Investigation of the Uptake and Transport of Two Novel Camptothecin Derivatives in Caco-2 Cell Monolayers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Cell Culture
2.3. Cytotoxicity Assay
2.4. Establishment and Evaluation of Caco-2 Cell Monolayers
2.5. Transport Studies
2.6. Uptake Experiment
2.7. HPLC Analysis of Samples
2.8. Data Analysis
2.9. Statistical Analysis
3. Results
3.1. Cytotoxicity Test
3.2. Evaluation of Caco-2 Cell Monolayer Integrity
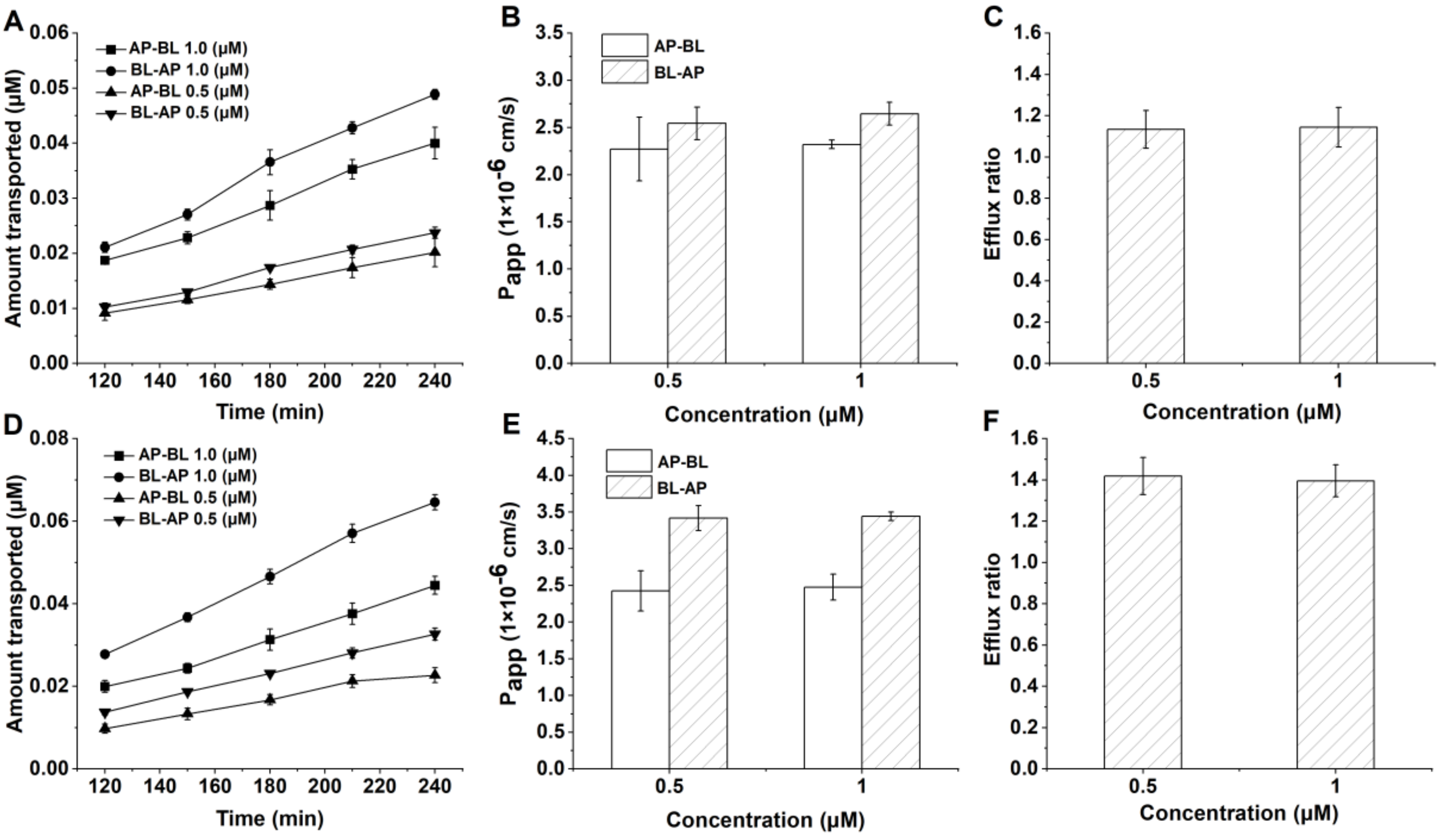
3.3. Transport of FL77-28 and FL77-29 across Caco-2 Cell Monolayers
3.4. Cellular Uptake Kinetic
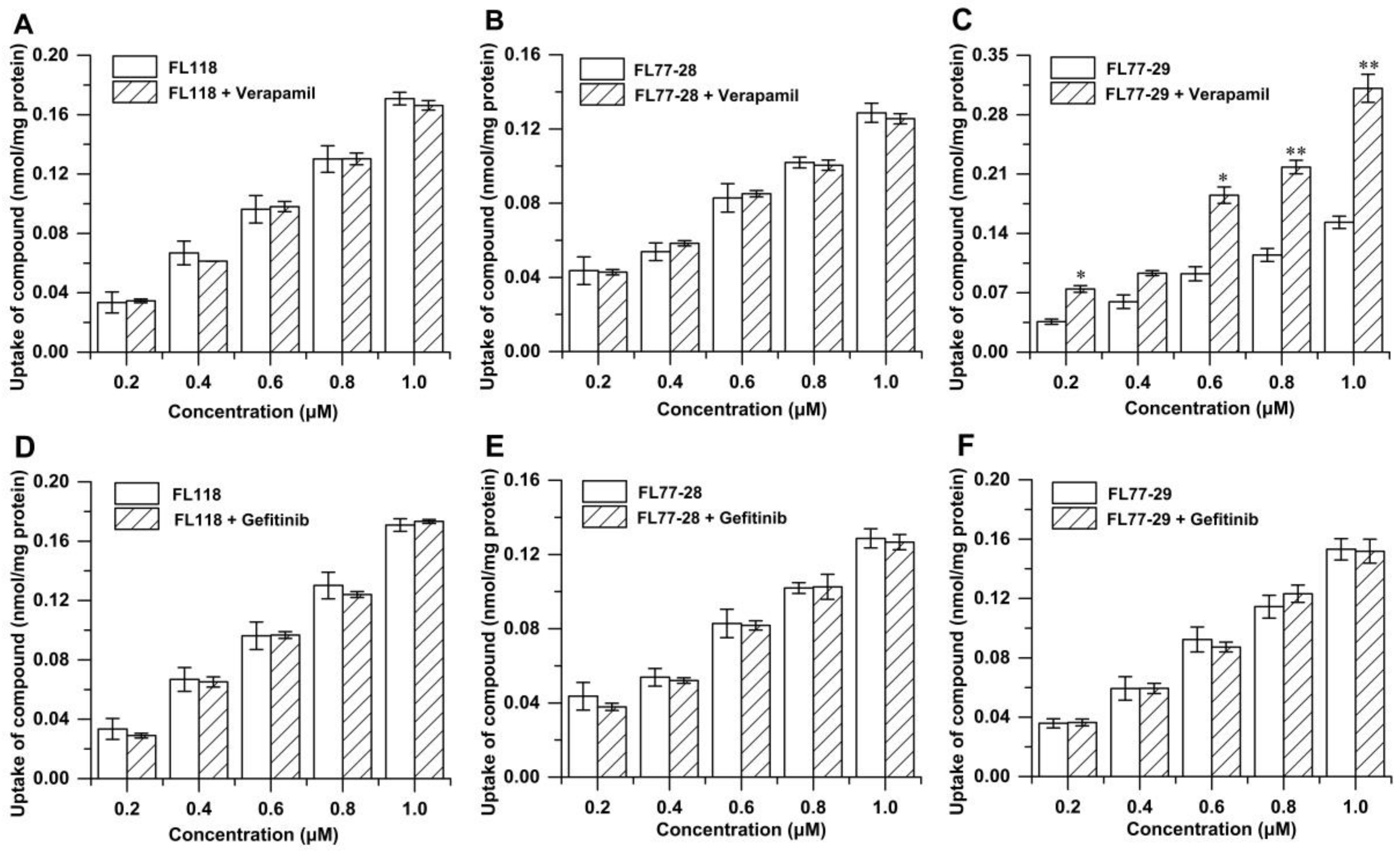
3.4.1. Effect of Temperature on the Uptake of FL118, FL77-28, and FL77-29
3.4.2. Effects of Transporter Inhibitors on the Uptake of FL118, FL77-28, and FL77-29
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Li, W.; Zhang, H.; Assaraf, Y.G.; Zhao, K.; Xu, X.; Xie, J.; Yang, D.-H.; Chen, Z.-S. Overcoming ABC transporter-mediated multidrug resistance: Molecular mechanisms and novel therapeutic drug strategies. Drug Resist. Updates 2016, 27, 14–29. [Google Scholar] [CrossRef]
- Mohammad, I.S.; He, W.; Yin, L. Understanding of human ATP binding cassette superfamily and novel multidrug resistance modulators to overcome MDR. Biomed. Pharmacother. 2018, 100, 335–348. [Google Scholar] [CrossRef]
- Nussinov, R.; Tsai, C.-J.; Jang, H. Anticancer drug resistance: An update and perspective. Drug Resist. Updates 2021, 59, 100796. [Google Scholar] [CrossRef] [PubMed]
- Estudante, M.; Morais, J.G.; Soveral, G.; Benet, L.Z. Intestinal drug transporters: An overview. Adv. Drug Deliv. Rev. 2013, 65, 1340–1356. [Google Scholar] [CrossRef]
