Nanoemulsions of Jasminum humile L. and Jasminum grandiflorum L. Essential Oils: An Approach to Enhance Their Cytotoxic and Antiviral Effects
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Capillary Gas Chromatography-Mass Spectrometry (GC/MS) Analysis
2.3. Preparation of Nanoemulsion Formulations
2.4. Determination of Sample Cytotoxicity on Cancer Cells
2.5. Determination of Antiviral Activity
2.6. MTT Protocol
2.7. Calculation of the Selectivity Index (SI)
2.8. Statistical Analysis
3. Results and Discussion
3.1. GC-MS Analysis of the Essential Oil
3.2. Characterization of Nanoemuslion Formulations (Physical Characterization)
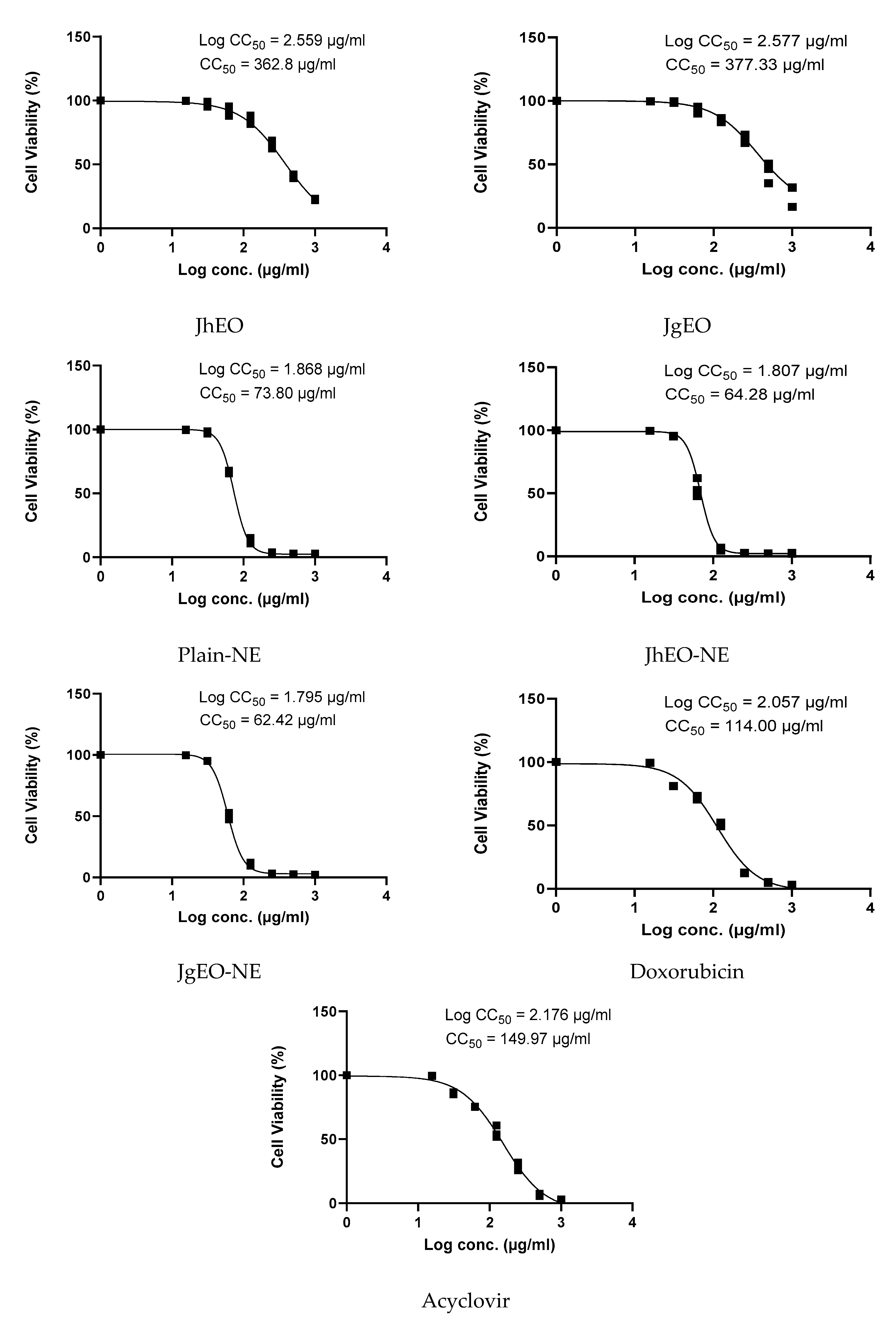
3.3. Cytotoxic Concentrations of Tested Samples on Normal Cells
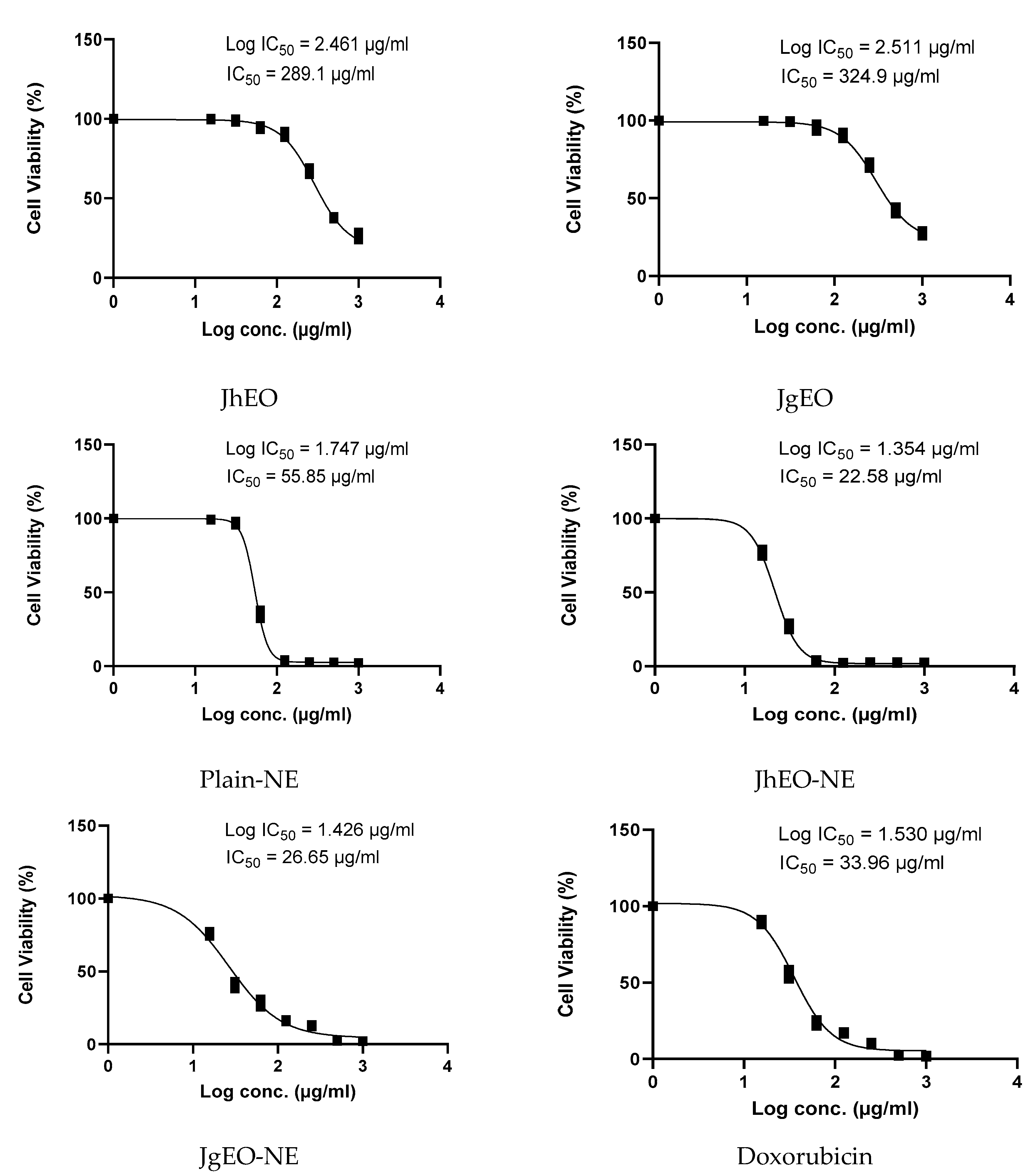
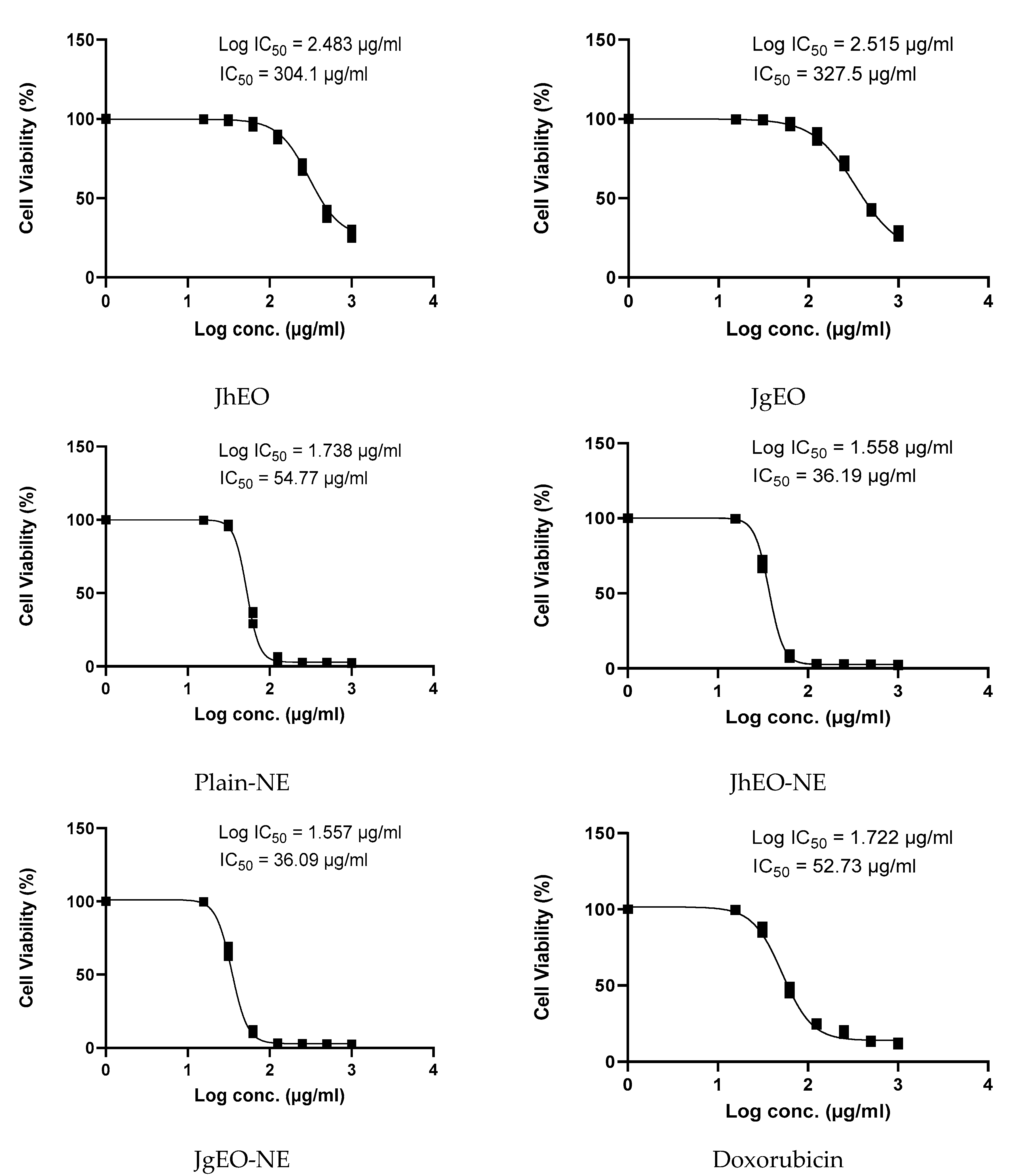
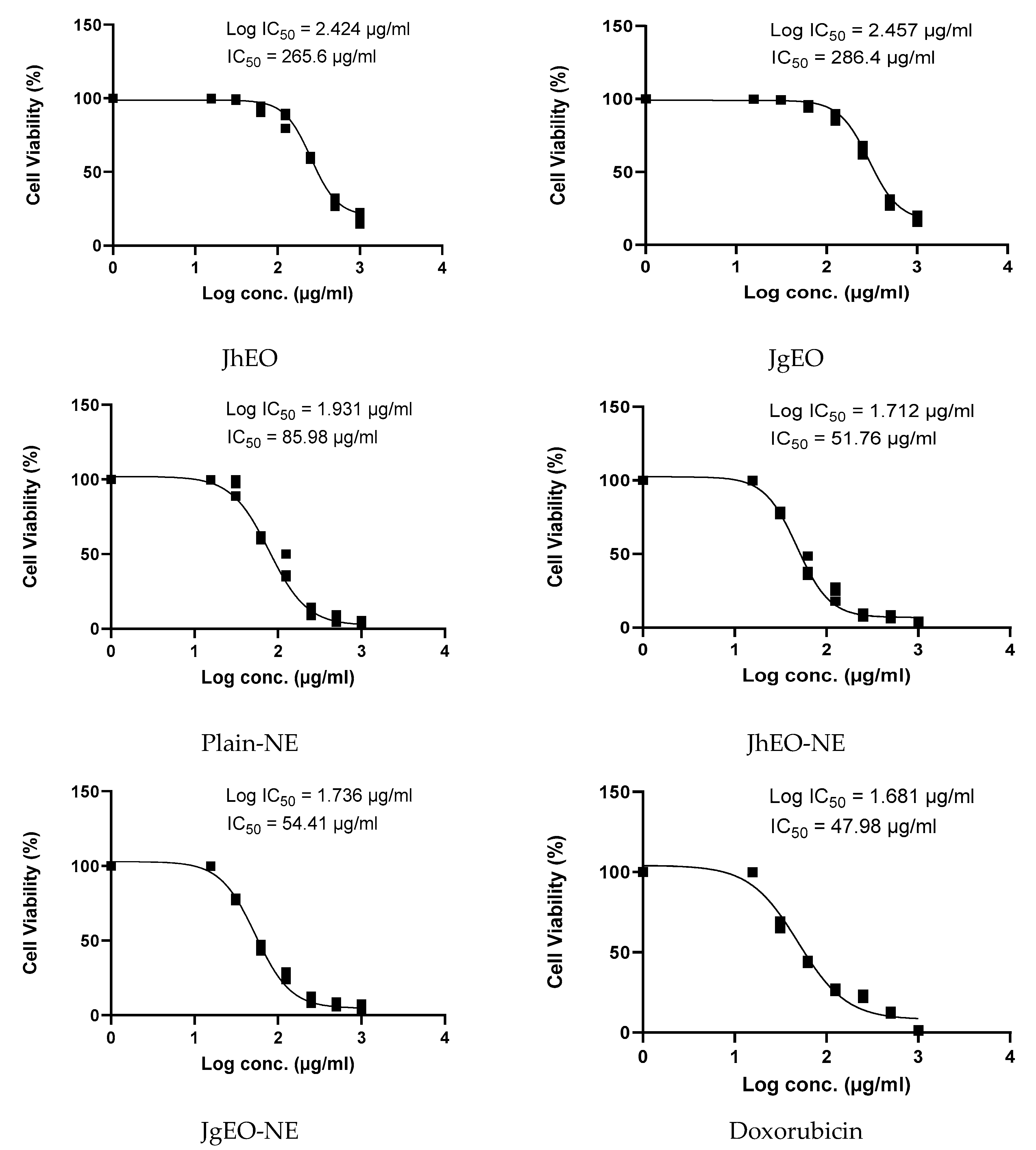
