Ultrastructural Damages to H1N1 Influenza Virus Caused by Vapor Essential Oils
Abstract
:1. Introduction
2. Results and Discussion
2.1. Headspace-Gas Chromatography/Mass Spectrometry (HS-GC/MS)
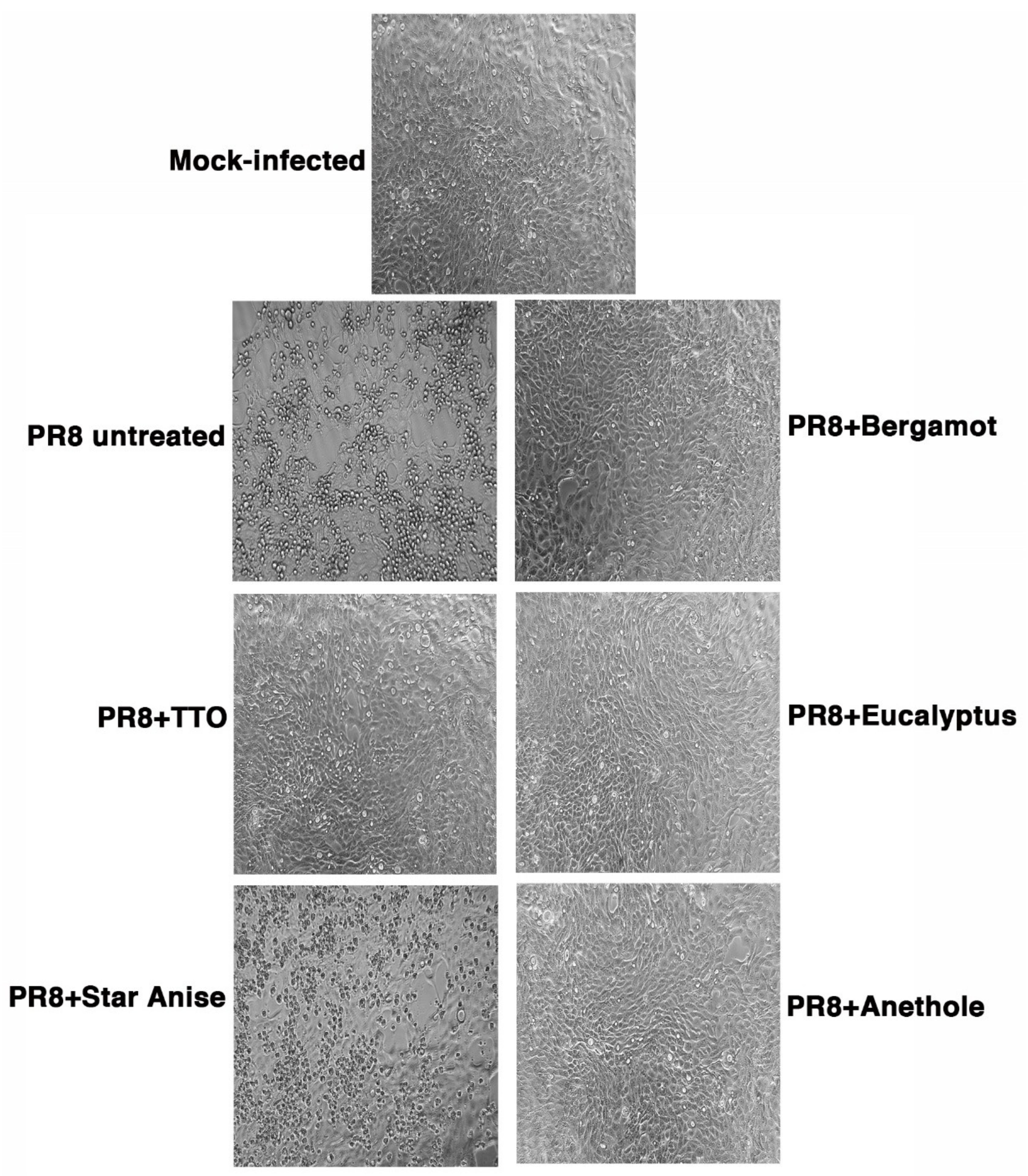
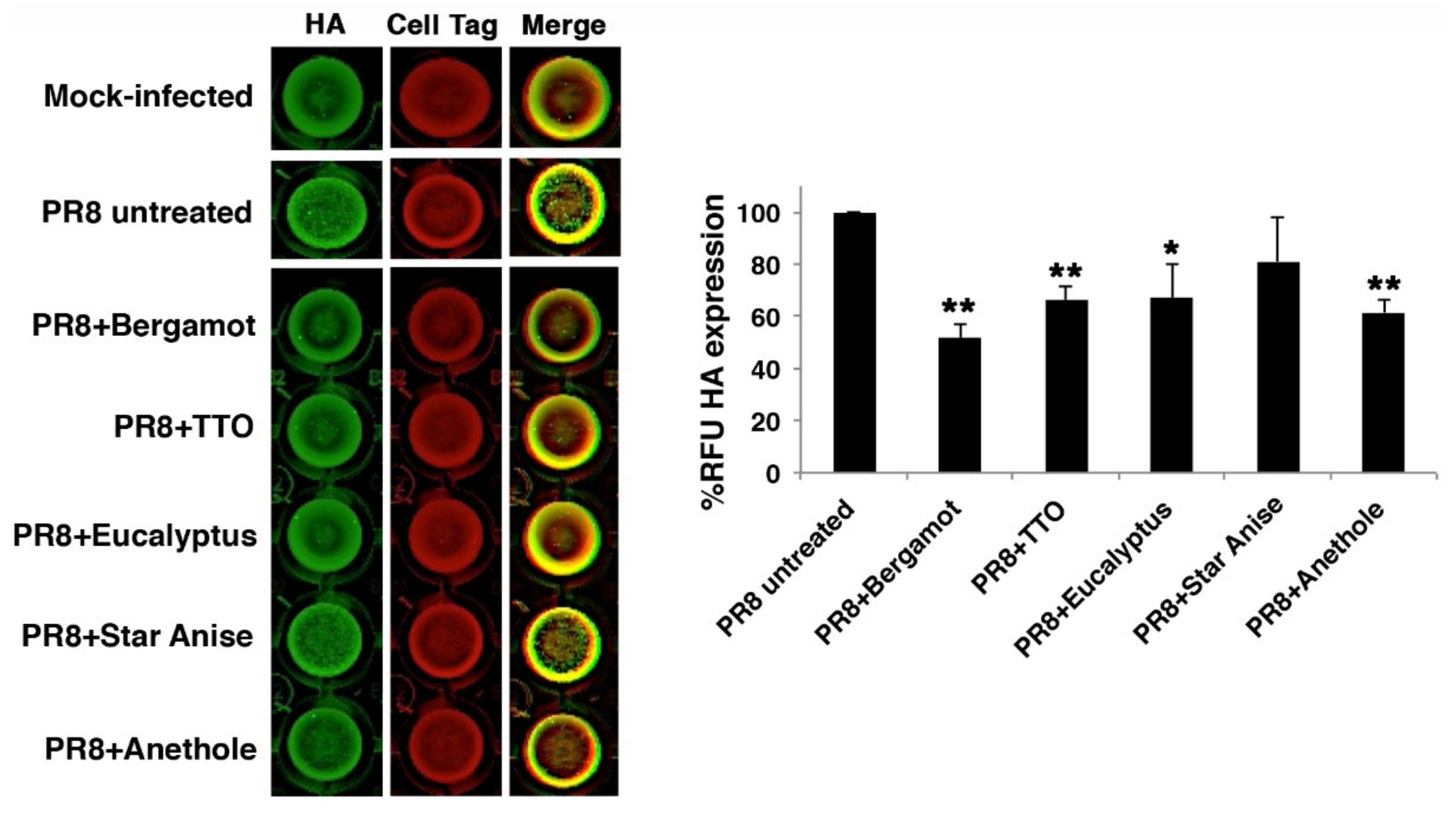
2.2. Antiviral Activity
2.3. Transmission Electron Microscopy (TEM) Imaging
3. Materials and Methods
3.1. Materials
3.2. Gas Chromatography–Mass Spectrometry (GC–MS) Analysis
3.3. Head Space GC/MS Analysis
3.4. Cell Cultures
3.5. Cell Toxicity Assays
3.6. TCID50 (Tissue Culture Infectious Dose 50%) and Cytopathic Effect Assays
3.7. In-Cell Western (ICW) Assay
3.8. Transmission Electron Microscopy (TEM) Imaging
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Petrova, V.N.; Russell, C.A. The evolution of seasonal influenza viruses. Nat. Rev. Microbiol. 2018, 16, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Simmerman, J.M.; Suntarattiwong, P.; Levy, J.; Gibbons, R.V.; Cruz, C.; Shaman, J.; Jarman, R.G.; Chotpitayasunondh, T. Influenza virus contamination of common household surfaces during the 2009 influenza A (H1N1) pandemic in Bangkok, Thailand: Implications for contact transmission. Clin. Infect. Dis. 2010, 51, 1053–1061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Benedictis, P.; Beato, M.S.; Capua, I. Inactivation of avian influenza viruses by chemical agents and physical conditions: A review. Zoonoses Public Health 2007, 54, 51–68. [Google Scholar] [CrossRef] [PubMed]
- Gomes, I.B.; Malheiro, J.; Mergulhão, F.; Maillard, J.-Y.; Simões, M. Comparison of the efficacy of natural-based and synthetic biocides to disinfect silicone and stainless steel surfaces. Pathog. Dis. 2016, 74, ftw014. [Google Scholar] [CrossRef] [Green Version]
- Jang, Y.; Lee, J.; So, B.; Lee, K.; Yun, S.; Lee, M.; Choe, N. Evaluation of changes induced by temperature, contact time, and surface in the efficacies of disinfectants against avian influenza virus. Poult. Sci. 2014, 93, 70–76. [Google Scholar] [CrossRef]
- Pyankov, O.V.; Usachev, E.V.; Pyankova, O.; Agranovski, I.E. Inactivation of airborne influenza virus by tea tree and eucalyptus oils. Aerosol Sci. Technol. 2012, 46, 1295–1302. [Google Scholar] [CrossRef]