- Gottesman, M.; Lavi, O.; Hall, M.; Gillet, J.-P. Toward a Better Understanding of the Complexity of Cancer Drug Resistance. Annu. Rev. Pharmacol. Toxicol. 2016, 56, 85–102. [Google Scholar] [CrossRef]
- Kümler, I.; Stenvang, J.; Moreira, J.; Brünner, N.; Nielsen, D.L. Drug transporters in breast cancer: Response to anthracyclines and taxanes. Expert Rev. Anticancer Ther. 2015, 15, 1075–1092. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, B.M.F.; Cardoso, D.S.P.; Ferreira, M.-J.U. Overcoming Multidrug Resistance: Flavonoid and Terpenoid Nitrogen-Containing Derivatives as ABC Transporter Modulators. Molecules 2020, 25, 3364. [Google Scholar] [CrossRef] [PubMed]
- Martino, E.; Della Volpe, S.; Terribile, E.; Benetti, E.; Sakaj, M.; Centamore, A.; Sala, A.; Collina, S. The long story of camptothecin: From traditional medicine to drugs. Bioorg. Med. Chem. Lett. 2017, 27, 701–707. [Google Scholar] [CrossRef]
- Khaiwa, N.; Maarouf, N.R.; Darwish, M.H.; Alhamad, D.W.M.; Sebastian, A.; Hamad, M.; Omar, H.A.; Orive, G.; Al-Tel, T.H. Camptothecin’s journey from discovery to WHO Essential Medicine: Fifty years of promise. Eur. J. Med. Chem. 2021, 223, 113639. [Google Scholar] [CrossRef]
- Bailly, C. Irinotecan: 25 years of cancer treatment. Pharmacol. Res. 2019, 148, 104398. [Google Scholar] [CrossRef]
- Lorusso, D.; Pietragalla, A.; Mainenti, S.; Masciullo, V.; Di Vagno, G.; Scambia, G. Review role of topotecan in gynaecological cancers: Current indications and perspectives. Crit. Rev. Oncol. Hematol. 2010, 74, 163–174. [Google Scholar] [CrossRef]
- Nakatomi, K.; Yoshikawa, M.; Oka, M.; Ikegami, Y.; Hayasaka, S.; Sano, K.; Shiozawa, K.; Kawabata, S.; Soda, H.; Ishikawa, T.; et al. Transport of 7-ethyl-10-hydroxycamptothecin (SN-38) by breast cancer resistance protein ABCG2 in human lung cancer cells. Biochem. Biophys. Res. Commun. 2001, 288, 827–832. [Google Scholar] [CrossRef]
