The New Di-Gold Metallotweezer Based on an Alkynylpyridine System
Abstract
:1. Introduction
2. Results and Discussion
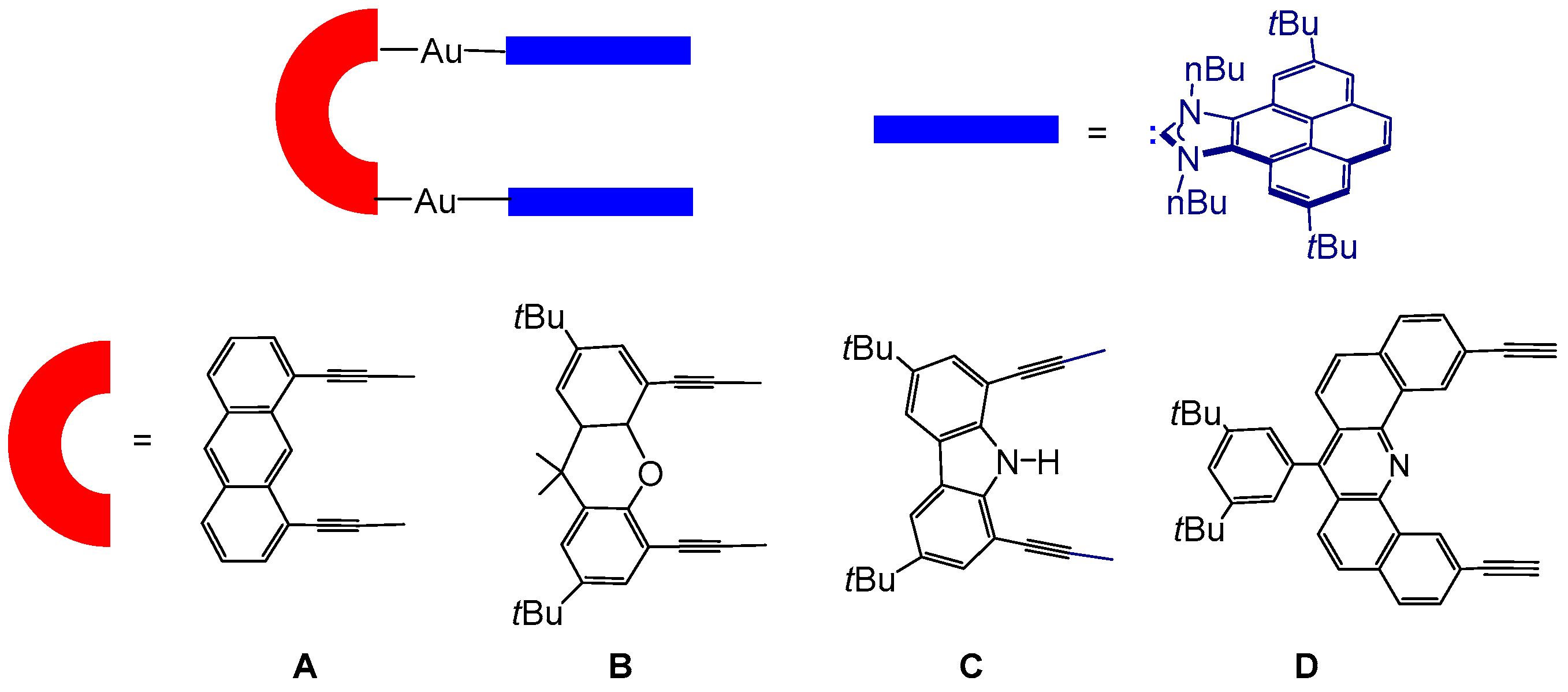
2.1. Synthesis and Characterization
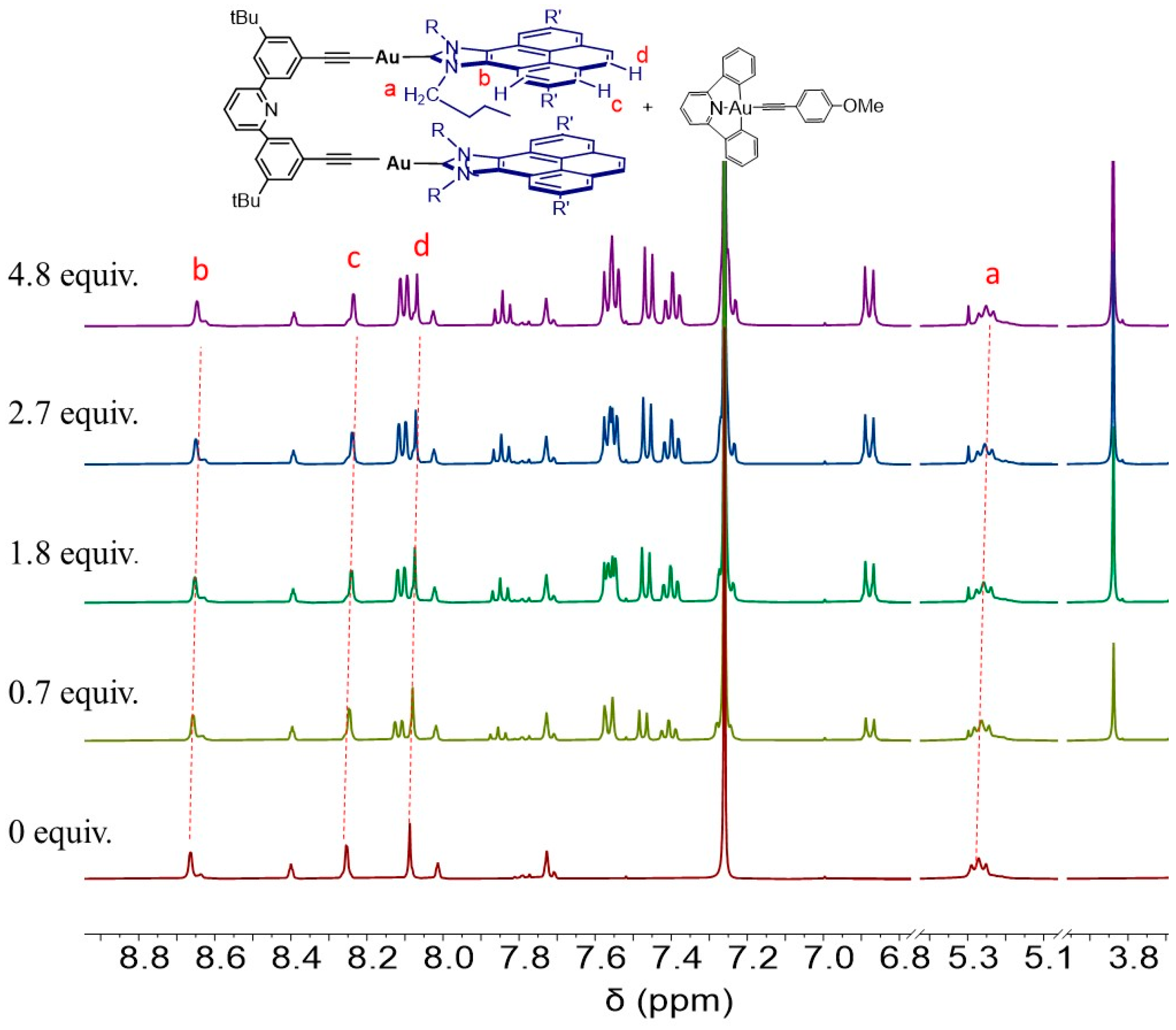
2.2. Molecular Recognition: Determination of Binding Affinities
2.3. Photophysical Characterization
3. Conclusions
4. Materials and Methods
4.1. General Procedures
4.2. Physical Measurements
4.3. Synthesis and Characterization
4.3.1. Synthesis of 2
4.3.2. Synthesis of 3@2
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Galan, A.; Ballester, P. Stabilization of reactive species by supramolecular encapsulation. Chem. Soc. Rev. 2016, 45, 1720–1737. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, M.; Brunaghim, J.L.; Raymond, K.N. Stabilization of a Reactive Cationic Species by Supramolecular Encapsulation. Angew. Chem. Int. Ed. 2000, 39, 4119–4121. [Google Scholar] [CrossRef]
- Koshland, D.E. Correlation of Structure and Function in Enzyme Action. Science 1963, 142, 1533–1541. [Google Scholar] [CrossRef] [PubMed]
- Koshland, D.E. The Key—Lock Theory and the Induced Fit Theory. Angew. Chem. Int. Ed. 1994, 33, 2375–2378. [Google Scholar] [CrossRef]
- Wang, S.T.; Sawada, T.; Ohara, K.; Yamaguchi, K.; Fujita, M. Capsule–Capsule Conversion by Guest Encapsulation. Angew. Chem. Int. Ed. 2016, 55, 2063–2066. [Google Scholar] [CrossRef]
- Hiraoka, S.; Harano, K.; Nakamura, T.; Shiro, M.; Shionoya, M. Induced-Fit Formation of a Tetrameric Organic Capsule Consisting of Hexagram-Shaped Amphiphile Molecules. Angew. Chem. Int. Ed. 2009, 48, 7006–7009. [Google Scholar] [CrossRef]
- Kasai, K.; Aoyagi, M.; Fujita, M. Flexible Coordination Networks with Fluorinated Backbones. Remarkable Ability for Induced-Fit Enclathration of Organic Molecules. J. Am. Chem. Soc. 2000, 122, 2140–2141. [Google Scholar] [CrossRef]