3.4. Cytotoxic Activity of Tested Samples
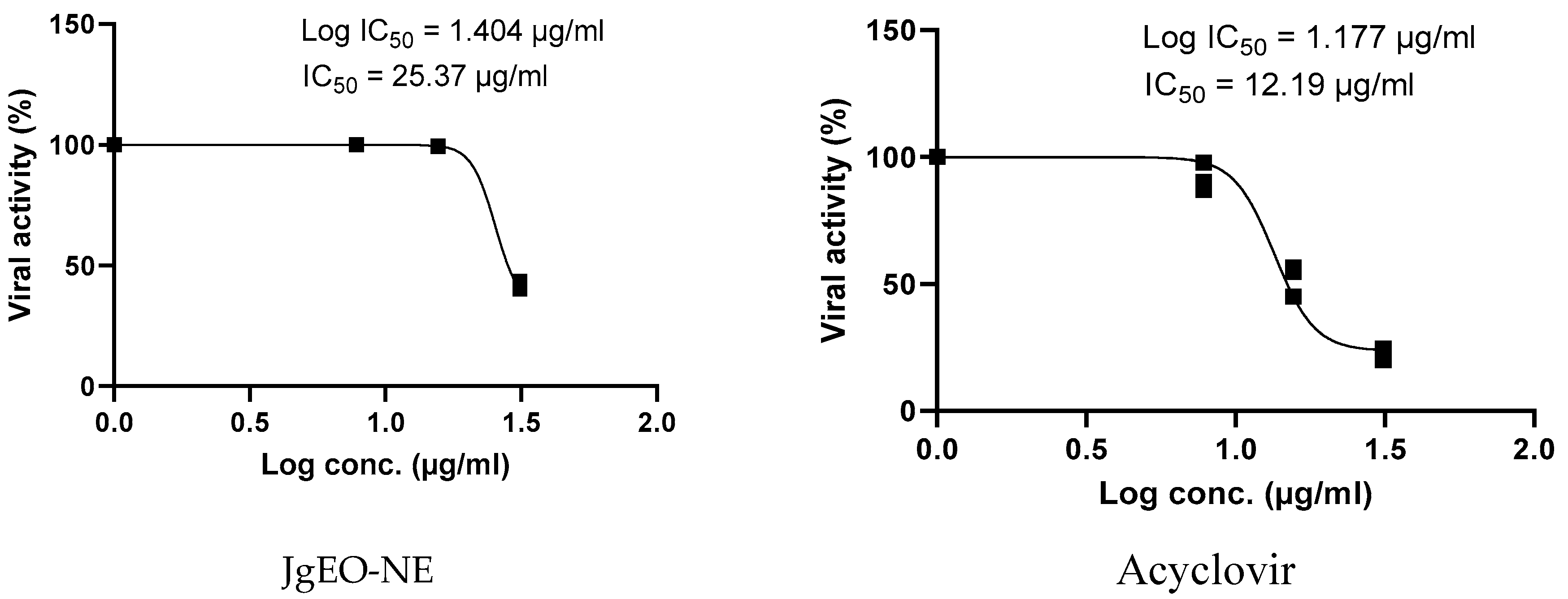
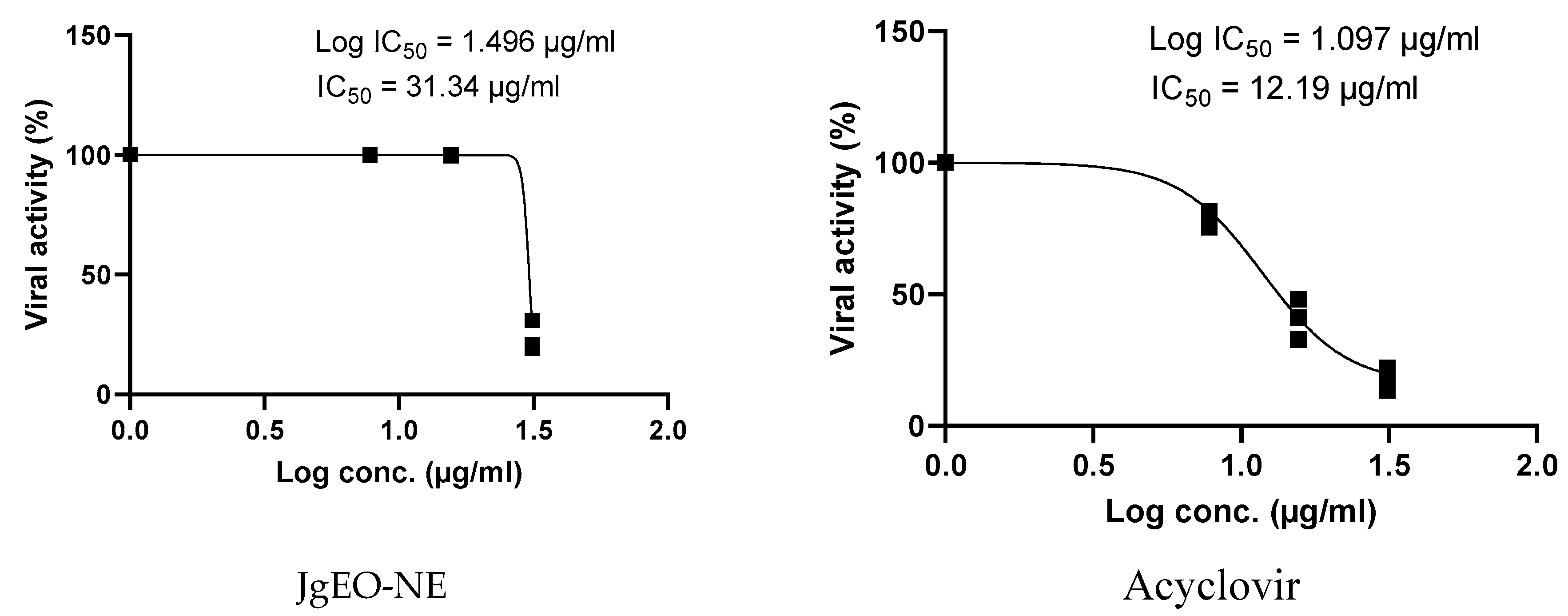
3.5. Antiviral Activity of Tested Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Neeraj Mourya, N.M.; Devendra Bhopte, D.B.; Rakesh Sagar, R.S. A review on Jasminum sambac: A potential medicinal plant. Int. J. Indig. Herbs Drugs 2017, 2, 13–16. [Google Scholar]
- Kunhachan, P.; Banchonglikitkul, C.; Kajsongkram, T.; Khayungarnnawee, A.; Leelamanit, W. Chemical composition, toxicity and vasodilatation effect of the flowers extract of Jasminum sambac (L.) Ait. “G. Duke of Tuscany”. Evid. Based Complement. Altern. Med. 2012, 2012, 1–7. [Google Scholar] [CrossRef]
- Hongratanaworakit, T. Stimulating effect of aromatherapy massage with jasmine oil. Nat. Prod. Commun. 2010, 5, 157–162. [Google Scholar] [CrossRef]