- de Vries, N.A.; Zhao, J.; Kroon, E.; Buckle, T.; Beijnen, J.H.; van Tellingen, O. P-glycoprotein and breast cancer resistance protein: Two dominant transporters working together in limiting the brain penetration of topotecan. Clin. Cancer Res. 2007, 13, 6440–6449. [Google Scholar] [CrossRef] [Green Version]
- Filipski, E.; Berland, E.; Ozturk, N.; Guettier, C.; van der Horst, G.T.; Lévi, F.; Okyar, A. Optimization of irinotecan chronotherapy with P-glycoprotein inhibition. Toxicol. Appl. Pharmacol. 2014, 274, 471–479. [Google Scholar] [CrossRef]
- Ling, X.; Cao, S.; Cheng, Q.; Keefe, J.T.; Rustum, Y.M.; Li, F. A novel small molecule FL118 that selectively inhibits survivin, Mcl-1, XIAP and cIAP2 in a p53-independent manner, shows superior antitumor activity. PLoS ONE 2012, 7, e45571. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Liu, Z.; Zhang, D.; Liu, R.; Lin, Q.; Liu, J.; Yang, Z.; Ma, Q.; Sun, D.; Zhou, X.; et al. FL118, a novel survivin inhibitor, wins the battle against drug-resistant and metastatic lung cancers through inhibition of cancer stem cell-like properties. Am. J. Transl. Res. 2017, 9, 3676–3686. [Google Scholar]
- Westover, D.; Ling, X.; Lam, H.; Welch, J.; Jin, C.; Gongora, C.; Del Rio, M.; Wani, M.; Li, F. FL118, a novel camptothecin derivative, is insensitive to ABCG2 expression and shows improved efficacy in comparison with irinotecan in colon and lung cancer models with ABCG2-induced resistance. Mol. Cancer 2015, 14, 92–103. [Google Scholar] [CrossRef] [Green Version]
- Ling, X.; Liu, X.; Zhong, K.; Smith, N.; Prey, J.; Li, F. FL118, a novel camptothecin analogue, overcomes irinotecan and topotecan resistance in human tumor xenograft models. Am. J. Transl. Res. 2015, 7, 1765–1781. [Google Scholar]