- Zhan, Y.Y.; Kojima, T.; Nakamura, T.; Takahashi, T.; Takahashi, S.; Habeta, Y.; Shoji, Y.; Maeda, H.; Fukushima, T.; Hiraoka, S. Induced-fit expansion and contraction of a self-assembled nanocube finely responding to neutral and anionic guests. Nat. Commun. 2018, 9, 4530. [Google Scholar] [CrossRef] [Green Version]
- Ibáñez, S.; Poyatos, M.; Peris, E. Cation-Driven Self-Assembly of a Gold(I)-Based Metallo-Tweezer. Angew. Chem. Int. Ed. 2017, 56, 9786–9790. [Google Scholar] [CrossRef]
- Ibáñez, S.; Peris, E. Chemically Tunable Formation of Different Discrete, Oligomeric, and Polymeric Self-Assembled Structures from Digold Metallotweezers. Chem. Eur. J. 2018, 24, 8424–8431. [Google Scholar] [CrossRef]
- Ibáñez, S.; Poyatos, M.; Peris, E. The Complex Coordination Landscape of a Digold(I) U-Shaped Metalloligand. Angew. Chem. Int. Ed. 2018, 57, 16816–16820. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, S.; Peris, E. A Matter of Fidelity: Self-Sorting Behavior of Di-Gold Metallotweezers. Chem. Eur. J. 2019, 25, 8254–8258. [Google Scholar] [CrossRef] [PubMed]
- Biz, C.; Ibáñez, S.; Poyatos, M.; Gusev, D.; Peris, E. Gold(I) Metallo-Tweezers for the Recognition of Functionalized Polycyclic Aromatic Hydrocarbons by Combined pi-pi Stacking and H-Bonding. Chem. Eur. J. 2017, 23, 14439–14444. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, S.; Peris, E. Shape-Adaptability and Redox-Switching Properties of a Di-Gold Metallotweezer. Chem. Eur. J. 2021, 27, 9661–9665. [Google Scholar] [CrossRef]
- Ibáñez, S.; Vicent, C.; Peris, E. Clippane: A Mechanically Interlocked Molecule (MIM) Based on Molecular Tweezers. Angew. Chem. Int. Ed. 2022, 61, e202112513. [Google Scholar] [CrossRef]
- Tiekink, E.R.T. Supramolecular assembly of molecular gold(I) compounds: An evaluation of the competition and complementarity between aurophilic (Au⋯Au) and conventional hydrogen bonding interactions. Coord. Chem. Rev. 2014, 41, 370–412. [Google Scholar] [CrossRef]
