Abstract
Acanthocereus tetragonus (L.) Hummelinck is used as an alternative food source in some Mexican communities. It has been shown that the young stems of A. tetragonus provide crude protein, fiber, and essential minerals for humans. In this work, we analyzed the phytochemical profile, the total phenolic content (TPC), and the antioxidant activity of cooked and crude samples of A. tetragonus to assess its functional metabolite contribution to humans. The phytochemical profile was analyzed using Ultra-High-Performance Liquid Chromatography coupled to High-Resolution Mass Spectrometry (UHPLC-PDA-HESI-Orbitrap-MS/MS). Under the proposed conditions, 35 metabolites were separated and tentatively identified. Of the separated metabolites, 16 occurred exclusively in cooked samples, 6 in crude samples, and 9 in both crude and cooked samples. Among the detected compounds, carboxylic acids, such as threonic, citric, and malic acids, phenolic acids, and glycosylated flavonoids (luteolin-O-rutinoside) were detected. The TPC and antioxidant activity were analyzed using the Folin–Ciocalteu method and the 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical inhibition method, respectively. The TPC and antioxidant activity were significantly reduced in the cooked samples. We found that some metabolites remained intact after the cooking process, suggesting that A. tetragonus represents a source of functional metabolites for people who consume this plant species.
1. Introduction
The arid and semi-arid zones of Mexico possess ecosystems with a high diversity of plants with potential applications; one of the main representative species from the arid Mexican environments are cacti species [1], which are traditionally used for food, traditional medicine, and construction [2]. It has been proposed that the Cactaceae family is native to the American continent, grouping around 2000 species, being Mexico the country with the highest diversity [3]. Cacti species are succulent plants that have suffered anatomical and physiological changes to adapt to the harsh environment [4] and whose morphology varies according to each genus and species. The Cactaceae Family includes four subfamilies, i.e., Opuntioideae, Pereskioideae, Cactoideae, and Maihuenioideae, and 63 genera [4]. It has been proposed that about 78% of cacti species are endemic; nevertheless, around 57% of the cactofloristic diversity is used by man for horticulture, food, medicine, forage, and handicrafts production [5]. Within the cacti family, the Opuntia genus is one of the most studied due to its nutritional interest.
Cacti species are characterized by the production of mucilage, which, in conjunction with different tissues, represents a source of functional metabolites for humans. Within the functional metabolites, phenolics have gained strong attention since they impact the quality of both fresh and processed plant-based foodstuffs, and their contribution to the maintenance of human health has been described [6]. Thus, analytical techniques such as liquid chromatography coupled with high-resolution mass spectrometry represent a tool for analyzing and discovering compounds with functional properties [7,8].
In Mexican gastronomy, the young stems of Acanthocereus tetragonus, traditionally known as cruzeta or jacube, are also used as a food source [9]. A. tetragonus is a columnar cactus distributed in coastal areas of the American continent in warm, sub-humid, and semi-arid regions [10,11]. A. tetragonus is traditionally collected from wild populations, and the young stems are sold at prices of around USD 1 per 150 g of fresh chopped young stems in local markets and urban venues (see Figure 1a–c), especially in the state of Veracruz, Mexico [12], where they represent a source of alternative food in some isolated regions. Regarding their chemical composition, it has been demonstrated that they are rich in proteins, fiber, and minerals such as phosphorus, potassium, magnesium, sodium, and copper, in addition to containing a high level of calcium [13]; nevertheless, as far as we know, no information exists regarding the phytochemical profile of A. tetragonus. In this study, we analyzed, by means of Ultra-High-Performance Liquid Chromatography coupled to High-Resolution Mass Spectrometry (UHPLC-PDA-HESI-Orbitrap-MS/MS), the phytochemical profile of cooked and crude samples of A. tetragonus to assess its functional metabolite contribution to the human diet.

Figure 1.
(a): Young stems of Acanthocereus tetragonus growing in the wild. (b,c): A. tetragonus and other vegetables produced by familiar farms and sold in local markets in northern Veracruz, México.
2. Results and Discussion
2.1. Total Phenolic Content and Antioxidant Activity of Crude and Cooked Samples of A. tetragonus
Table 1 shows the results obtained for the total phenolic content and antioxidant activity of cooked and crude samples of A. tetragonus. The crude samples of A. tetragonus showed a higher phenolic content (40.79 ± 1.00 µg GAE per mg of DW) when compared with the cooked samples (27.52 ± 1.36 µg GAE per mg of DW). Statistically (p ≤ 0.05), there were significant differences between crude and cooked samples. Regarding the antioxidant activity, the crude samples showed the highest value (86.44%). Our results agree with findings for other plant species, such as Prunus mahaleb (Rosaceae), where the TPC and antioxidant activity were significantly reduced after heating treatment [14]. In this regard, for Bixa orellana (Bixaceae), it has been proposed that the thermal degradation rate of phenolic compounds depends on the temperature applied to the samples, and the degradation curve shows a first-order kinetic behavior [15]. Our evidence suggests that A. tetragonus TPC and antioxidant activity are reduced by the traditional food preparation process. Nevertheless, further investigations are required to evaluate the degradation rate of A. tetragonus compounds and the limits of temperature and exposure time within which food materials should be processed to ensure a minor degradation of the functional metabolites and a balance between their functional and sensory characteristics.

Table 1.
Total phenolic content and antioxidant activity of cooked and crude samples of A. tetragonus. The values represent the mean (n = 3) ± standard deviation. Equal letters indicate no statistically significant differences (p ≤ 0.05).