- Li, Q.Y.; Zu, Y.G.; Shi, R.Z.; Yao, L.P. Review camptothecin: Current perspectives. Curr. Med. Chem. 2006, 13, 2021–2039. [Google Scholar] [CrossRef]
- Fan, S.; Cao, Y.-X.; Li, G.-Y.; Lei, H.; Attiogbe, M.K.I.; Yao, J.-C.; Yang, X.-Y.; Liu, Y.-J.; Hei, Y.-Y.; Zhang, H.; et al. F10, a new camptothecin derivative, was identified as a new orally–bioavailable, potent antitumor agent. Eur. J. Med. Chem. 2020, 202, 112528. [Google Scholar] [CrossRef]
- Dallavalle, S.; Merlini, L.; Morini, G.; Musso, L.; Penco, S.; Beretta, G.L.; Tinelli, S.; Zunino, F. Synthesis and cytotoxic activity of substituted 7-aryliminomethyl derivatives of camptothecin. Eur. J. Med. Chem. 2004, 39, 507–513. [Google Scholar] [CrossRef]
- Dallavalle, S.; Giannini, G.; Alloatti, D.; Casati, A.; Marastoni, E.; Musso, L.; Merlini, L.; Morini, G.; Penco, S.; Pisano, C.; et al. Synthesis and cytotoxic activity of polyamine analogues of camptothecin. J. Med. Chem. 2006, 49, 5177–5186. [Google Scholar] [CrossRef]
- Zhou, L.; Weng, Q.; Zheng, Y.; Zhou, Y.; Li, Q.; Li, F. Uptake and efflux of FL118 and two FL118 derivatives in 3D cell model. Cytotechnology 2019, 71, 785–795. [Google Scholar] [CrossRef]
- Pires, C.L.; Praça, C.; Martins, P.A.T.; Batista de Carvalho, A.L.M.; Ferreira, L.; Marques, M.P.M.; Moreno, M.J. Re-Use of Caco-2 Monolayers in Permeability Assays—Validation Regarding Cell Monolayer Integrity. Pharmaceutics 2021, 13, 1563. [Google Scholar] [CrossRef]
- Zeng, Z.; Shen, Z.L.; Zhai, S.; Xu, J.L.; Liang, H.; Shen, Q.; Li, Q.Y. Transport of curcumin derivatives in Caco-2 cell monolayers. Eur. J. Pharm. Biopharm. 2017, 117, 123–131. [Google Scholar] [CrossRef]
