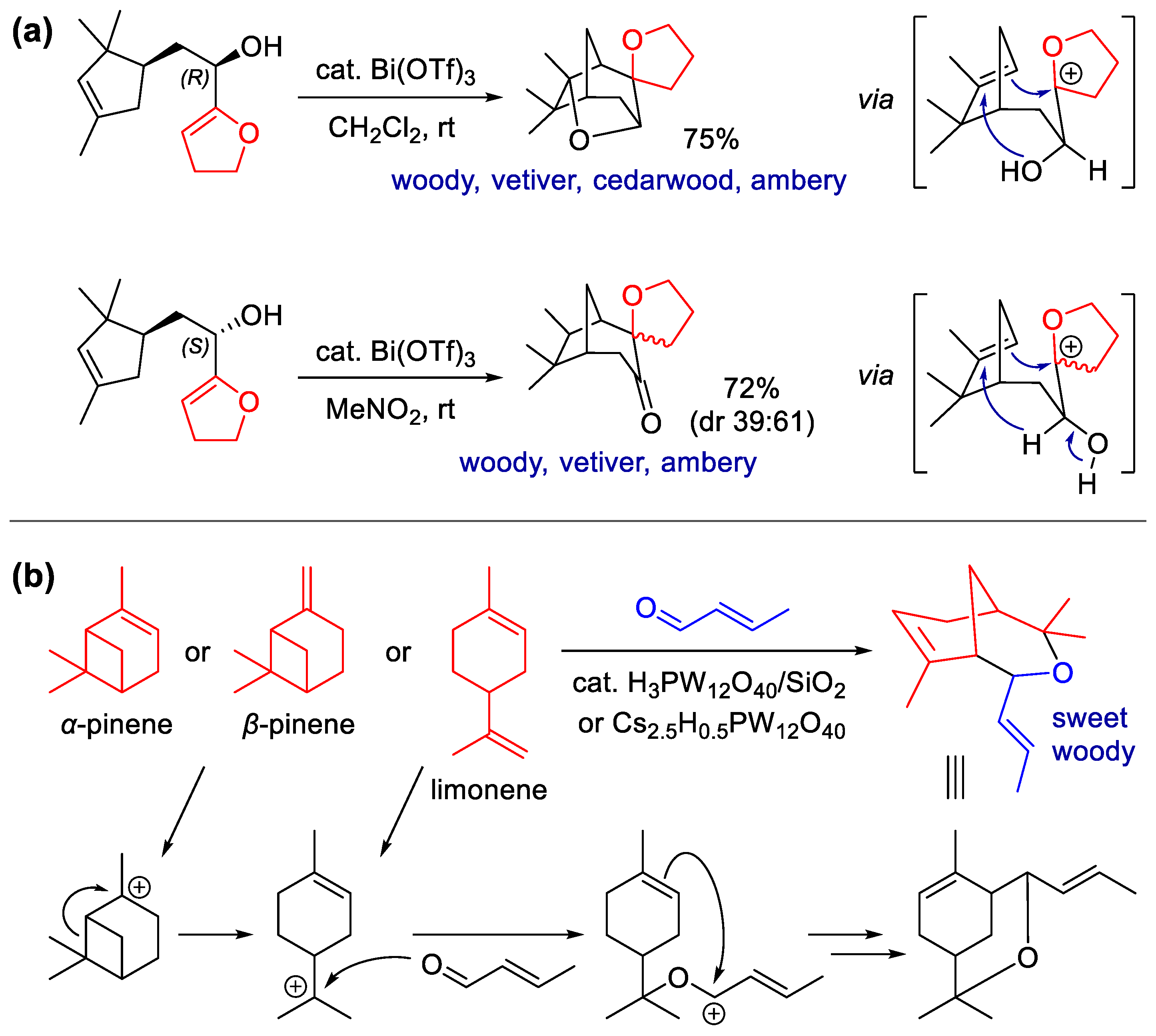
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Z.; Huang, B.; Ouyang, L.; Zheng, L. Synthesis of Cyclic Fragrances via Transformations of Alkenes, Alkynes and Enynes: Strategies and Recent Progress. Molecules 2022, 27, 3576. https://doi.org/10.3390/molecules27113576
Lin Z, Huang B, Ouyang L, Zheng L. Synthesis of Cyclic Fragrances via Transformations of Alkenes, Alkynes and Enynes: Strategies and Recent Progress. Molecules. 2022; 27(11):3576. https://doi.org/10.3390/molecules27113576
Chicago/Turabian StyleLin, Zhigeng, Baoying Huang, Lufeng Ouyang, and Liyao Zheng. 2022. "Synthesis of Cyclic Fragrances via Transformations of Alkenes, Alkynes and Enynes: Strategies and Recent Progress" Molecules 27, no. 11: 3576. https://doi.org/10.3390/molecules27113576
APA StyleLin, Z., Huang, B., Ouyang, L., & Zheng, L. (2022). Synthesis of Cyclic Fragrances via Transformations of Alkenes, Alkynes and Enynes: Strategies and Recent Progress. Molecules, 27(11), 3576. https://doi.org/10.3390/molecules27113576