2.2. Phytochemical Analysis of A. tetragonus Young Stems
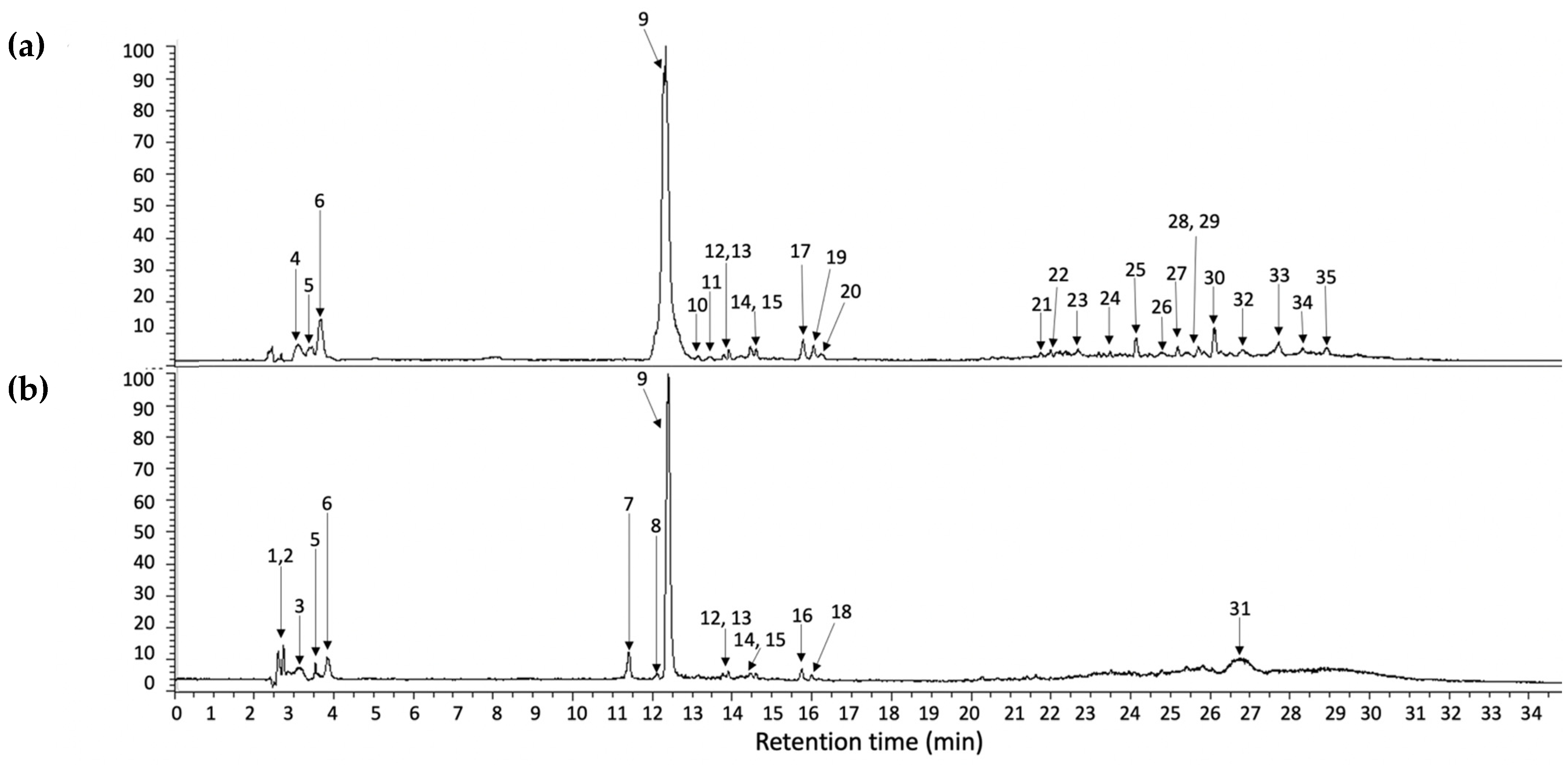
The methanolic extracts of crude and cooked stems of A. tetragonus were analyzed by UHPLC-PDA-HESI-Orbitrap-MS/MS. Under the chromatographic conditions, 35 metabolites were separated and tentatively identified. Of the separated metabolites, 16 occurred in cooked samples, 6 in crude samples, and 9 in both crude and cooked samples. Among the detected compounds, carboxylic acids, such as threonic, citric, and malic acids, phenolic acids, and glycosylated flavonoids (luteolin-O-rutinoside) were tentatively identified. All metabolites showed a mass accuracy below 5 ppm, except for compound 14.
It has been proposed that the cooking process may lead to two main phenomena: (1) the degradation of phytocompounds and (2) an increase in the extractability of compounds. Thus, the degradation or enhanced extractability may vary according to: (1) the food material, (2) the processing parameters, and (3) the chemical nature of the compounds and their thermal stability [16]. In our research, compounds such as simple organic acids (citric acid), phenolic acids (sinapic and eucomic acid), and one flavonoid (luteolin rutinoside) remained present after the thermal process.
Compounds 1 (rt: 2.63 min), 2 (rt: 2.74 min), and 3 (rt: 3.10 min) were only detected in crude samples of A. tetragonus and corresponded to simple organic acids. Compound 1 was identified as threonic acid since the pseudomolecular ion at m/z 135.0293 yielded fragments at m/z 119.0350 and m/z 103.0390, which were generated due to the loss of one and two molecules of water, respectively. This compound has been proposed to have potential as an androgen-driven balding prevention agent [17]. On the other hand, compounds 2 and 3 were identified as 2-hydroxy-succinic acid (malic acid), as proposed by Ledesma-Escobar, et al. [18]. In addition to citric acid, these carboxylic acids are responsible for some fruits’ sourness [19]. Although these compounds may be present in concentrations below the detection limits in cooked samples, our results suggest that the cooking process led to the breakdown of these organic acids. For other systems, it has been demonstrated that the cooking process strongly influences the modification of the pH, mainly in association with the heating rate [20].
Citric acid (compound 5) and three of its derivatives (compounds 4, 6, and 8) were detected in both crude and cooked samples. Citric acid was identified as reported previously [18,21], and its derivatives were identified since the characteristic fragment ions of citric acid were detected within the mass spectrum (see Table 2). Citric acid is a highly demanded organic acid by the food and beverages, pharmaceutical, and personal care industries. In addition, despite being one of the most widely employed and industrially demanded compound, this carboxylic acid still has potential growth in the global market; thus, new sources and alternatives for its production need to be investigated [22]. On the other hand, compound 7 (rt: 11.41 min) was only detected in crude samples and was identified as piscidic acid, since the pseudomolecular ion at m/z 255.0506 yielded fragments at m/z 193.0498 ([M-H-CHO2-OH]−), 165.0549 ([M-H-C2H3O3-OH]−), and 149.0600 ([M-H-2CHO2-OH]−). Piscidic acid has been previously detected in other cacti species such as Coryphantha macromeris [23] and prickly pear cactus (Opuntia ficus indica) [24], and its occurrence in green vegetables has been proposed [25]. This metabolite has also been detected in Opuntia ficus indica cladodes and proposed as one of the metabolites that exert a photoprotective effect against UVA-induced oxidative stress in human keratinocytes [26].

Table 2.
Metabolites identified in cooked and crude young stems of A. tetragonus by UHPLC-PDA-HESI-Orbitrap-MS/MS.