- Zhao, G.; Yin, Z.; Dong, J. Antiviral efficacy against hepatitis B virus replication of oleuropein isolated from Jasminum officinale L. var. grandiflorum. J. Ethnopharmacol. 2009, 125, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Jirovetz, L.; Buchbauer, G.; Schweiger, T.; Denkova, Z.; Slavchev, A.; Stoyanova, A.; Schmidt, E.; Geissler, M. Chemical composition, olfactory evaluation and antimicrobial activities of Jasminum grandiflorum L. absolute from India. Nat. Prod. Commun. 2007, 2, 407–412. [Google Scholar] [CrossRef]
- Manjunath, C.; Mahurkar, N. In vitro cytotoxicity of cardamom oil, lemon oil, and jasmine oil on human skin, gastric, and brain cancer cell line. J. Cancer Res. Ther. 2021, 17, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Nayak, M.; Sahoo, G.C.; Pandey, K.; Sarkar, M.C.; Ansari, Y.; Das, V.N.R.; Topno, R.K.; Madhukar, M.; Das, P. Iron oxide nanoparticles based antiviral activity of H1N1 influenza A virus. J. Infect. Chemother. 2019, 25, 325–329. [Google Scholar] [CrossRef]
- Zhao, Z.; Xiao, Y.; Xu, L.; Liu, Y.; Jiang, G.; Wang, W.; Li, B.; Zhu, T.; Tan, Q.; Tang, L.; et al. Glycyrrhizic acid nanoparticles as antiviral and anti-inflammatory agents for COVID-19 treatment. ACS Appl. Mater. Interfaces 2021, 13, 20995–21006. [Google Scholar] [CrossRef] [PubMed]
- Donsì, F.; Ferrari, G. Essential oil nanoemulsions as antimicrobial agents in food. J. Biotechnol. 2016, 233, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Egypt-Global Cancer Observatory; World Health Organization: Geneva, Switzerland, 2020; Available online: https://gco.iarc.fr/today/data/factsheets/populations/818-egypt-fact-sheets.pdf (accessed on 18 November 2021).