- Cheung, S.K.; Yip, S.K.; Yam, V.W.W. Synthesis, characterization, electrochemistry and luminescence studies of heterometallic gold(I)–rhenium(I) alkynyl complexes. J. Organomet. Chem. 2004, 689, 4451–4462. [Google Scholar] [CrossRef]
- McArdle, C.P.; Van, S.; Jennings, M.C.; Puddephatt, R.J. Gold(I) Macrocycles and Topologically Chiral [2] Catenanes. J. Am. Chem. Soc. 2002, 124, 3959–3965. [Google Scholar] [CrossRef]
- Lu, W.; Xiang, H.F.; Zhu, N.Y.; Che, C.M. The 3(ππ*) Emission of Cy3PAu(C⋮C)nAuPCy3 (n = 3, 4). Effect of Chain Length upon Acetylenic 3(ππ*) Emission. Organometallics 2002, 21, 2343–2346. [Google Scholar] [CrossRef]
- Puddephatt, R.J. Coordination polymers: Polymers, rings and oligomers containing gold(I) centres. Coord. Chem. Rev. 2001, 216, 313–332. [Google Scholar] [CrossRef]
- Stoddart, J.F. The chemistry of the mechanical bond. Chem. Soc. Rev. 2009, 38, 1802–1820. [Google Scholar] [CrossRef] [PubMed]
- Leblond, J.; Petitjean, A. Molecular Tweezers: Concepts and Applications. ChemPhysChem 2011, 12, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Hardouin-Lerouge, M.; Hudhomme, P.; Salle, M. Molecular clips and tweezers hosting neutral guests. Chem. Soc. Rev. 2011, 40, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Wong, K.M.C.; Yam, V.W.W. Platinum-based phosphorescent double-decker tweezers: A strategy for extended heterologous metal-metal interactions. Angew. Chem. Int. Ed. 2013, 52, 14117–14120. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.K.W.; Yam, V.W.W. Precise Modulation of Molecular Building Blocks from Tweezers to Rectangles for Recognition and Stimuli-Responsive Processes. Acc. Chem. Res. 2018, 51, 3041–3051. [Google Scholar] [CrossRef]
- Kong, F.K.W.; Chan, A.K.W.; Ng, M.; Low, K.H.; Yam, V.W.W. Construction of Discrete Pentanuclear Platinum(II) Stacks with Extended Metal-Metal Interactions by Using Phosphorescent Platinum(II) Tweezers. Angew. Chem. Int. Ed. 2017, 56, 15103–15107. [Google Scholar] [CrossRef]