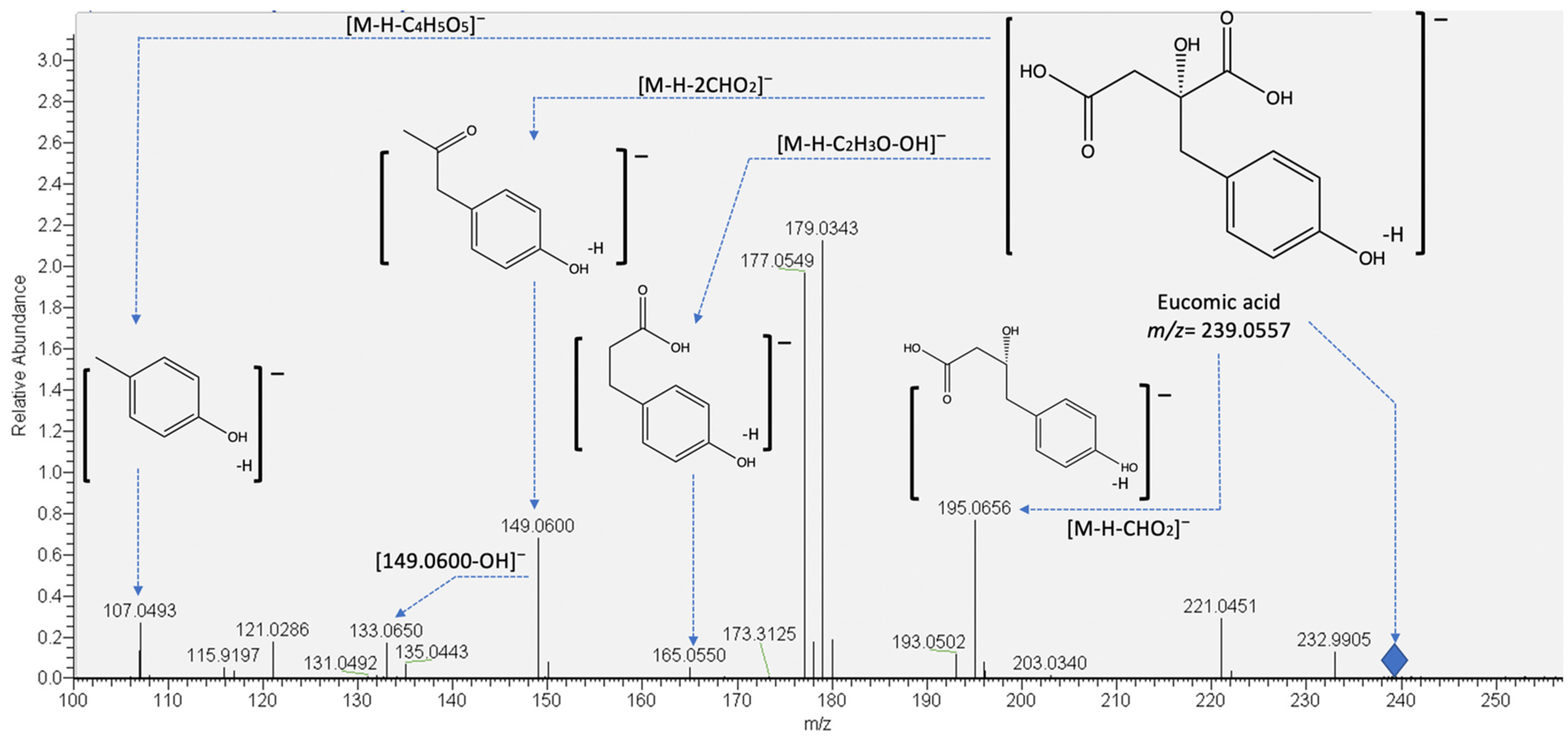
On the other hand, compounds 9 and 10 were identified as eucomic acid isomers, as reported by Hernández, et al. [27]. This metabolite showed the highest relative abundance in the chromatogram (see Figure 2). For this compound, the pseudomolecular ion generated fragments at m/z 195.0656, 149.0600 133.0654, and 107.0502 (see Figure 3), and according to its relative abundance, it may correspond to one of the main metabolites present in crude and cooked samples of A. tetragonus, similar to that found for Opuntia ficus indica cladodes [28] and peel [29]. Eucomic acid has been previously reported in Opuntia ficus indica cladodes [30] and fruits [27] and as a key compound in leaf-opening processes in other plant species such as Lotus japonicus (Fabaceae) [31].
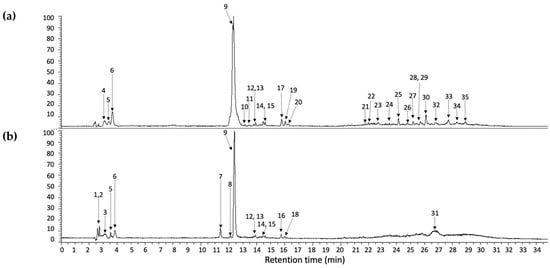
Figure 2.
UHPLC chromatogram integrated at 254, 280, 320, and 440 nm of cooked (a) and crude (b) samples prepared from A. tetragonus. The peak numbers refer to those metabolites indicated in Table 1. The same numbers in “(a,b)” refer to metabolites found in both samples.
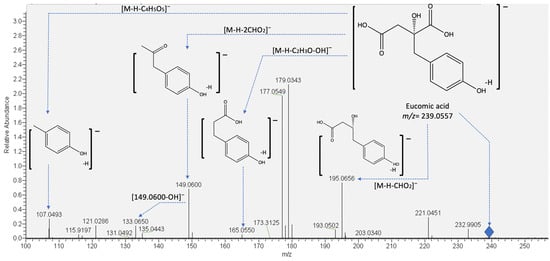
Figure 3.
Proposed fragmentation pattern of eucomic acid (peaks 9 and 10).
Compound 11 was identified as dihydroxybenzoic acid, as reported by Taamalli, et al. [32]. In our research, two flavonoid isomers were also detected. Compounds 12 and 13 were identified as luteolin-O-rutinoside, as proposed by García-Salas, et al. and Taamalli et al. [32,33], being the cleavage of the glycosidic moiety one of the first fragmentation steps followed by the cleavage of the C-C bond in the C ring of the basic flavonoid structure. Our results suggest that the consumption of A. tetragonus young stems contributes to luteolin derivative intake, since these isomers remained present in the samples after the thermal process. Further studies are required to evaluate the concentration of selected compounds after the heating or digestion processes. Luteolin-O-rutinoside has also been reported in other plants such as Setaria italica (Poaceae) [34] and in species of the genus Cynara (Asteraceae) [35]. Compound 14 is also of phenolic nature, and it was identified as diphenylphenol, since the pseudomolecular ion at m/z 245.0928 lost one water molecule, generating one fragment at m/z 229.1076. For this metabolite, no information exists regarding cacti species.
The occurrence of phenolic compounds and flavonoids has been previously reported in other edible cacti species such as Opuntia humifusa fruits [36] and cladodes [37] Hylocereus polyrhizus and H. undatus seeds [38], and Opuntia spp. [39]. For this group of metabolites, the antioxidant properties have been proposed [40,41], which can be influenced by the employed solvents and extraction processes. It has been proposed that the most polar solvents have the best extractive capacity [42,43]. Yahia and Mondragon-Jacobo [44] evaluated the antioxidant capacity of 10 varieties of Opuntia spp., using polar and nonpolar extractions. The polar extracts showed the highest biological activity. It has been demonstrated that the functional properties may also vary depending on the analyzed species [45] and can be affected by maturation process [46] and storage conditions [47] or vary in different sections of the plant [36,37].