- Buriani, A.; Fortinguerra, S.; Sorrenti, V.; Caudullo, G.; Carrara, M. Essential oil phytocomplex activity, a review with a focus on multivariate analysis for a network pharmacology-informed phytogenomic approach. Molecules 2020, 25, 1833. [Google Scholar] [CrossRef] [Green Version]
- Sadgrove, N.J.; Padilla-González, G.F.; Phumthum, M. Fundamental chemistry of essential oils and volatile organic compounds, methods of analysis and authentication. Plants 2022, 11, 789. [Google Scholar] [CrossRef]
- Mancianti, F.; Ebani, V.V. Biological activity of essential oils. Molecules 2020, 25, 678. [Google Scholar] [CrossRef] [Green Version]
- Mehdizadeh, L.; Moghaddam, M. Chapter 10—Essential Oils: Biological Activity and Therapeutic Potential. In Therapeutic, Probiotic, and Unconventional Foods; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 167–179. [Google Scholar]
- Madia, V.N.; De Angelis, M.; De Vita, D.; Messore, A.; De Leo, A.; Ialongo, D.; Tudino, V.; Saccoliti, F.; De Chiara, G.; Garzoli, S.; et al. Investigation of Commiphora myrrha (Nees) Engl. oil and its main components for antiviral activity. Pharmaceuticals 2021, 14, 243. [Google Scholar] [CrossRef]
- Navarra, M.; Mannucci, C.; Delbò, M.; Calapai, G. Citrus bergamia essential oil: From basic research to clinical application. Front. Pharmacol. 2015, 6, 36. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.W.; Hu, W.T.; Huang, B.K.; Qin, L.P. Illicium verum: A review on its botany, traditional use, chemistry and pharmacology. J. Ethnopharmacol. 2011, 136, 10–20. [Google Scholar] [CrossRef]
- Carson, C.F.; Hammer, K.A.; Riley, T.V. Melaleuca alternifolia (Tea Tree) oil: A review of antimicrobial and other medicinal properties. Clin. Microbiol. Rev. 2006, 19, 50–62. [Google Scholar] [CrossRef] [Green Version]
- Dhakad, A.K.; Pandey, V.V.; Beg, S.; Rawat, J.M.; Singh, A. Biological, medicinal and toxicological significance of Eucalyptus leaf essential oil: A review. J. Sci. Food Agric. 2018, 98, 833–848. [Google Scholar] [CrossRef]
- Setzer, W.N. Essential oils as complementary and alternative medicines for the treatment of influenza. Am. J. Essent. Oil Nat. Prod. 2016, 4, 16–22. [Google Scholar]
- Horváth, G.; Ács, K. Essential oils in the treatment of respiratory tract diseases highlighting their role in bacterial infections and their anti-inflammatory action: A review. Flavour Fragr. J. 2015, 30, 331–341. [Google Scholar] [CrossRef]