- Alburaiki, T.A.; Hany, A.M.; Riad, K.F.; Osman, D.M. Association of parental, child, and environmental factors with the occurrence of childhood leukemia in Upper Egypt. J. High Inst. Pub. Health 2021, 51, 10–18. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Braun, N.A.; Kohlenberg, B.; Sim, S.; Meier, M.; Hammerschmidt, F.-J. Jasminum flexile flower absolute from India—A detailed comparison with three other jasmine absolutes. Nat. Prod. Commun. 2009, 4, 1239–1250. [Google Scholar] [CrossRef]
- Prakash, O.; Sahoo, D.; Rout, P.K. Liquid CO2 extraction of Jasminum grandiflorum and comparison with conventional processes. Nat. Prod. Commun. 2012, 7, 89–92. [Google Scholar] [CrossRef]
- Wei, F.H.; Chen, F.L.; Tan, X.M. Gas chromatographic-mass spectrometric analysis of essential oil of Jasminum officinale L. var grandiflorum flower. Trop. J. Pharm. Res. 2015, 14, 149–152. [Google Scholar] [CrossRef]
- Zhou, H.-C.; Hou, Z.-W.; Wang, D.-X.; Ning, J.-M.; Wei, S. Large scale preparation, stress analysis, and storage of headspace volatile condensates from Jasminum sambac flowers. Food Chem. 2019, 286, 170–178. [Google Scholar] [CrossRef]
- Aman, R.M.; Abu Hashim, I.I.; Meshali, M.M. Novel clove essential oil nanoemulgel tailored by taguchi′s model and scaffold-based nanofibers: Phytopharmaceuticals with promising potential as cyclooxygenase-2 inhibitors in external inflammation. Int. J. Nanomed. 2020, 15, 2171–2195. [Google Scholar] [CrossRef]
- Mehrbod, P.; Safari, H.; Mollai, Z.; Fotouhi, F.; Mirfakhraei, Y.; Entezari, H.; Goodarzi, S.; Tofighi, Z. Potential antiviral effects of some native Iranian medicinal plants extracts and fractions against influenza A virus. BMC Complement. Med. Ther. 2021, 21, 1–12. [Google Scholar] [CrossRef]
- Wang, H.-X.; Zeng, M.-S.; Ye, Y.; Liu, J.-Y.; Xu, P.-P. Antiviral activity of puerarin as potent inhibitor of influenza virus neuraminidase. Phytother. Res. 2021, 35, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, P.F.D.; Alves, J.M.; Damasceno, J.L.; Oliveira, R.A.M.; Dias, H.; Crotti, A.E.M.; Tavares, D.C. Cytotoxicity screening of essential oils in cancer cell lines. Rev. Bras. Farmacogn. 2015, 25, 183–188. [Google Scholar] [CrossRef]
- Tsemeugne, J.; Shinyuy, L.M.; Djeukoua, S.K.D.; Sopbue, E.F.; Ngemenya, M.N. Evaluation of macrofilaricidal and microfilaricidal activities against Onchocerca ochengi and cytotoxicity of some synthesized azo compounds containing thiophene backbone. Parasitol. Res. 2021, 120, 2087–2094. [Google Scholar] [CrossRef] [PubMed]
- Yousefbeyk, F.; Dabirian, S.; Ghanbarzadeh, S.; Eghbali Koohi, D.; Yazdizadeh, P.; Ghasemi, S. Green synthesis of silver nanoparticles from Stachys byzantina K. Koch: Characterization, antioxidant, antibacterial, and cytotoxic activity. Part. Sci. Technol. 2021, 40, 219–232. [Google Scholar] [CrossRef]
- Sun, X.-Y.; Gan, Q.-Z.; Ouyang, J.-M. Size-dependent cellular uptake mechanism and cytotoxicity toward calcium oxalate on Vero cells. Sci. Rep. 2017, 7, 41949. [Google Scholar] [CrossRef]
- Gonçalves, E.M.; Ventura, C.Â.; Yano, T.; Rodrigues Macedo, M.L.; Genari, S.C. Morphological and growth alterations in Vero cells transformed by cisplatin. Cell Biol. Int. 2006, 30, 485–494. [Google Scholar] [CrossRef]