In nature, compounds are usually found forming conjugates with carbohydrates. In this regard, for closely related plant species to A. tetragonus, De Leo, et al. [48] analyzed the phytochemical profile and flavonoid content of Opuntia ficus indica flowers, finding that glycosylated flavonoids such as isorhamnetin 3-O-robinobioside were present in high concentrations (42.69 mg/g), followed by isorhamnetin 3-O-galactoside (9.79 mg/g) and quercetin 3-O-ruthinoside (7.09 mg/g). Similarly, for Opuntia ficus indica and Opuntia joconostle byproducts, Mata, et al. [49] and Morales, et al. [50], respectively, found the presence of glycosylated phenolics, being the glycosylated derivatives of isorhamnetin the major compounds in O. joconostle. Jiménez-Aspee, et al. [51] evaluated the presence of metabolites in Eulychinia acida fruits, finding six main compounds, with glycosylated isorhamnetin in the highest concentration. This metabolite has also been detected in Opuntia monacantha extracts, as well as in kaempferol [52]. In our investigation, only one metabolite was a glycosylated flavonoid. Its relative abundance in the chromatogram was similar for crude and cooked samples, suggesting that this compound remains intact after the heating process.
Among the detected compounds, cinnamic and benzoic acids were also identified. Compounds 15, 17, and 19 corresponded to cinnamic acids: compounds 17 and 19 occurred exclusively in cooked samples and were identified as 4-hydroxycinnamic acid isomers since the pseudomolecular ion at m/z 163.0395 yielded one characteristic fragment ion at 119.0495 generated by the loss of CO2. On the other hand, compound 15 occurred in both cooked and crude samples and was identified as sinapic acid. For this metabolite, the pseudomolecular ion at m/z 223.0607 yielded fragments at m/z 193.0501 ([M-H-CH3O]−) and 179.0343 ([M-H-CH3O-CH3]−). Regarding the occurrence of benzoic acids in the samples, compound 20 was identified as gallic acid, as proposed by Zhang, et al. [53]. This metabolite has been reported in commercial and wild Opuntia species [54], and its commercial and potential industrial applications [55], as well as its pharmacological ability to suppress inflammatory responses in induced gastrointestinal disorders in mice [56], have been proposed.
Compound 23 was identified as di-tert-butyl 4-amino-4-(3-(tert-butoxy)-3-oxopropyl) heptanedioate. For this metabolite, the pseudomolecular ion yielded fragments at m/z 342.2284 ([M-H-C4H9O]−) and 270.1710 ([M-H-2C4H9O]−). Compound 24 was identified as 1,4-benzenediol, 2,2’-(6-dodecyne-1,12-diyl) bis [3,6-dimethoxy]− since the pseudomolecular ion at m/z 501.2493 generated fragments at m/z 193.0867 ([C11H13O3]−). Compound 30 was identified as a linolenic acid derivative (Dirhamnosyl linolenic acid), as proposed by Li, et al. [57], and compound 33 as N,N-dihexyl-4-hydroxy-3,5-dimethoxybenzamide, since the parental ion at m/z 364.2494 yielded fragments at m/z 334.2388 ([M-H-CH3O]−) and at m/z 288.0328 ([M-H-2CH3O-OH]−). Similarly, compound 34 was identified as dodecyl 4-O-acetyl-2-O-(4-O-acetyl-6-deoxy-α-L-mannopyranosyl)-6-deoxy-β-D-galactopyranoside. For this metabolite, the pseudomolecular ion at m/z 561.3280 yielded fragments at m/z 515.3221, generated by the loss of two water molecules and one methyl group. As far as we know, this is the first time that these metabolites are reported in A. tetragonus and cacti species.
Compounds 21, 22, 26–32, and 35 were not identified, since the spectrometric evidence did not match the literature’s information. This is interesting, since these metabolites may correspond to new unreported compounds. Furthermore, according to the UV absorption pattern of these compounds (ca. 275 nm), they may contain a phenolic ring within their structure. Our results indicate, for the first time, the metabolite profile of A. tetragonus; further studies are required to assess each compound’s total content and elucidate the structure of unknown metabolites.
3. Materials and Methods
3.1. Sample Collection and Preparation for Phytochemical Analysis
The young stems of Acanthocereus tetragonus were collected near the “Cerro de Horcones” community from the municipality of Alamo-Temapache, Veracruz, Mexico (21.073783, −97.782116) in November 2020. The collected samples were dethorned, and then the cuticle was removed manually. For the cooking process, each sample (2 kg) was sliced and boiled in water (1:3 p/v) at 90 °C for 15 min. Once the cooking process was achieved, the crude and cooked samples were dried in an oven at 40 °C (Gallenkamp, London, UK) for one week in dark conditions and then pulverized in a mortar. The resultant powder was subjected to extraction with methanol (three times, 1:3 p/v) in an ultrasonic bath (30 min each time). The resulting extract was filtered and concentrated by rotary evaporation (Heidolph Instruments, Schwabach, Germany) under reduced pressure at 40 °C. The samples were freeze-dried (FreeZone 4.5; Labconco Corporation, Kansas City, MO, USA), and each freeze-dried sample was resuspended (2.5 mg mL−1) in HPLC-Mass Spectrometry-grade methanol, sonicated for 10 min, filtered, and then used for the phytochemical analysis.