- Choi, H.J. Chemical constituents of essential oils possessing anti-influenza A/WS/33 virus activity. Osong Public Health Res. Perspect. 2018, 9, 348–353. [Google Scholar] [CrossRef]
- Cermelli, C.; Fabio, A.; Fabio, G.; Quaglio, P. Effect of eucalyptus essential oil on respiratory bacteria and viruses. Curr. Microbiol. 2008, 56, 89–92. [Google Scholar] [CrossRef]
- Yang, Z.C.; Wang, B.C.; Yang, X.S.; Wang, Q. Chemical composition of the volatile oil from Cynanchum stauntonii and its activities of anti-influenza virus. Colloids Surf. B Biointerfaces 2005, 43, 198–202. [Google Scholar] [CrossRef]
- Corona, A.; di Leva, F.S.; Rigogliuso, G.; Pescatori, L.; Madia, V.N.; Subra, F.; Delelis, O.; Esposito, F.; Cadeddu, M.; Costi, R.; et al. New insights into the interaction between pyrrolyl diketoacids and HIV-1 integrase active site and comparison with RNase H. Antivir. Res. 2016, 134, 236–243. [Google Scholar] [CrossRef]
- Serkedjieva, J.; Gegova, G.; Mladenov, K. Protective efficacy of an aerosol preparation, obtained from Geranium sanguineum L., in experimental influenza infection. Pharmazie 2008, 63, 160–163. [Google Scholar]
- Superti, F.; Marchetti, M.; Rapetti Mogol, G. Inactivation of Influenza virus by a blend of essential plant oil vapour and aerosol. J. Biol. Regul. Homeost. Agents 2021, 35, 1667–1675. [Google Scholar] [CrossRef]
- De Vita, D.; Angeli, A.; Pandolfi, F.; Bortolami, M.; Costi, R.; Di Santo, R.; Suffredini, E.; Ceruso, M.; Del Prete, S.; Capasso, C.; et al. Inhibition of the α-carbonic anhydrase from Vibrio cholerae with amides and sulfonamides incorporating imidazole moieties. J. Enzyme Inhib. Med. Chem. 2017, 32, 798–804. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Patel, K.B.; Booth, L.J.; Metcalf, J.P.; Lin, H.K.; Wu, W. Protective essential oil attenuates influenza virus infection: An in vitro study in MDCK cells. BMC Complement. Altern. Med. 2010, 15, 69. [Google Scholar] [CrossRef] [Green Version]
- Najar, B.; Nardi, V.; Stincarelli, M.A.; Patrissi, S.; Pistelli, L.; Giannecchini, S. Screening of the essential oil effects on human H1N1 influenza virus infection: An in vitro study in MDCK cells. Nat. Prod. Res. 2021, 1–4. [Google Scholar] [CrossRef]
- Inouye, S.; Yamaguchi, H.; Takizawa, T. Screening of the antibacterial effects of a variety of essential oils on respiratory tract pathogens, using a modified dilution assay method. J. Infect. Chemother. 2001, 7, 251–254. [Google Scholar] [CrossRef]
