A Highly Efficient Bismuth Nitrate/Keto-ABNO Catalyst System for Aerobic Oxidation of Alcohols to Carbonyl Compounds under Mild Conditions
Abstract
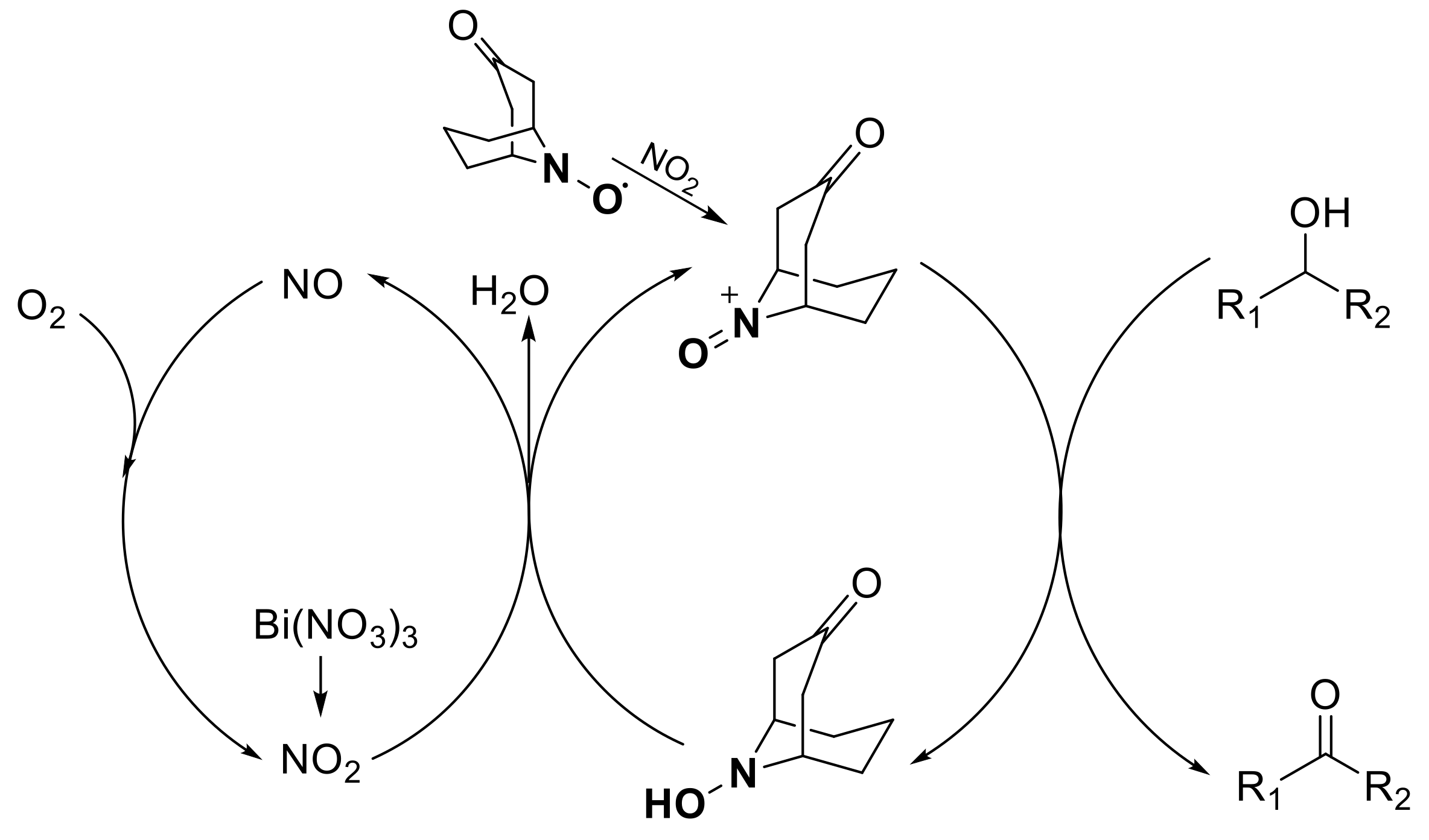
:1. Introduction
2. Results and Discussion
3. Experimental Section
3.1. General Information
3.2. General Procedure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Hudlicky, M. Oxidations in Organic Chemistry; American Chemical Society: Washington, DC, USA, 1990. [Google Scholar]
- Tojo, G.; Fernández, M.I. Oxidation of Alcohols to Aldehydes and Ketones: A Guide to Current Common Practice; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Sheldon, R.A.; Arends, I.W.C.E.; ten Brink, G.-J.; Dijksman, A. Green, Catalytic Oxidations of Alcohols. Acc. Chem. Res. 2002, 35, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.J.; Reid, M.; Foot, J.; Raw, S.A. Tandem oxidation processes using manganese dioxide: Discovery, applications, and current studies. Acc. Chem. Res. 2005, 38, 851–869. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Nunez, M.E.; Mello, R.; Olmos, A.; Acerete, R.; Asensio, G. Oxidation of alcohols to carbonyl compounds with CrO3·SiO2 in supercritical carbon dioxide. J. Org. Chem. 2006, 71, 1039–1042. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.V.; Hall, M. Activation of DMSO for Swern-type oxidation by 1,1-dichlorocycloheptatriene. Tetrahedron Lett. 2014, 55, 6895–6898. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.Q.; Zhao, Y.K.; Luo, H.Q.; Chai, L.Z.; Sheng, Q.J. I2O5: Mild and efficient reagents for the oxidation of alcohols in water. Tetrahedron Lett. 2007, 48, 3017–3019. [Google Scholar] [CrossRef]
- Punniyamurthy, T.; Velusamy, S.; Iqbal, J. Recent advances in transition metal catalyzed oxidation of organic substrates with molecular oxygen. Chem. Rev. 2005, 105, 2329–2364. [Google Scholar] [PubMed]