3.2. Phytochemical Analysis Using UHPLC-PDA-HESI-Orbitrap-MS/MS
Metabolite profiling was performed as reported previously [58,59,60], using a UHPLC system (Dionex™ UltiMate™ 3000; Thermo Fisher Scientific®, Waltham, MA, USA, hyphenated with a Thermo Scientific™ Q Exactive™ Focus Hybrid Quadrupole-Orbitrap™ mass spectrometer (Thermo Fisher Scientific®). The chromatographic system was equipped with a C18 column (ID: 150 × 4.6 mm, 5 µm; Restek Corporation, Bellefonte, PA, USA), a quaternary Series RS pump, and a Dionex™ UltiMate™ 3000 Series TCC-3000RS column compartments with an UltiMate™ 3000 Series WPS-3000RS autosampler (Thermo Fisher Scientific®). A Photodiode Array Detector (PDA) recording from 200 to 800 nm was also employed for peak characterization. The detection wavelengths were 254, 280, 320, and 440 nm for peak construction. The mobile phases consisted of a 1% formic aqueous solution (A) and acetonitrile (B). The elution program [time (min), %B] consisted of 5%B at time zero (0.00, 5), maintained for 5 min (5.00, 5), then %B was increased to 30% (10.00, 30) and maintained for 5 min (15.00, 30), further increased to 70% at min 20 (20.00, 70) and maintained for 5 min at the same proportion (25.00, 70); finally, the elution system returned to the initial conditions at min 35 (35.00, 5) and then was maintained for 12 min for column equilibration prior to each injection. The flow rate was set to 1.0 mL min−1, and the injection volume was 10 μL. The system was controlled by Thermo Scientific™ Chromeleon™ 7.2 Chromatography Data System (CDS) Software (Thermo Fisher Scientific® and Dionex Softron GmbH division of Thermo Fisher Scientific®, Olching-Geiselbullach, Germany). The UHPLC was coupled to the mass spectrometer with a Heated Electrospray Ionization Source II (HESI II) (Thermo Fisher Scientific®). Nitrogen (purity > 99.999%; obtained from a Genius NM32LA nitrogen generator, Peak Scientific®) was employed as a collision and damping gas. As reported previously [58], the mass calibration for Orbitrap was performed weekly, in both negative and positive modes using caffeine and N-butylamine (Sigma-Aldrich®) as positive ions, and buspirone hydrochloride, sodium dodecyl sulfate, and taurocholic acid sodium salt, as negative ions. These compounds were dissolved in a mixture of acetic acid, acetonitrile, water, and methanol (Merck Darmstadt, Hesse, Germany) and infused using a Chemyx Fusion 100 syringe pump (Chemyx Inc., Stafford, TX, USA). Xcalibur™ 2.3 and TraceFinder™ 3.2 software (Thermo Fisher Scientific®) were used for UHPLC control and data processing. Q Exactive™ 2.0 SP2 (Thermo Fisher Scientific®) was used to control the mass spectrometer.
MS Parameters
HESI parameters were chosen as reported previously [58,59,60], using a sheath gas flow rate 75 units; an auxiliary gas unit, flow rate 20; capillary temperature 400 °C; auxiliary gas heater temperature 500 °C; spray voltage 2500 V (for ESI-); and S-Lens RF level 30. Full scan data in negative mode were acquired at a resolving power of 70,000 full width half maximum (FWHM) at m/z of 200. A scan range of m/z 100–1000 was chosen for the compounds of interest. The automatic gain control (AGC) was set at 3 × 106, and the injection time was set to 200 milliseconds (ms). The scan rate was set at 2 scans s−1. The AGC target was set to 2 × 105, with the maximum injection time of 20 ms. The precursor ions were filtered by the quadrupole operating at an isolation window of m/z of 2. The fore vacuum, high vacuum, and ultrahigh vacuum were maintained at approximately 2 mbar, from 105 to below 1010 mbar. Collision energy (HCD cell) was operated at 30 eV. Detection was based on the calculated exact mass and retention time of the target compounds. The mass tolerance window was set to 5 ppm.
3.3. Total Phenolic Compound Determination
Total phenolic compound determination was carried out by the Folin–Ciocalteu method described by Agbor, et al. [61]. A standard curve (n = 3) with gallic acid was prepared at different concentrations (0, 2, 4, 6, 8, 10, 12, and 14 µg/mL). Once the solution was prepared, 2.5 mL of deionized water and 0.1 mL of 1N Folin-Ciocalteu reagent (Sigma-Aldrich) were added to each tube. After 6 min, 0.25 mL of 10% aqueous sodium carbonate (Na2CO3) was added. After 30 min of reaction in dark conditions, the absorbance of the samples was monitored at 760 nm with a spectrophotometer (Jenway, Genova, Switzerland). The straight-line equation (y = 0.0159x − 0.0017) and the correlation coefficient (r2 = 0.9932) were obtained in Microsoft Excel version 16.60.
3.4. Antioxidant Activity
The antioxidant activity was determined using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical method proposed by Brand-Williams, et al. [62]. To 0.1 mL of the extract (each extract at a concentration of 10 mg mL−1), 3.9 mL of DPPH+ methanolic solution (60 µM) was added. After 30 min of reaction, the absorbance of the samples was measured at 510 nm. The results were expressed as percentage of inhibition of the DPPH+ radical, with the formula: % inhibition = (Ai − Af)/Ai × 100, where Ai is the absorbance of the blank, and Af is the absorbance of the sample.
3.5. Statistical Analysis
Statistical analysis was performed using SigmaPlot software (Systat software, version 11.0). Sample differences were determined using one-way ANOVA. Statistical significance of the means was considered at p ≤ 5%.
4. Conclusions
Under the proposed conditions, 35 metabolites were separated and tentatively identified. The cooking process affected the antioxidant activity as well as the phytochemical profile, leading to the formation of new compounds and the degradation of organic acids. Our results indicate that some compounds such as citric acid, eucomic acid, luteolin rutinoside, and sinapic acid remained present after the cooking process. Among the detected compounds, eucomic acid showed the highest relative abundance in both crude and cooked samples. To the best of our knowledge, the secondary metabolite profiling of cooked and crude samples of A. tetragonus is reported here for the first time, as well as their fragmentation pattern. Our results show evidence of the kind of metabolites that are consumed when young stems of A. tetragonus are collected from wild populations, cooked, and used as alternative food by humans. Additionally, our findings offer the basis for future investigations focused on the isolation and quantification of target compounds as well as on the evaluation of their biological activities.
Author Contributions
Conceptualization, Y.A.G.-A. and E.C.-G.; Data curation, J.R.V.-M., O.J.R.-H., C.E.C.-M., F.C.-S., C.A. and E.C.-G.; Formal analysis, E.C.-G.; Funding acquisition, Y.A.G.-A. and E.C.-G.; Investigation, J.C.-C.; Methodology, C.A.; Supervision, Y.A.G.-A. and E.C.-G.; Validation, C.E.C.-M., F.C.-S. and C.A.; Writing—review & editing, Y.A.G.-A. and E.C.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Instituto Politécnico Nacional (Proyecto SIP: 20221180), by resources from the Autonomous University of Aguascalientes (PIBT19-2), and by the National Fund of Scientific and Technological Development of Chile (ANID; Fondecyt Regular No. 1150745).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Acknowledgments
We thank to Ruben Muñoz and Jorge Bórquez Ramirez from the University of Antofagasta, Chile, and Samson Soleil from the Universidad Autónoma de Aguascalientes for technical support.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Plant extracts are available from the authors.