- Goñi, P.; López, P.; Sánchez, C.; Gómez-Lus, R.; Becerril, R.; Nerín, C. Antimicrobial activity in the vapour phase of a combination of cinnamon and clove essential oils. Food Chem. 2009, 116, 982–989. [Google Scholar] [CrossRef]
- Hudson, J.; Kuo, M.; Vimalanathan, S. The antimicrobial properties of cedar leaf (Thuja plicata) oil; a safe and efficient decontamination agent for buildings. Int. J. Environ. Res. Public Health 2011, 8, 4477–4487. [Google Scholar] [CrossRef] [PubMed]
- Vimalanathan, S.; Hudson, J. The activity of cedar leaf oil vapor against respiratory viruses: Practical applications. J. Appl. Pharm. Sci. 2013, 3, 11–15. [Google Scholar] [CrossRef]
- Vimalanathan, S.; Hudson, J. Anti-influenza virus activity of essential oils and vapors. Am. J. Essent. Oil. 2014, 2, 47–53. [Google Scholar]
- Ohira, H.; Torii, N.; Aida, T.M.; Watanabe, M.; Smith, R.L. Rapid separation of shikimic acid from Chinese star anise (Illicium verum Hook. f.) with hot water extraction. Sep. Purif. Technol. 2009, 69, 102–108. [Google Scholar] [CrossRef]
- Garozzo, A.; Timpanaro, R.; Stivala, A.; Bisignano, G.; Castro, A. Activity of Melaleuca alternifolia (tea tree) oil on Influenza virus A/PR/8: Study on the mechanism of action. Antivir. Res. 2011, 89, 83–88. [Google Scholar] [CrossRef]
- Xing, C.; Qin, C.; Li, X.; Zhang, F.; Linhardt, R.J.; Sun, P.; Zhang, A. Chemical composition and biological activities of essential oil isolated by HS-SPME and UAHD from fruits of bergamot. LWT 2019, 104, 38–44. [Google Scholar] [CrossRef]
- Wang, Q.; Jiang, L.; Wen, Q. Effect of three extraction methods on the volatile component of Illicium verum Hook. f. analyzed by GC-MS. Wuhan Univ. J. Nat. Sci. 2007, 12, 529–534. [Google Scholar] [CrossRef]
- Murbach Teles Andrade, B.F.; Nunes Barbosa, L.; Bérgamo Alves, F.C.; Albano, M.; Mores Rall, V.L.; Sforcin, J.M.; Henrique Fernandes, A.A.; Fernandes Júnior, A. The antibacterial effects of Melaleuca alternifolia, Pelargonium graveolens and Cymbopogon martinii essential oils and major compounds on liquid and vapor phase. J. Essent. Oil Res. 2015, 28, 227–233. [Google Scholar] [CrossRef] [Green Version]
- Tyagi, A.K.; Malik, A. Antimicrobial potential and chemical composition of Eucalyptus globulus oil in liquid and vapour phase against food spoilage microorganisms. Food Chem. 2011, 126, 228–235. [Google Scholar] [CrossRef]