- Saurav, K.; Kannabiran, K. Cytotoxicity and antioxidant activity of 5-(2,4-dimethylbenzyl)pyrrolidin-2-one extracted from marine Streptomyces VITSVK5 spp. Saudi J. Biol. Sci. 2012, 19, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Sahu, S.C.; Zheng, J.; Graham, L.; Chen, L.; Ihrie, J.; Yourick, J.J.; Sprando, R.L. Comparative cytotoxicity of nanosilver in human liver HepG2 and colon Caco2 cells in culture. J. Appl. Toxicol. 2014, 34, 1155–1166. [Google Scholar] [CrossRef] [PubMed]
- Camarillo, I.G.; Xiao, F.; Madhivanan, S.; Salameh, T.; Nichols, M.; Reece, L.M.; Leary, J.F.; Otto, K.; Natarajan, A.; Ramesh, A.; et al. Low and high voltage electrochemotherapy for breast cancer: An in vitro model study. In Electroporation-Based Therapies for Cancer: From Basics to Clinical Applications, 1st ed.; Woodhead Publishing: Sawston, UK, 2014; pp. 55–102. [Google Scholar]
- Liu, J.-J.; Zhang, Y.; Lin, D.-J.; Xiao, R.-Z. Tanshinone IIA inhibits leukemia THP-1 cell growth by induction of apoptosis. Oncol. Rep. 2009, 21, 1075–1081. [Google Scholar]
- Popovich, D.G.; Kitts, D.D. Structure–function relationship exists for ginsenosides in reducing cell proliferation and inducing apoptosis in the human leukemia (THP-1) cell line. Arch. Biochem. Biophys. 2002, 406, 1–8. [Google Scholar] [CrossRef]
- Gu, Y.; Ting, Z.; Qiu, X.; Zhang, X.; Gan, X.; Fang, Y.; Xu, X.; Xu, R. Linalool preferentially induces robust apoptosis of a variety of leukemia cells via upregulating p53 and cyclin-dependent kinase inhibitors. Toxicology 2010, 268, 19–24. [Google Scholar] [CrossRef]
- Kitts, D.D.; Popovich, D.G.; Hu, C. Characterizing the mechanism for ginsenoside-induced cytotoxicity in cultured leukemia (THP-1) cells. Can. J. Physiol. Pharmacol. 2007, 85, 1173–1183. [Google Scholar] [CrossRef]
- Ayesh, B.M.; Abed, A.A.; Faris, D.A. In vitro inhibition of human leukemia THP-1 cells by Origanum syriacum L. and Thymus vulgaris L. extracts. BMC Res Notes 2014, 7, 1–6. [Google Scholar] [CrossRef][Green Version]
- Kawagoe, H.; Kawagoe, R.; Sano, K. Targeted down-regulation of MLL-AF9 with antisense oligodeoxyribonucleotide reduces the expression of the HOXA7 and -A10 genes and induces apoptosis in a human leukemia cell line, THP-1. Leukemia 2001, 15, 1743–1749. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Giri, B.; Gupta, V.K.; Yaffe, B.; Modi, S.; Roy, P.; Sethi, V.; Lavania, S.P.; Vickers, S.M.; Dudeja, V.; Banerjee, S.; et al. Pre-clinical evaluation of Minnelide as a therapy for acute myeloid leukemia. J. Transl. Med. 2019, 17, 1–9. [Google Scholar]
- Dahham, S.S.; Tabana, Y.M.; Iqbal, M.A.; Ahamed, M.B.K.; Ezzat, M.O.; Majid, A.S.A.; Majid, A.M.S.A. The anticancer, antioxidant and antimicrobial properties of the sesquiterpene β-caryophyllene from the essential oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef]
- DAILEY, O.D.; Wang, X.; Chen, F.; Huang, G. Anticancer activity of branched-chain derivatives of oleic acid. Anticancer Res. 2011, 31, 3165–3169. [Google Scholar]
- Ahmed, S.A.; Rahman, A.A.; Elsayed, K.N.M.; Abd El-Mageed, H.R.; Mohamed, H.S.; Ahmed, S.A. Cytotoxic activity, molecular docking, pharmacokinetic properties and quantum mechanics calculations of the brown macroalga Cystoseira trinodis compounds. J. Biomol. Struct. Dyn. 2021, 39, 3855–3873. [Google Scholar] [CrossRef]