References
- de Araújo, F.F.; de Paulo Farias, D.; Neri-Numa, I.A.; Pastore, G.M. Underutilized plants of the Cactaceae family: Nutritional aspects and technological applications. Food Chem. 2021, 362, 130196. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Campohermoso, A.D.; López-Espinosa, A.; Ocampo-Fletes, I. Conocimiento y uso de Cactaceas por familias campesinas en Coxcatlán, Puebla. Ra Ximhai 2010, 6, 347–353. [Google Scholar] [CrossRef]
- Jimenez Sierra, C.L. Las cactáceas mexicanas y los riesgos que enfretan. Rev. Digit. Univ. 2011, 12, 1–23. [Google Scholar]
- Santos-Díaz, M.S.; Pérez-Molphe-Balch, E.; Ramírez-Malagón, R.; Núñez-Palenius, H.G.; Ochoa-Alejo, N.; Tepper, G.H. Species diversity and extinction. In Mexican Threatened Cacti: Current Status and Strategies for Their Conservation; Nova Science: New York, NY, USA, 2010; pp. 1–60. [Google Scholar]
- Goettsch, B.; Hilton-Taylor, C.; Cruz-Piñón, G.; Duffy, J.P.; Frances, A.; Hernández, H.M.; Inger, R.; Pollock, C.; Schipper, J.; Superina, M.; et al. High proportion of cactus species threatened with extinction. Nat. Plants 2015, 1, 15142. [Google Scholar] [CrossRef] [Green Version]
- Porras-Loaiza, A.P.; López-Malo, A. Importancia de los grupos fenólicos en los alimentos. Temas Selectos de Ingeniería de Alimentos 2009, 3, 121–134. [Google Scholar]
- Naz, S.; Gallart-Ayala, H.; Reinke, S.N.; Mathon, C.; Blankley, R.; Chaleckis, R.; Wheelock, C.E. Development of a Liquid Chromatography-High Resolution Mass Spectrometry Metabolomics Method with High Specificity for Metabolite Identification Using All Ion Fragmentation Acquisition. Anal. Chem. 2017, 89, 7933–7942. [Google Scholar] [CrossRef] [Green Version]
- Lacalle-Bergeron, L.; Izquierdo-Sandoval, D.; Sancho, J.V.; López, F.J.; Hernández, F.; Portolés, T. Chromatography hyphenated to high resolution mass spectrometry in untargeted metabolomics for investigation of food (bio)markers. Trends Anal. Chem. 2021, 135, 116161. [Google Scholar] [CrossRef]
- Juárez-Cruz, A.; Goytia-Jiménez, M.A.; Garcia, G. Acanthocereus tetragonus y A. subinermis: Parte de la Diversidad Fitogenética y Gastronómica de México. In Proceedings of the 1er Foro Estudiantil Indígena México, Washington, DC, USA, 6 October 2016. [Google Scholar]
- Nataren-Velazquez, J.; Del Angel-Pérez, A.L.; Megchún-García, J.V.; Ramírez-Herrera, E.; Ibarra-Pérez, F. Colecta y caracterización morfológica del izote (Yucca elephantipes) y cruceta (Acanthocereus tetragonus) del estado de Veracruz. In Prospectiva de la Investigación Agrícola en el Siglo XXI en México; Avendaño Ruiz, B.D., Bautista Ortega, J., Del Angel-Pérez, A.L., Ireta Paredes, A.d.R., Martinez Trejo, G., Pérez Hernández, P., Schwentesius Ridermann, R., Eds.; Universidad Autónoma de Chapingo and Plaza y Valdés, S.L.: Texcoco, México, 2020; pp. 137–149. [Google Scholar]
- Arias, S.; Aquino, D. Familia Cactaceae I. Flora Del Bajío Reg. Adyac. 2019, 209, 1–295. [Google Scholar] [CrossRef]
- Peddler; Potrero del Llano, municipio de Álamo-te mapache, Veracruz, México. Personal communication, 2022.
- Juárez-Cruz, A.; Livera-Muñoz, M.; Sosa-Montes, E.; Goytia-Jiménez, M.A.; González-Hernández, V.A.; Bárcena-Gama, R. Composición química de tallos inmaduros de Acanthocereus spp. e Hylocereus undatus (Haw.) Britton & Rose. Rev. Fitotec. Mex. 2012, 35, 171–175. [Google Scholar]
- Ghafoor, K.; Ahmed, I.A.M.; Doğu, S.; Uslu, N.; Fadimu, G.J.; Juhaimi, F.A.; Babiker, E.E.; Özcan, M.M. The effect of heating temperature on total phenolic content, antioxidant activity, and phenolic compounds of plum and mahaleb fruits. Int. J. Food Eng. 2019, 15, 1–11. [Google Scholar] [CrossRef]
- Zapata, J.E.; Sepúlveda, C.T.; Álvarez, A.C. Kinetics of the thermal degradation of phenolic compounds from achiote leaves (Bixa orellana L.) and its effect on the antioxidant activity. Food Sci. Technol. 2022, 42, 1–8. [Google Scholar] [CrossRef]
- Palermo, M.; Pellegrini, N.; Fogliano, V. The effect of cooking on the phytochemical content of vegetables. J. Sci. Food Agric. 2014, 94, 1057–1070. [Google Scholar] [CrossRef] [PubMed]
- Kwack, M.H.; Ahn, J.S.; Kim, M.K.; Kim, J.C.; Sung, Y.K. Preventable effect of L-threonate, an ascorbate metabolite, on androgen-driven balding via repression of dihydrotestosterone-induced dickkopf-1 expression in human hair dermal papilla cells. BMB Rep. 2010, 43, 688–692. [Google Scholar] [CrossRef] [PubMed]
- Ledesma-Escobar, C.A.; Priego-Capote, F.; Luque de Castro, M.D. Characterization of lemon (Citrus limon) polar extract by liquid chromatography-tandem mass spectrometry in high resolution mode. J. Mass Spectrom. 2015, 50, 1196–1205. [Google Scholar] [CrossRef]
- Füzfai, Z.; Molnár-Perl, I. Gas chromatographic–mass spectrometric fragmentation study of flavonoids as their trimethylsilyl derivatives: Analysis of flavonoids, sugars, carboxylic and amino acids in model systems and in citrus fruits. J. Chromatogr. A 2007, 1149, 88–101. [Google Scholar] [CrossRef]
- Best, I.; Casimiro-Gonzales, S.; Portugal, A.; Olivera-Montenegro, L.; Aguilar, L.; Muñoz, A.M.; Ramos-Escudero, F. Phytochemical screening and DPPH radical scavenging activity of three morphotypes of Mauritia flexuosa L.f. from Peru, and thermal stability of a milk-based beverage enriched with carotenoids from these fruits. Heliyon 2020, 6, e05209. [Google Scholar] [CrossRef]