- Szczerbanik, M.; Jobling, J.; Morris, S.; Holford, P. Essential oil vapours control some common postharvest fungal pathogens. Aust. J. Exp. Agric. 2007, 47, 103–109. [Google Scholar] [CrossRef]
- Li, Y.; Lai, Y.; Wang, Y.; Liu, N.; Zhang, F.; Xu, P. 1,8-Cineol protect against influenza-virus-induced pneumonia in mice. Inflammation 2016, 39, 1582–1593. [Google Scholar] [CrossRef]
- Nadjib, B.M. Effective antiviral activity of essential oils and their characteristic terpenes against Coronaviruses: An update. J. Pharmacol. Clin. Toxicol. 2020, 8, 1138. [Google Scholar]
- Asif, M.; Saleem, M.; Saadullah, M.; Yaseen, H.S.; Al Zarzour, R. COVID-19 and therapy with essential oils having antiviral, anti-inflammatory, and immunomodulatory properties. Inflammopharmacology 2020, 28, 1153–1161. [Google Scholar] [CrossRef]
- Wink, M. Potential of DNA intercalating alkaloids and other plant secondary metabolites against SARS-CoV-2 causing COVID-19. Diversity 2020, 12, 175. [Google Scholar] [CrossRef]
- Lai, W.L.; Chuang, H.S.; Lee, M.H.; Wei, C.L.; Lin, C.F.; Tsai, Y.C. Inhibition of herpes simplex virus type 1 by thymol-related monoterpenoids. Planta Med. 2012, 78, 1636–1638. [Google Scholar] [CrossRef] [Green Version]
- Astani, A.; Reichling, J.; Schnitzler, P. Comparative study on the antiviral activity of selected monoterpenes derived from essential oils. Phytother. Res. 2010, 24, 673–679. [Google Scholar] [CrossRef]
- Astani, A.; Schnitzler, P. Antiviral activity of monoterpenes beta-pinene and limonene against herpes simplex virus in vitro. Iran. J. Microbiol. 2014, 6, 149–155. [Google Scholar]
- Astani, A.; Reichling, J.; Schnitzler, P. Screening for antiviral activities of isolated compounds from essential oils. Evid.-Based Complement. Altern. Med. 2011, 2011, 253643. [Google Scholar] [CrossRef] [Green Version]
- Garzoli, S.; Masci, V.; Caradonna, V.; Tiezzi, A.; Giacomello, P.; Ovidi, E. Liquid and vapor phase of four conifer-derived essential oils: Comparison of chemical compositions and antimicrobial and antioxidant properties. Pharmaceuticals 2021, 14, 134. [Google Scholar] [CrossRef]