- Tanaka, Y.; Wong, K.M.C.; Yam, V.W.W. Phosphorescent molecular tweezers based on alkynylplatinum(ii) terpyridine system: Turning on of NIR emission via heterologous Pt⋯M interactions (M = PtII, PdII, AuIII and AuI). Chem. Sci. 2012, 3, 1185–1191. [Google Scholar] [CrossRef]
- Wong, K.M.C.; Yam, V.W.W. Self-Assembly of Luminescent Alkynylplatinum(II) Terpyridyl Complexes: Modulation of Photophysical Properties through Aggregation Behavior. Acc. Chem. Res. 2011, 44, 424–434. [Google Scholar] [CrossRef]
- Yuan, M.; Zhang, X.; Han, Y.; Wang, F.; Wang, F. Organoplatinum(II)-Based Self-Complementary Molecular Tweezers with Guest-Induced Fluorochromic Behaviors. Inorg. Chem. 2020, 59, 14134–14140. [Google Scholar] [CrossRef]
- Zhang, X.; Han, Y.; Liu, G.; Wang, F. Macrocyclic versus acyclic preorganization in organoplatinum(II)-based host–guest complexes. Chin. Chem. Lett. 2019, 30, 1927–1930. [Google Scholar] [CrossRef]
- Zhang, X.; Ao, L.; Han, Y.; Gao, Z.; Wang, F. Modulating Pt⋯Pt metal–metal interactions through conformationally switchable molecular tweezer/guest complexation. Chem. Commun. 2018, 54, 1754–1757. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Han, Y.; Gao, Z.; Wang, F. Multicomponent Assembled Systems Based on Platinum(II) Terpyridine Complexes. Acc. Chem. Res. 2018, 51, 2719–2729. [Google Scholar] [CrossRef] [PubMed]
- Lui, M.; Han, Y.; Zhong, H.; Zhang, X.; Wang, F. Supramolecular Chirogenesis Induced by Platinum(II) Tweezers with Excellent Environmental Tolerance. Angew. Chem. Int. Ed. 2021, 60, 3498–3503. [Google Scholar] [CrossRef]
- Li, Z.; Han, Y.; Gao, Z.; Wang, F. Supramolecular Engineering of Discrete Pt(II)···Pt(II) Interactions for Visible-Light Photocatalysis. ACS Catal. 2017, 7, 4676–4681. [Google Scholar] [CrossRef]
- Ibáñez, S.; Poyatos, M.; Peris, E. Gold Catalysts with Polyaromatic-NHC ligands. Enhancement of Activity by Addition of Pyrene. Organometallics 2017, 36, 1447–1451. [Google Scholar] [CrossRef]