- Cabañas-García, E.; Areche, C.; Gómez-Aguirre, Y.A.; Jáuregui-Rincon, J.; Cruz-Sosa, F.; Pérez-Molphe-Balch, E. Phytochemical profile of Coryphantha macromeris (Engelm.) Britton & Rose (Cactaceae) obtained from in vitro cultures. Rev. Mex. Ing. Quim. 2019, 19, 239–249. [Google Scholar] [CrossRef] [Green Version]
- Mores, S.; Vandenberghe, L.P.S.; Magalhães Júnior, A.I.; de Carvalho, J.C.; de Mello, A.F.M.; Pandey, A.; Soccol, C.R. Citric acid bioproduction and downstream processing: Status, opportunities, and challenges. Bioresour. Technol. 2021, 320, 124426. [Google Scholar] [CrossRef]
- Cabañas-García, E.; Areche, C.; Jáuregui-Rincón, J.; Cruz-Sosa, F.; Pérez-Molphe Balch, E. Phytochemical Profiling of Coryphantha macromeris (Cactaceae) Growing in Greenhouse Conditions Using Ultra-High-Performance Liquid Chromatography–Tandem Mass Spectrometry. Molecules 2019, 24, 705. [Google Scholar] [CrossRef] [Green Version]
- Feitosa Teles, F.F.; Warren Stull, J.; Brown, W.H.; Whiting, F.M. Amino and organic acids of the prickly pear cactus (Opuntia ficus indica L.). J. Sci. Food Agric. 1984, 35, 421–425. [Google Scholar] [CrossRef]
- Wishart, D.S.; Guo, A.; Oler, E.; Wang, F.; Anjum, A.; Peters, H.; Dizon, R.; Sayeeda, Z.; Tian, S.; Lee, B.L.; et al. HMDB 5.0: The Human Metabolome Database for 2022. Nucleic Acids Res. 2021, 50, D622–D631. [Google Scholar] [CrossRef] [PubMed]
- Petruk, G.; Di Lorenzo, F.; Imbimbo, P.; Silipo, A.; Bonina, A.; Rizza, L.; Piccoli, R.; Monti, D.M.; Lanzetta, R. Protective effect of Opuntia ficus-indica L. cladodes against UVA-induced oxidative stress in normal human keratinocytes. Bioorg. Med. Chem. Lett. 2017, 27, 5485–5489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández, F.; Andreu-Coll, L.; Bento-Silva, A.; Serra, A.T.; Mena, P.; Legua, P.; Bronze, M.R. Phytochemical profile of Opuntia ficus-indica (L.) Mill fruits (cv. ´Orito´) stored at different conditions. Foods 2022, 11, 160. [Google Scholar] [CrossRef] [PubMed]
- Blando, F.; Russo, R.; Negro, C.; De Bellis, L.; Frassinetti, S. Antimicrobial and antibiofilm activity against Staphylococcus aureus of Opuntia ficus-indica (L.) Mill. cladode polyphenolic extracts. Antioxidants 2019, 8, 117. [Google Scholar] [CrossRef] [Green Version]
- Alexandre, E.M.C.; Coelho, M.C.; Ozcan, K.; Pinto, C.A.; Teixeira, J.A.; Saraiva, J.A.; Pintado, M. Emergent technologies for the extraction of antioxidants from prickly pear peel and their antimicrobial activity. Foods 2021, 10, 570. [Google Scholar] [CrossRef] [PubMed]
- Missaoui, M.; D’Antuono, I.; D’Imperio, M.; Linsalata, V.; Boukhchina, S.; Logrieco, A.F.; Cardinali, A. Characterization of micronutrients, bioaccessibility and antioxidant activity of prickly pear cladodes as functional ingredient. Molecules 2020, 25, 2176. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Park, S.; Koshizawa, T.; Ueda, M. (R)-Eucomic acid, a leaf-opening factor of the model organism, Lotus japonicus. Tetrahedron 2009, 65, 2136–2141. [Google Scholar] [CrossRef]
- Taamalli, A.; Arráez-Román, D.; Abaza, L.; Iswaldi, I.; Fernández-Gutiérrez, A.; Zarrouk, M.; Segura-Carretero, A. LC-MS-based metabolite profiling of methanolic extracts from the medicinal and aromatic species Mentha pulegium and Origanum majorana. Phytochem. Anal. 2015, 26, 320–330. [Google Scholar] [CrossRef]
- García-Salas, P.; Gómez-Caravaca, A.M.; Morales-Soto, A.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Identification and quantification of phenolic compounds in diverse cultivars of eggplant grown in different seasons by high-performance liquid chromatography coupled to diode array detector and electrospray-quadrupole-time of flight-mass spectrometry. Food Res. Int. 2014, 57, 114–122. [Google Scholar] [CrossRef]
- Gluchoff-Fiasson, K.; Jay, M.; Viricel, M.R. Flavone O- and C-glycosides from Setaria italica. Phytochemistry 1989, 28, 2471–2475. [Google Scholar] [CrossRef]
- Mohamed, A.I.E.-A.; El-Negoumy, S.I.; Nabiel, A.; Saleh, M. The flavonoids of Cynara sibthorpiana. Biochem. Syst. Ecol. 1988, 16, 285. [Google Scholar] [CrossRef]
- Cha, M.-N.; Jun, H.-I.; Lee, W.-J.; Kim, M.-J.; Kim, M.-K.; Kim, Y.-S. Chemical composition and antioxidant activity of Korean cactus (Opuntia humifusa) fruit. Food Sci. Biotechnol. 2013, 22, 523–529. [Google Scholar] [CrossRef]
- Jun, H.-I.; Cha, M.-N.; Yang, E.-I.; Choi, D.G.; Kim, Y.-S. Physicochemical properties and antioxidant activity of Korean cactus (Opuntia humifusa) cladodes. Hortic Environ. Biotechnol. 2013, 54, 288–295. [Google Scholar] [CrossRef]
- Lim, H.K.; Tan, C.P.; Karim, R.; Ariffin, A.A.; Bakar, J. Chemical composition and DSC thermal properties of two species of Hylocereus cacti seed oil: Hylocereus undatus and Hylocereus polyrhizus. Food Chem. 2010, 119, 1326–1331. [Google Scholar] [CrossRef]
- Chahdoura, H.; Barreira, J.C.M.; Barros, L.; Santos-Buelga, C.; Ferreira, I.C.F.R.; Achour, L. Seeds of Opuntia spp. as a novel high potential by-product: Phytochemical characterization and antioxidant activity. Ind. Crops Prod. 2015, 65, 383–389. [Google Scholar] [CrossRef] [Green Version]
- Fernández-López, J.A.; Almela, L.; Obón, J.M.; Castellar, R. Determination of Antioxidant Constituents in Cactus Pear Fruits. Plant Foods Hum. Nutr. 2010, 65, 253–259. [Google Scholar] [CrossRef]