- Garzoli, S.; Laghezza Masci, V.; Franceschi, S.; Tiezzi, A.; Giacomello, P.; Ovidi, E. Headspace/GC–MS Analysis and Investigation of Antibacterial, Antioxidant and Cytotoxic Activity of Essential Oils and Hydrolates from Rosmarinus officinalis L. and Lavandula angustifolia Miller. Foods 2021, 10, 1768. [Google Scholar] [CrossRef]
- De Angelis, M.; Casciaro, B.; Genovese, A.; Brancaccio, D.; Marcocci, M.E.; Novellino, E.; Carotenuto, A.; Palamara, A.T.; Mangoni, M.L.; Nencioni, L. Temporin G, an amphibian antimicrobial peptide against influenza and parainfluenza respiratory viruses: Insights into biological activity and mechanism of action. FASEB J. 2021, 35, e21358. [Google Scholar] [CrossRef]
- De Angelis, M.; Amatore, D.; Checconi, P.; Zevini, A.; Fraternale, A.; Magnani, M.; Hiscott, J.; De Chiara, G.; Palamara, A.T.; Nencioni, L. Influenza virus down-modulates G6PD expression and activity to induce oxidative stress and promote its replication. Front. Cell Infect. Microbiol. 2022, 11, 804976. [Google Scholar] [CrossRef]


| N° | Component 1 | LRI 2 | LRI 3 | BEO 4 (%) | IV-EO 5 (%) | TTO 6 (%) | EEO 7 (%) |
|---|---|---|---|---|---|---|---|
| 1 | α-thujene | 920 | 923 | 0.3 ± 0.03 | - | - | 1.7 ± 0.02 |
| 2 | α-pinene | 941 | 943 | 1.3 ± 0.02 | 0.3 ± 0.03 | 0.2 ± 0.02 | - |
| 3 | sabinene | 973 | 972 | - | - | tr | - |
| 4 | β-myrcene | 981 | 983 | 1.5 ± 0.03 | tr | 0.1 ± 0.00 | 0.6 ± 0.02 |
| 5 | β-pinene | 988 | 986 | 7.3 ± 0.02 | - | 0.1 ± 0.00 | - |
| 6 | α-phellandrene | 1008 | 1005 | - | 0.2 ± 0.02 | tr | 0.5 ± 0.02 |
| 7 | α-terpinene | 1013 | 1010 | - | - | 1.1 ± 0.02 | - |
| 8 | p-cymene | 1020 | 1016 | 0.7 ± 0.02 | tr | 0.3 ± 0.04 | 1.7 ± 0.01 |
| 9 | limonene | 1021 | 1023 | 31.9 ± 0.06 | 0.1 ± 0.02 | - | - |
| 10 | 1,8-cineole | 1026 | 1025 | - | - | 0.5 ± 0.02 | 93.7 ± 0.02 |
| 11 | cis-β-ocimene | 1035 | 1032 | 0.5 ± 0.02 | - | - | - |
| 12 | β-terpinene | 1040 | 1036 | - | tr | - | - |
| 13 | trans-β-ocimene | 1044 | 1043 | 0.2 ± 0.02 | - | - | - |
| 14 | trans-sabinene hydrate | 1057 | 1053 | - | 0.2 ± 0.02 | - | - |
| 15 | γ-terpinene | 1059 | 1054 | 7.5 ± 0.02 | - | 2.7 ± 0.03 | 1.6 ± 0.02 |
| 16 | cis-sabinene hydrate | 1072 | 1069 | - | tr | - | - |
| 17 | terpinolene | 1079 | 1080 | - | - | tr | tr |
| 18 | linalool | 1090 | 1092 | 17.0 ± 0.03 | 1.0 ± 0.02 | - | - |
| 19 | terpinen-4-ol | 1164 | 1160 | - | - | 92.4 ± 0.03 | tr |
| 20 | estragole | 1180 | 1177 | - | 3.2 ± 0.02 | - | - |