- Fossati, L.; Dechaume, R.; Hardillier, E.; Chevillon, D.; Prevost, C.; Bolze, S.; Maubon, N. Use of simulated intestinal fluid for Caco-2 permeability assay of lipophilic drugs. Int. J. Pharm. 2008, 360, 148–155. [Google Scholar] [CrossRef]
- Hubatsch, I.; Ragnarsson, E.G.; Artursson, P. Determination of drug permeability and prediction of drug absorption in Caco-2 monolayers. Nat. Protoc. 2007, 2, 2111–2119. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Hu, W.; Zhang, X.; Wang, Y.; Zhuang, W.; Li, F.; Li, Q. Cellular Uptake and Transport Characteristics of FL118 Derivatives in Caco-2 Cell Monolayers. Chem. Pharm. Bull. 2021, 69, 1054–1060. [Google Scholar] [CrossRef]
- Weng, Q.; Zhou, L.; Xia, L.; Zheng, Y.; Zhang, X.; Li, F.; Li, Q. In vitro evaluation of FL118 and 9-Q20 cytotoxicity and cellular uptake in 2D and 3D different cell models. Cancer Chemother. Pharmacol. 2019, 84, 527–537. [Google Scholar] [CrossRef]
- Biganzoli, E.; Cavenaghi, L.A.; Rossi, R.; Brunati, M.C.; Nolli, M.L. Use of a Caco-2 cell culture model for the characterization of intestinal absorption of antibiotics. Farmaco 1999, 54, 594–599. [Google Scholar] [CrossRef]
- Antunes, F.; Andrade, F.; Ferreira, D.; Nielsen, H.M.; Sarmento, B. Models to predict intestinal absorption of therapeutic peptides and proteins. Curr. Drug Metab. 2013, 14, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Proctor, W.R.; Ming, X.; Thakker, D.R. Enzyme and Transporter Based Drug–Drug Interactions; Springer: New York, NY, USA, 2010; p. 268. [Google Scholar]
- Troutman, M.D.; Thakker, D.R. Efflux ratio cannot assess P-glycoprotein-mediated attenuation of absorptive transport: Asymmetric effect of P-glycoprotein on absorptive and secretory transport across Caco-2 cell monolayers. Pharm. Res. 2003, 20, 1200–1209. [Google Scholar] [CrossRef] [PubMed]