- Abouseadaa, H.H.; Atia, M.A.M.; Younis, I.Y.; Issa, M.Y.; Ashour, H.A.; Saleh, I.; Osman, G.H.; Arif, I.A.; Mohsen, E. Gene-targeted molecular phylogeny, phytochemical profiling, and antioxidant activity of nine species belonging to family Cactaceae. Saudi J. Biol. Sci. 2020, 27, 1649–1658. [Google Scholar] [CrossRef]
- Ammar, I.; Ennouri, M.; Attia, H. Phenolic content and antioxidant activity of cactus (Opuntia ficus-indica L.) flowers are modified according to the extraction method. Ind. Crops Prod. 2015, 64, 97–104. [Google Scholar] [CrossRef]
- Mokrani, A.; Madani, K. Effect of solvent, time and temperature on the extraction of phenolic compounds and antioxidant capacity of peach (Prunus persica L.) fruit. Sep. Purif. Technol. 2016, 162, 68–76. [Google Scholar] [CrossRef]
- Yahia, E.M.; Mondragon-Jacobo, C. Nutritional components and anti-oxidant capacity of ten cultivars and lines of cactus pear fruit (Opuntia spp.). Food Res. Int. 2011, 44, 2311–2318. [Google Scholar] [CrossRef]
- Kuti, J.O. Antioxidant compounds from four Opuntia cactus pear fruit varieties. Food Chem. 2004, 85, 527–533. [Google Scholar] [CrossRef]
- Saïdani Tounsi, M.; Ouerghemmi, I.; Ksouri, R.; Aidi Wannes, W.; Hammrouni, I.; Marzouk, B. HPLC-Determination of Phenolic Composition and Antioxidant Capacity of Cactus Prickly Pears Seeds. Asian J. Chem. 2011, 11, 1006–1010. [Google Scholar]
- Cruz-Bravo, R.K.; Guzmán-Maldonado, S.H.; Araiza-Herrera, H.A.; Zegbe, J.A. Storage alters physicochemical characteristics, bioactive compounds and antioxidant capacity of cactus pear fruit. Postharvest Biol. Technol. 2019, 150, 105–111. [Google Scholar] [CrossRef]
- De Leo, M.; Abreu, M.B.D.; Pawlowska, A.M.; Cioni, P.L.; Braca, A. Profiling the chemical content of Opuntia ficus-indica flowers by HPLC–PDA-ESI-MS and GC/EIMS analyses. Phytochem. Lett. 2010, 3, 48–52. [Google Scholar] [CrossRef]
- Mata, A.; Ferreira, J.P.; Semedo, C.; Serra, T.; Duarte, C.M.M.; Bronze, M.R. Contribution to the characterization of Opuntia spp. juices by LC–DAD–ESI-MS/MS. Food Chem. 2016, 210, 558–565. [Google Scholar] [CrossRef]
- Morales, P.; Barros, L.; Ramírez-Moreno, E.; Santos-Buelga, C.; Ferreira, I.C.F.R. Exploring xoconostle by-products as sources of bioactive compounds. Food Res. Int. 2014, 65, 437–444. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Aspee, F.; Quispe, C.; Soriano, M.d.P.C.; Fuentes Gonzalez, J.; Hüneke, E.; Theoduloz, C.; Schmeda-Hirschmann, G. Antioxidant activity and characterization of constituents in copao fruits (Eulychnia acida Phil., Cactaceae) by HPLC–DAD–MS/MSn. Food Res. Int. 2014, 62, 286–298. [Google Scholar] [CrossRef]
- Valente, L.M.M.; da Paixão, D.; do Nascimento, A.C.; dos Santos, P.F.P.; Scheinvar, L.A.; Moura, M.R.L.; Tinoco, L.W.; Gomes, L.N.F.; da Silva, J.F.M. Antiradical activity, nutritional potential and flavonoids of the cladodes of Opuntia monacantha (Cactaceae). Food Chem. 2010, 123, 1127–1131. [Google Scholar] [CrossRef]
- Zhang, L.; Tu, Z.-C.; Xie, X.; Lu, Y.; Wang, Z.-C.; Wang, H.; Sha, X.-M. Antihyperglycemic, antioxidant activities of two Acer palmatum cultivars, and identification of phenolics profile by UPLC-QTOF-MS/MS: New natural sources of functional constituents. Ind. Crops Prod. 2016, 89, 522–532. [Google Scholar] [CrossRef]
- Guevara-Figueroa, T.; Jiménez-Islas, H.; Reyes-Escogido, M.L.; Mortensen, A.G.; Laursen, B.B.; Lin, L.-W.; De León-Rodríguez, A.; Fomsgaard, I.S.; Barba de la Rosa, A.P. Proximate composition, phenolic acids, and flavonoids characterization of commercial and wild nopal (Opuntia spp.). J. Food Compos. Anal. 2010, 23, 525–532. [Google Scholar] [CrossRef]
- Badhani, B.; Sharma, N.; Kakkar, R. Gallic acid: A versatile antioxidant with promising therapeutic and industrial applications. RSC Adv. 2015, 5, 27540–27557. [Google Scholar] [CrossRef]
- Pandurangan, A.K.; Mohebali, N.; Esa, N.M.; Looi, C.Y.; Ismail, S.; Saadatdoust, Z. Gallic acid suppresses inflammation in dextran sodium sulfate-induced colitis in mice: Possible mechanisms. Int. Immunopharmacol. 2015, 28, 1034–1043. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Tu, Z.; Wang, H.; Zhang, L. Ultrasound-Assisted Extraction Optimization of alpha-Glucosidase Inhibitors from Ceratophyllum demersum L. and Identification of Phytochemical Profiling by HPLC-QTOF-MS/MS. Molecules 2020, 25, 4507. [Google Scholar] [CrossRef] [PubMed]
- Cornejo, A.; Salgado, F.; Caballero, J.; Vargas, R.; Simirgiotis, M.; Areche, C. Secondary Metabolites in Ramalina terebrata Detected by UHPLC/ESI/MS/MS and Identification of Parietin as Tau Protein Inhibitor. Int. J. Mol. Sci. 2016, 17, 1303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simirgiotis, M.J.; Quispe, C.; Bórquez, J.; Areche, C.; Sepúlveda, B. Fast Detection of Phenolic Compounds in Extracts of Easter Pears (Pyrus communis) from the Atacama Desert by Ultrahigh-Performance Liquid Chromatography and Mass Spectrometry (UHPLC–Q/Orbitrap/MS/MS). Molecules 2016, 21, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simirgiotis, M.J.; Quispe, C.; Areche, C.; Sepúlveda, B. Phenolic Compounds in Chilean Mistletoe (Quintral, Tristerix tetrandus) Analyzed by UHPLC–Q/Orbitrap/MS/MS and Its Antioxidant Properties. Molecules 2016, 21, 245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agbor, G.A.; Vinson, J.A.; Donnelly, P.E. Folin-Ciocalteau Reagent for Polyphenolic Assay. Int. J. Food Sci. 2014, 3, 147–156. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).