| 21 | α-terpineol | 1185 | 1183 | tr | - | 0.6 ± 0.02 | 0.1 ± 0.00 |
| 22 | carveol | 1210 | 1201 | tr | - | - | - |
| 23 | cis-piperitol | 1219 | 1215 | - | - | tr | -- |
| 24 | p-anisaldehyde | 1232 | 1229 | - | 0.2 ± 0.03 | - | - |
| 25 | linalyl acetate | 1259 | 1252 | 31.4 ± 0.02 | - | - | - |
| 26 | anethole | 1269 | 1260 | - | 93.0 ± 0.07 | - | - |
| 27 | α-citral | 1291 | 1287 | 0.1 ± 0.02 | - | - | - |
| 28 | α-copaene | 1373 | 1370 | - | - | 0.1 ± 0.00 | - |
| 29 | β-elemene | 1410 | 1406 | - | - | tr | - |
| 30 | β-caryophyllene | 1426 | 1424 | - | 0.7 ± 0.03 | 0.1 ± 0.00 | - |
| 31 | α-bergamotene | 1435 | 1430 | - | tr | - | - |
| 32 | aromadendrene | 1459 | 1460 | - | tr | 0.4 ± 0.03 | - |
| 33 | humulene | 1470 | 1465 | - | tr | tr | - |
| 34 | γ-muurolene | 1490 | 1486 | - | - | 0.2 ± 0.02 | - |
| 35 | ledene | 1499 | 1492 | - | - | 0.5 ± 0.02 | - |
| 36 | β-bisabolene | 1501 | 1501 | 0.1 ± 0.02 | tr | - | - |
| 37 | α-farnesene | 1510 | 1506 | 0.1 ± 0.01 | tr | - | - |
| 38 | δ-cadinene | 1528 | 1530 | - | - | 0.4 ± 0.02 | - |
| 39 | spathulenol | 1573 | 1571 | - | - | tr | - |
| 40 | viridiflorol | 1580 | 1583 | - | - | 0.1 ± 0.01 | - |
| 41 | globulol | 1596 | 1594 | - | - | 0.1 ± 0.01 | - |
| 42 | cubenol | 1635 | 1631 | - | - | 0.1 ± 0.01 | - |
| SUM | 99.9 | 98.9 | 100.0 | 99.9 | |||
| Terpenoids | 68.3 | 1.8 | 98.0 | 99.9 | |||
| Sesquiterpenoids | 0.2 | 0.7 | 1.9 | - | |||
| Other | 31.4 | 96.4 | 0.1 | - |
| N° | Component 1 | LRI 2 | LRI 3 | BEO 4 (%) | IV-EO 5 (%) | TTO 6 (%) | EEO 7 (%) |
|---|---|---|---|---|---|---|---|
| 1 | α-thujene | 920 | 923 | 1.5 ± 0.02 | 0.4 ± 0.02 | 3.5 ± 0.04 | 5.6 ± 0.02 |
| 2 | α-pinene | 941 | 943 | 6.5 ± 0.02 | 19.1 ± 0.06 | 10.0 ± 0.04 | - |
| 3 | camphene | 950 | 946 | 0.3 ± 0.02 | 0.3 ± 0.03 | 0.1 ± 0.00 | - |
| 4 | sabinene | 974 | 972 | - | - | 0.3 ± 0.02 | - |
| 5 | β-myrcene | 981 | 983 | 3.9 ± 0.03 | 2.3 ± 0.04 | 1.5 ± 0.04 | 0.8 ± 0.00 |
| 6 | β-pinene | 988 | 986 | 20.4 ± 0.05 | - | 1.7 ± 0.06 | - |
| 7 | α-phellandrene | 1008 | 1005 | 0.2 ± 0.01 | 5.9 ± 0.07 | 1.4 ± 0.01 | 0.6 ± 0.01 |
| 8 | α-terpinene | 1013 | 1010 | - | - | 18.1 ± 0.02 | - |
| 9 | p-cymene | 1020 | 1016 | 1.1 ± 0.03 | 0.5 ± 0.03 | 10.7 ± 0.04 | 1.9 ± 0.04 |
| 10 | limonene | 1021 | 1023 | 51.2 ± 0.03 | 3.8 ± 0.06 | - | - |
| 11 | 1,8-cineole | 1026 | 1025 | - | - | 8.3 ± 0.03 | 89.8 ± 0.03 |