- Wong, K.M.C.; Hung, L.L.; Lam, W.H.; Zhu, N.; Yam, V.W.W. A Class of Luminescent Cyclometalated Alkynylgold(III) Complexes: Synthesis, Characterization, and Electrochemical, Photophysical, and Computational Studies of [Au(C∧N∧C)(C⋮CR)] (C∧N∧C = κ3C,N,C Bis-cyclometalated 2,6-Diphenylpyridyl). J. Am. Chem. Soc. 2007, 129, 4350–4365. [Google Scholar] [CrossRef]
- Lowe, A.J.; Pfeffer, F.M.; Thordarson, P. Determining binding constants from 1H NMR titration data using global and local methods: A case study using [n]polynorbornane-based anion hosts. Supramol. Chem. 2012, 24, 585–594. [Google Scholar] [CrossRef]
- Thordarson, P. Determining association constants from titration experiments in supramolecular chemistry. Chem. Soc. Rev. 2011, 40, 1305–1323. [Google Scholar] [CrossRef]
- Dale, E.J.; Vermeuken, N.A.; Thomas, A.A.; Barnes, J.C.; Juricek, M.; Blackburn, A.K.; Strutt, N.L.; Sarjeant, A.A.; Stern, C.L.; Denmark, S.E.; et al. ExCage. J. Am. Chem. Soc. 2014, 136, 10669–10682. [Google Scholar] [CrossRef]
- Barnes, J.C.; Juricek, M.; Strutt, N.L.; Frasconi, M.; Sampath, S.; Giesener, M.A.; McGrier, P.L.; Bruns, C.J.; Stern, C.L.; Sarjeant, A.A.; et al. ExBox: A Polycyclic Aromatic Hydrocarbon Scavenger. J. Am. Chem. Soc. 2013, 135, 183–192. [Google Scholar] [CrossRef]
- Tanaka, Y.; Wong, K.M.-C.; Yam, V.W.W. Host-guest interactions of phosphorescent molecular tweezers based on an alkynylplatinum(II) terpyridine system with polyaromatic hydrocarbons. Chem. Eur. J. 2013, 19, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, S.; Peris, E. “Lock and Key” and “Induced-Fit” Host-Guest Models in Two Digold(I)-Based Metallotweezers. Inorg. Chem. 2022, 61. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Raghuvanshi, A.; Saini, A.K.; Mobin, S.M. Anthracene derived dinuclear gold(I) diacetylide complexes: Synthesis, photophysical properties and supramolecular interactions. J. Organomet. Chem. 2016, 813, 103–109. [Google Scholar] [CrossRef]