- Usta, J.; Shatha, S.; Racha, K.; Yolla, B.; Omar, R.; Sawsan, K.; Karim, E. Possible mediators underlying linalool effect on HepG-2 but not primary hepatocytes: Comparative study. Planta Med. 2011, 77, 1–11. [Google Scholar] [CrossRef]
- Zhao, Y.; Meng, X.; Zeng, Y.; Wang, C.; Chen, J.; She, Z. Linalool inhibits MCF-7 tumor growth in a xenograft model by apoptosis induction and immune modulation. Nat. Prod. Commun. 2021, 16, 1–9. [Google Scholar] [CrossRef]
- Chiang, L.-C.; Ng, L.-T.; Cheng, P.-W.; Chiang, W.; Lin, C.-C. Antiviral activities of extracts and selected pure constituents of Ocimum basilicum. Clin. Exp. Pharmacol. Physiol. 2005, 32, 811–816. [Google Scholar] [CrossRef]
- Ryabchenko, B.; Tulupova, E.; Schmidt, E.; Wlcek, K.; Buchbauer, G.; Jirovetz, L. Investigation of anticancer and antiviral properties of selected aroma samples. Nat. Prod. Commun. 2008, 3, 1085–1088. [Google Scholar] [CrossRef]
- Chathuranga, K.; Weerawardhana, A.; Dodantenna, N.; Ranathunga, L.; Cho, W.-K.; Ma, J.Y.; Lee, J.-S. Inhibitory effect of Sargassum fusiforme and its components on replication of respiratory syncytial virus in vitro and in vivo. Viruses 2021, 13, 548. [Google Scholar] [CrossRef]
- Chidewe, C.; Castillo, U.F.; Sem, D.S. Structural analysis and antimicrobial activity of chromatographically separated fractions of leaves of Sesamum angustifolium. J. Biol. Act. Prod. Nat. 2017, 7, 463–474. [Google Scholar]
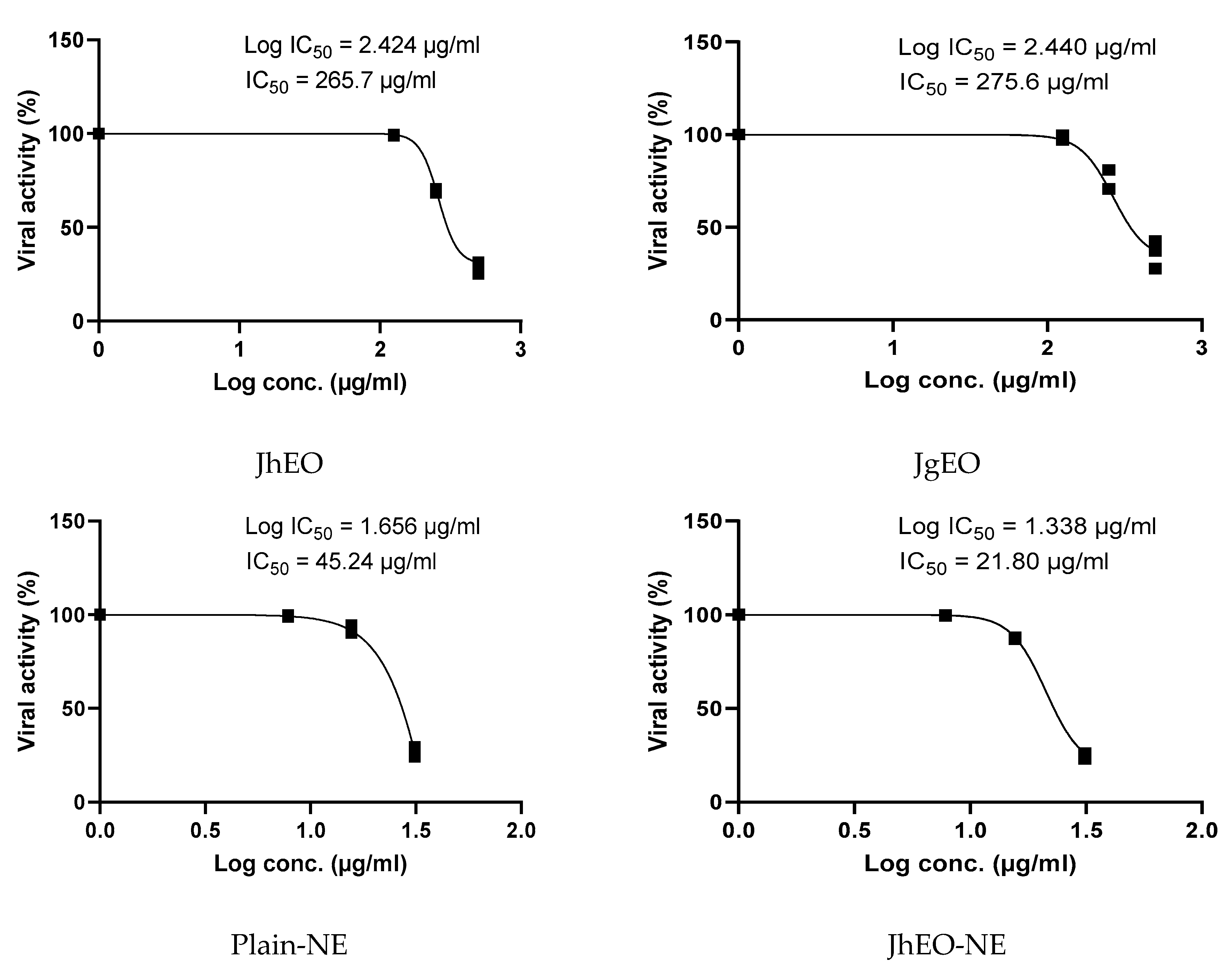
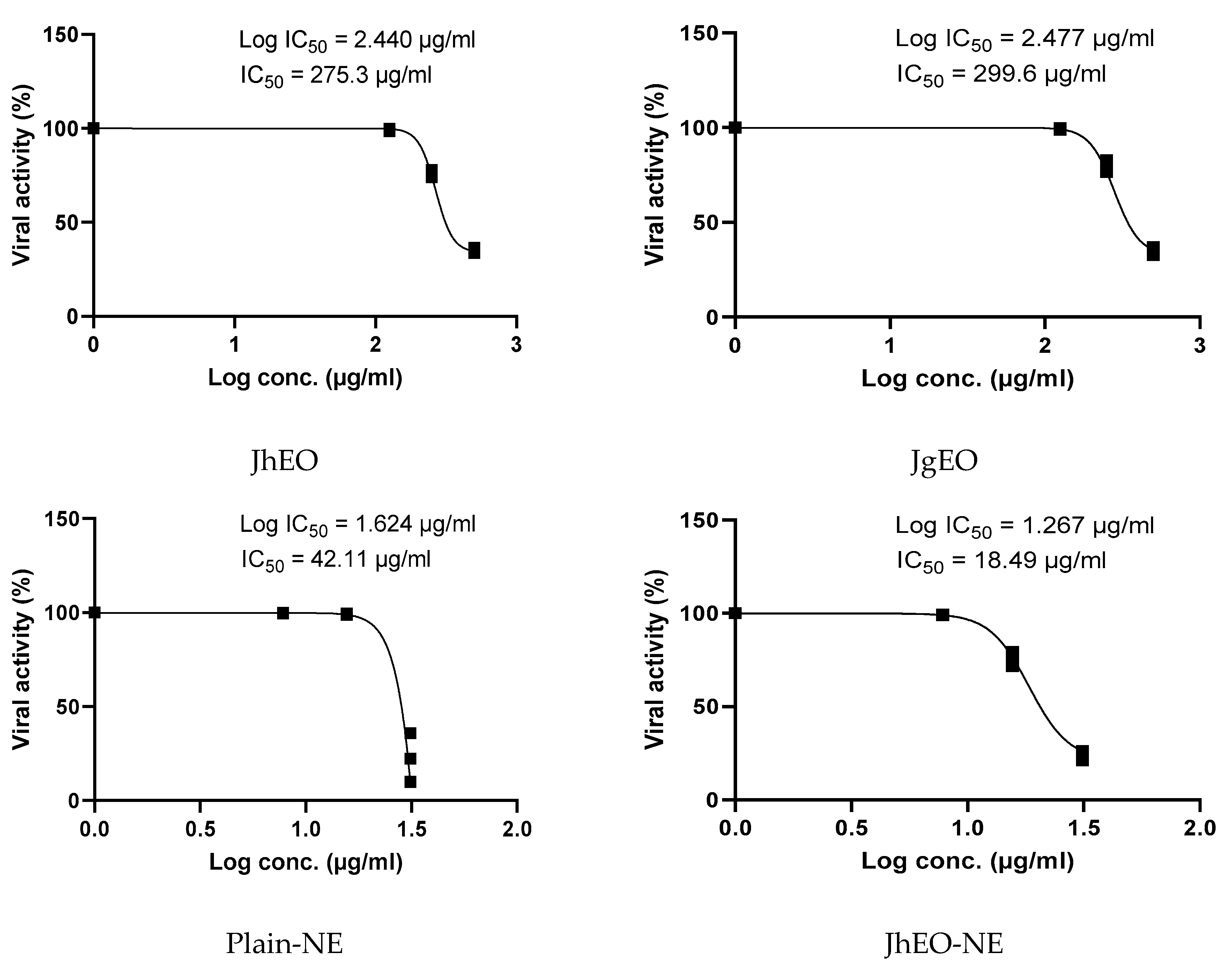
| Tentative Identification | R.R.I. calc. a | R.R.I. lit. b | Content % | Base Peak | M. Wt. | M. Formula | ||
|---|---|---|---|---|---|---|---|---|
| J. humile | J. grandiflorum | |||||||
| 1 | Linalool | 1095 | 1096 | 17.2 | 3.6 | 71.1 | 154.0 | C10H18O |
| 2 | Benzyl acetate | 1161 | 1162 | - | 32.4 | 108.0 | 150.0 | C9H10O2 |
| 3 | Carvone | 1240 | 1243 | 2.2 | - | 82.0 | 150.0 | C10H14O |
| 4 | Methyl anthranilate | 1338 | 1337 | 2.1 | 2.5 | 119.1 | 151.1 | C8H9NO2 |
| 5 | (Z)-jasmone | 1390 | 1392 | 6.6 | 8.5 | 79.1 | 164.0 | C11H16O |
| 6 | (Z)-caryophyllene | 1407 | 1408 | 5.6 | 6.2 | 41.1 | 204.0 | C15H24 |
| 7 | (E, E)-α-farnesene | 1506 | 1505 | 6.9 | 7.6 | 41.1 | 204.0 | C15H24 |
| 8 | (Z)-nerolidol | 1532 | 1532 | 5.0 | 11.9 | 69.1 | 222.0 | C15H26O |
| 9 | (3Z)-hexenyl benzoate | 1567 | 1566 | 7.4 | 1.1 | 105.1 | 204.0 | C13H16O2 |
| 10 | n-hexadecane | 1601 | 1600 | - | 1.3 | 57.1 | 226.0 | C16H34 |
| 11 | epi-α-cadinol | 1639 | 1640 | 3.0 | - | 161.1 | 222.0 | C15H26O |
| 12 | epi-α-muurolol | 1642 | 1642 | 2.9 | - | 43.1 | 222.0 | C15H26O |
| 13 | (Z)-methyl jasmonate | 1648 | 1649 | - | 1.8 | 83.1 | 224.0 | C13H20O3 |
| 14 | (Z)-methyl epijasmonate | 1679 | 1679 | - | 1.6 | 83.1 | 224.0 | C13H20O3 |
| 15 | (2E, 6Z)-farnesol | 1714 | 1715 | 2.5 | - | 69.1 | 222.0 | C15H26O |
| 16 | Benzyl benzoate | 1759 | 1759 | 6.9 | 7.4 | 105.1 | 212.0 | C14H12O2 |
| 17 | (2E, 6E)-farnesyl acetate | 1845 | 1846 | 2.0 | - | 69.1 | 264.0 | C17H28O2 |
| 18 | Benzyl salicylate | 1864 | 1865 | 2.6 | 2.5 | 91.1 | 228.0 | C14H12O3 |
| 19 | n-nonadecane | 1899 | 1900 | - | 1.2 | 57.1 | 268.0 | C19H40 |
| 20 | Phytol | 1941 | 1943 | - | 3.5 | 71.1 | 296.0 | C20H40O |
| 21 | Isophytol | 1947 | 1947 | 2.4 | - | 71.1 | 296.0 | C20H40O |
| 22 | Geranyl benzoate | 1957 | 1959 | 1.7 | - | 105.1 | 258.1 | C17H22O2 |