| 12 | cis-β-ocimene | 1035 | 1032 | 0.7 ± 0.03 | 0.4 ± 0.05 | - | - |
| 13 | β-terpinene | 1040 | 1036 | - | 0.7 ± 0.03 | - | - |
| 14 | trans-β-ocimene | 1044 | 1043 | 0.3 ± 0.04 | - | - | - |
| 15 | γ-terpinene | 1059 | 1054 | 9.2 ± 0.04 | 2.7 ± 0.03 | 29.3 ± 0.03 | 1.1 ± 0.02 |
| 16 | cis-sabinene-hydrate | 1072 | 1069 | - | 5.0 ± 0.05 | - | - |
| 17 | terpinolene | 1079 | 1080 | - | - | 4.6 ± 0.04 | tr |
| 18 | linalool | 1090 | 1092 | 4.6 ± 0.02 | 3.9 ± 0.05 | - | - |
| 19 | terpinen-4-ol | 1164 | 1160 | 0.1 ± 0.02 | - | 10.2 ± 0.05 | - |
| 20 | estragole | 1180 | 1177 | - | 5.5 ± 0.06 | - | - |
| 21 | α-terpineol | 1185 | 1183 | - | - | 0.3 ± 0.04 | 0.2 ± 0.01 |
| 22 | anethole | 1269 | 1260 | - | 49.1 ± 0.05 | - | - |
| 23 | α-farnesene | 1510 | 1506 | - | 0.3 ± 0.02 | - | - |
| SUM | 100.0 | 99.9 | 100.0 | 100.0 | |||
| Terpenoids | 100.0 | 45.0 | 100.0 | 100.0 | |||
| Sesquiterpenoids | - | 0.3 | - | - | |||
| Other | - | 54.6 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madia, V.N.; Toscanelli, W.; De Vita, D.; De Angelis, M.; Messore, A.; Ialongo, D.; Scipione, L.; Tudino, V.; D’Auria, F.D.; Di Santo, R.; et al. Ultrastructural Damages to H1N1 Influenza Virus Caused by Vapor Essential Oils. Molecules 2022, 27, 3718. https://doi.org/10.3390/molecules27123718
Madia VN, Toscanelli W, De Vita D, De Angelis M, Messore A, Ialongo D, Scipione L, Tudino V, D’Auria FD, Di Santo R, et al. Ultrastructural Damages to H1N1 Influenza Virus Caused by Vapor Essential Oils. Molecules. 2022; 27(12):3718. https://doi.org/10.3390/molecules27123718
Chicago/Turabian StyleMadia, Valentina Noemi, Walter Toscanelli, Daniela De Vita, Marta De Angelis, Antonella Messore, Davide Ialongo, Luigi Scipione, Valeria Tudino, Felicia Diodata D’Auria, Roberto Di Santo, and et al. 2022. "Ultrastructural Damages to H1N1 Influenza Virus Caused by Vapor Essential Oils" Molecules 27, no. 12: 3718. https://doi.org/10.3390/molecules27123718
APA StyleMadia, V. N., Toscanelli, W., De Vita, D., De Angelis, M., Messore, A., Ialongo, D., Scipione, L., Tudino, V., D’Auria, F. D., Di Santo, R., Garzoli, S., Stringaro, A., Colone, M., Marchetti, M., Superti, F., Nencioni, L., & Costi, R. (2022). Ultrastructural Damages to H1N1 Influenza Virus Caused by Vapor Essential Oils. Molecules, 27(12), 3718. https://doi.org/10.3390/molecules27123718