- Mukhametov, A.; Raevsky, O.A. On the mechanism of substrate/non-substrate recognition by P-glycoprotein. J. Mol. Graph. Model. 2017, 71, 227–232. [Google Scholar] [CrossRef]
- Sugano, K.; Kansy, M.; Artursson, P.; Avdeef, A.; Bendels, S.; Di, L.; Ecker, G.F.; Faller, B.; Fischer, H.; Gerebtzoff, G.; et al. Coexistence of passive and carrier-mediated processes in drug transport. Nat. Rev. Drug Discov. 2010, 9, 597–614. [Google Scholar] [CrossRef] [PubMed]

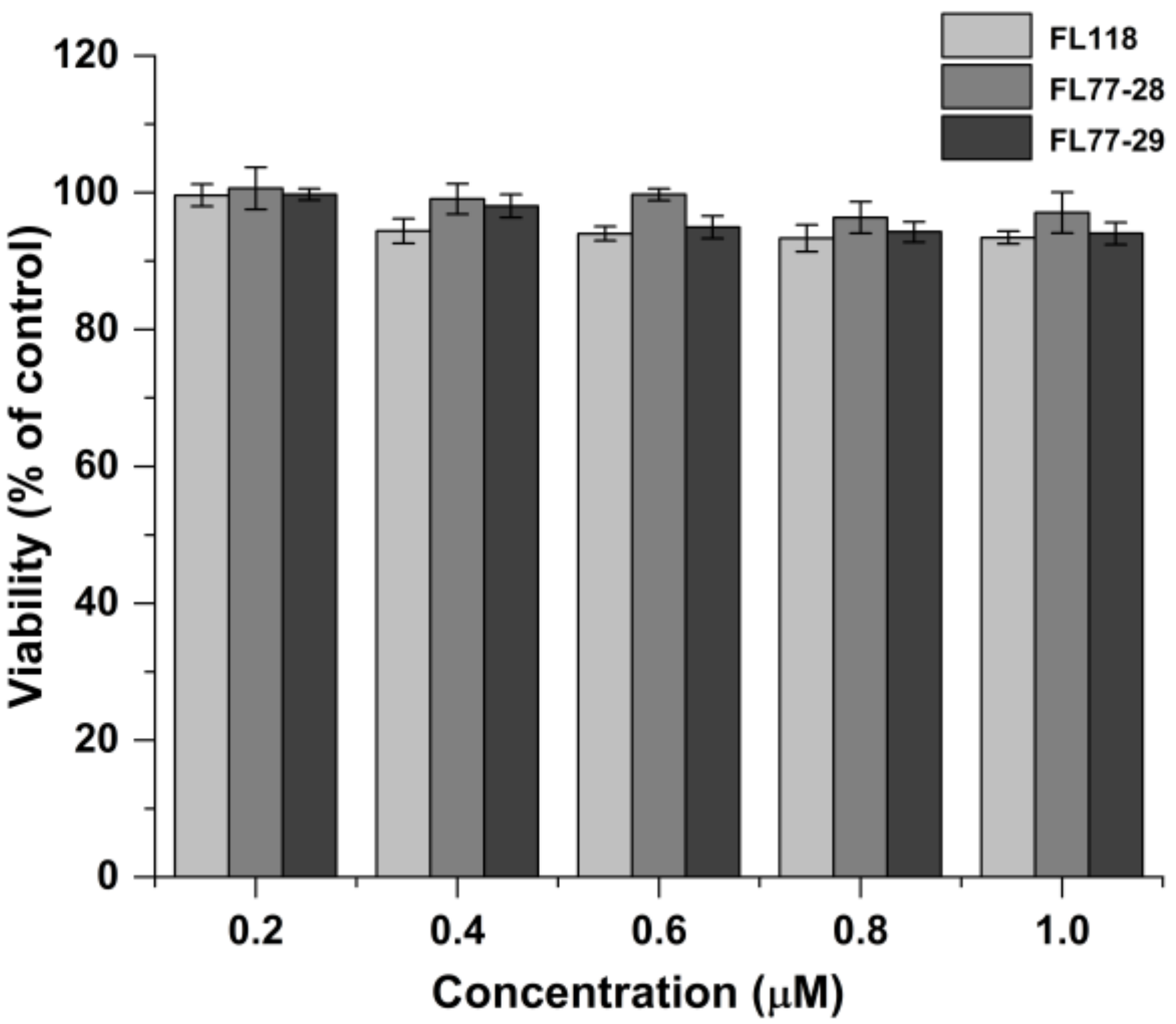

| Compound | HCT 116 | Hep G2 | A549 | HeLa |
|---|---|---|---|---|
| IC50 (μM) | ||||
| FL77-28 | 0.045 ± 0.014 | 0.065 ± 0.001 | 0.048 ± 0.007 | 0.022 ± 0.007 |
| FL77-29 | 0.025 ± 0.003 | 0.034 ± 0.002 | 0.028 ± 0.004 | 0.036 ± 0.004 |
| FL118 | 0.050 ± 0.004 | 0.104 ± 0.002 | 0.043 ± 0.004 | 0.040 ± 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhang, X.; Zhuang, W.; Yu, Y.; Sun, X.; Wang, H.; Li, F.; Li, Q. Investigation of the Uptake and Transport of Two Novel Camptothecin Derivatives in Caco-2 Cell Monolayers. Molecules 2022, 27, 3669. https://doi.org/10.3390/molecules27123669
Wang Y, Zhang X, Zhuang W, Yu Y, Sun X, Wang H, Li F, Li Q. Investigation of the Uptake and Transport of Two Novel Camptothecin Derivatives in Caco-2 Cell Monolayers. Molecules. 2022; 27(12):3669. https://doi.org/10.3390/molecules27123669
Chicago/Turabian StyleWang, Yi, Xiangli Zhang, Wenya Zhuang, Yanlei Yu, Xuanrong Sun, Hong Wang, Fengzhi Li, and Qingyong Li. 2022. "Investigation of the Uptake and Transport of Two Novel Camptothecin Derivatives in Caco-2 Cell Monolayers" Molecules 27, no. 12: 3669. https://doi.org/10.3390/molecules27123669