| 23 | Hexadecanoic acid | 1960 | 1960 | 3.4 | 1.2 | 41.1 | 256.0 | C16H32O2 |
| 24 | Methyl linoleate | 2084 | 2085 | 3.1 | - | 67.1 | 294.1 | C19H34O2 |
| 25 | n-heneicosane | 2099 | 2100 | - | 3.4 | 57.1 | 296.0 | C21H44 |
| 26 | Oleic acid | 2141 | 2142 | 1.6 | - | 41.1 | 282.1 | C18H34O2 |
| 27 | Phytol acetate | 2218 | 2218 | 1.5 | - | 43.1 | 338.1 | C22H42O2 |
| 28 | Tricosane | 2300 | 2300 | 2.8 | - | 57.1 | 324.0 | C23H48 |
| 29 | Tetracosane | 2400 | 2400 | 2.2 | - | 57.1 | 338.1 | C24H50 |
| 30 | Hexacosane | 2600 | 2600 | 4.6 | - | 57.1 | 366.1 | C26H54 |
| 31 | Nonacosane | 2900 | 2900 | 1.8 | - | 57.1 | 408.0 | C29H60 |
| Sample | CC50 ± SD (µg/mL) | Cytotoxic Activity | ||||||
|---|---|---|---|---|---|---|---|---|
| HepG-2 | MCF-7 | THP-1 | ||||||
| IC50 ± SD (µg/mL) | SI | IC50 ± SD (µg/mL) | SI | IC50 ± SD (µg/mL) | SI | |||
| Media | DMSO (-Ve Control) | NT | NA | - | NA | - | NA | - |
| Pure EOs | J. humile | 362.83 ± 15.31 | 289.10 ± 5.61 | 1.26 | 304.13 ± 3.55 | 1.19 | 265.60 ± 10.85 | 1.37 |
| J. grandiflorum | 377.33 ± 10.82 | 324.90 ± 22.85 | 1.16 | 327.53 ± 6.06 | 1.15 | 286.37 ± 7.63 | 1.32 | |
| Nano-emulsion | J. humile | 64.28 ± 4.17 | 22.58 ± 0.86 | 2.85 | 36.19 ± 0.77 | 1.78 | 51.76 ± 6.68 | 1.24 |
| J. grandiflorum | 62.42 ± 2.07 | 26.65 ± 0.06 | 2.34 | 36.09 ± 1.44 | 1.73 | 54.41 ± 0.60 | 1.15 | |
| Standard | Doxorubicin | 114.00 ± 2.17 | 33.96 ± 2.03 | 3.36 | 52.73 ± 2.44 | 2.16 | 47.98 ± 2.35 | 2.38 |
| Sample | CC50 ± SD (µg/mL) | MNTC (µg/mL) | HAV | HSV-1 | |||
|---|---|---|---|---|---|---|---|
| IC50 ± SD (µg/mL) | SI | IC50 ± SD (µg/mL) | SI | ||||
| Media | DMSO (-Ve Control) | NT | NT | NA | - | NA | - |
| Pure EOs | J. humile | 362.83 ± 15.31 | 500 | 265.70 ± 4.62 | 1.36 | 275.33 ± 7.41 | 1.32 |
| J. grandiflorum | 377.33 ± 10.82 | 500 | 275.57 ± 7.51 | 1.37 | 299.63 ± 19.76 | 1.26 | |
| Nano-emulsion | J. humile | 64.28 ± 4.17 | 31.25 | 21.80 ± 1.17 | 2.94 | 18.49 ± 1.20 | 3.48 |
| J. grandiflorum | 62.42 ± 2.07 | 31.25 | 25.37 ± 1.26 | 2.45 | 31.34 ± 2.72 | 1.99 | |
| Standard | Acyclovir | 149.97 ± 5.46 | 31.25 | 15.04 ± 1.38 | 9.98 | 12.49 ± 1.98 | 12.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mansour, K.A.; El-Neketi, M.; Lahloub, M.-F.; Elbermawi, A. Nanoemulsions of Jasminum humile L. and Jasminum grandiflorum L. Essential Oils: An Approach to Enhance Their Cytotoxic and Antiviral Effects. Molecules 2022, 27, 3639. https://doi.org/10.3390/molecules27113639
Mansour KA, El-Neketi M, Lahloub M-F, Elbermawi A. Nanoemulsions of Jasminum humile L. and Jasminum grandiflorum L. Essential Oils: An Approach to Enhance Their Cytotoxic and Antiviral Effects. Molecules. 2022; 27(11):3639. https://doi.org/10.3390/molecules27113639
Chicago/Turabian StyleMansour, Khaled Ahmed, Mona El-Neketi, Mohamed-Farid Lahloub, and Ahmed Elbermawi. 2022. "Nanoemulsions of Jasminum humile L. and Jasminum grandiflorum L. Essential Oils: An Approach to Enhance Their Cytotoxic and Antiviral Effects" Molecules 27, no. 11: 3639. https://doi.org/10.3390/molecules27113639
APA StyleMansour, K. A., El-Neketi, M., Lahloub, M.-F., & Elbermawi, A. (2022). Nanoemulsions of Jasminum humile L. and Jasminum grandiflorum L. Essential Oils: An Approach to Enhance Their Cytotoxic and Antiviral Effects. Molecules, 27(11), 3639. https://doi.org/10.3390/molecules27113639