- Gonell, S.; Poyatos, M.; Peris, E. Pyrene-Based Bisazolium Salts: From Luminescence Properties to Janus-Type Bis-N-Heterocyclic Carbenes. Chem. Eur. J. 2014, 20, 9716–9724. [Google Scholar] [CrossRef] [PubMed]
- Ibañez, S.; Guerrero, A.; Poyatos, M.; Peris, E. Fluorescent Pyrene-Based Bis-azole Compounds: Synthesis and Photophysical Analysis. Chem. Eur. J. 2015, 21, 10566–10575. [Google Scholar] [CrossRef]
- Liang, Y.; Zhang, P.; Chen, J. Function-oriented design of conjugated carbonyl compound electrodes for high energy lithium batteries. Chem. Sci. 2013, 4, 1330–1337. [Google Scholar] [CrossRef]
- Cave, G.W.V.; Fanizzi, F.P.; Deeth, R.J.; Errington, W.; Rourke, J.P. C−H Activation Induced by Water. Monocyclometalated to Dicyclometalated: C∧N∧C Tridentate Platinum Complexes. Organometallics 2000, 19, 1355–1364. [Google Scholar] [CrossRef]

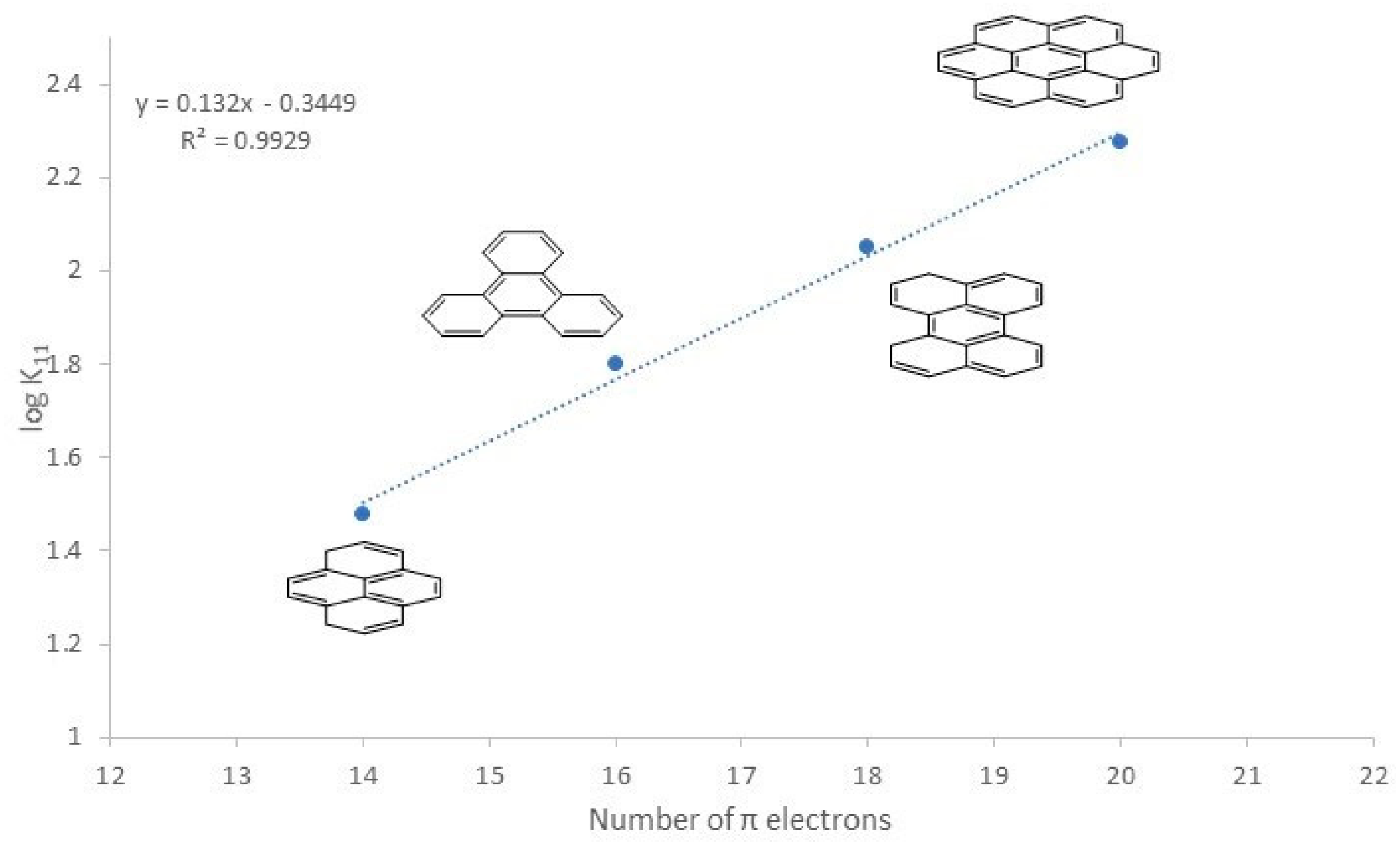
| Entry | Guest | K11 | logK11 |
|---|---|---|---|
| 1 | pyrene | 30 ± 2 | 1.36 |
| 2 | triphenylene | 63 ± 8 | 1.80 |
| 3 | perylene | 112 ± 8 | 2.05 |
| 4 | coronene | 188 ± 24 | 2.28 |
| 5 | 1-pyrenyl-methanol | 65 ± 8 | 1.82 |
| 6 | 3-perylenyl-methanol | 169 ± 12 | 2.23 |
| 7 | TNFLU | 0 | 0 |
| 8 | NTCDI | 155 ± 8 | 2.19 |
| 9 | Pt(C^N^C)(CO) | 31 ± 0,5 | 1.49 |
| 10 | 3 | 946 ± 51 | 2.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibáñez, S. The New Di-Gold Metallotweezer Based on an Alkynylpyridine System. Molecules 2022, 27, 3699. https://doi.org/10.3390/molecules27123699
Ibáñez S. The New Di-Gold Metallotweezer Based on an Alkynylpyridine System. Molecules. 2022; 27(12):3699. https://doi.org/10.3390/molecules27123699
Chicago/Turabian StyleIbáñez, Susana. 2022. "The New Di-Gold Metallotweezer Based on an Alkynylpyridine System" Molecules 27, no. 12: 3699. https://doi.org/10.3390/molecules27123699