- Shi, Z.; Zhang, C.; Tang, C.; Jiao, N. Recent advances in transition-metal catalyzed reactions using molecular oxygen as the oxidant. Chem. Soc. Rev. 2012, 41, 3381–3430. [Google Scholar] [CrossRef]
- Tian, Y.; Guo, X.; Li, M.; Li, C.; Hu, X.; Jin, L.; Sun, N.; Hu, B.; Shen, Z. SBA-15 Supported 1-Methyl-2-azaadamanane N-Oxyl (1-Me-AZADO) as Recyclable Catalyst for Oxidation of Alcohol. Org. Lett. 2021, 23, 3928–3932. [Google Scholar] [CrossRef]
- Kim, M.J.; Jung, Y.E.; Lee, C.Y.; Kim, J. HKUST-1/ABNO-catalyzed aerobic oxidation of secondary benzyl alcohols at room temperature. Tetrahedron Lett. 2018, 59, 2722–2725. [Google Scholar]
- Wang, L.; Shang, S.; Li, G.; Ren, L.; Lv, Y.; Gao, S. Iron/ABNO-Catalyzed Aerobic Oxidation of Alcohols to Aldehydes and Ketones under Ambient Atmosphere. J. Org. Chem. 2016, 81, 2189–2193. [Google Scholar] [CrossRef]
- Zhai, D.; Ma, S. Copper catalysis for highly selective aerobic oxidation of alcohols to aldehydes/ketones. Org. Chem. Front. 2019, 6, 3101–3106. [Google Scholar] [CrossRef]
- Stahl, S.S. Palladium oxidase catalysis: Selective oxidation of organic chemicals by direct dioxygen-coupled turnover. Angew. Chem. Int. Ed. 2004, 43, 3400–3420. [Google Scholar] [CrossRef]
- Choi, E.; Lee, C.; Na, Y.; Chang, S. [RuCl2(p-cymene)]2 on carbon: An efficient, selective, reusable, and environmentally versatile heterogeneous catalyst. Org. Lett. 2002, 4, 2369–2371. [Google Scholar] [CrossRef]
- Ide, M.S.; Davis, R.J. The important role of hydroxyl on oxidation catalysis by gold nanoparticles. Acc. Chem. Res. 2014, 47, 825–833. [Google Scholar] [CrossRef]
- Shen, D.; Miao, C.; Xu, D.; Xia, C.; Sun, W. Highly efficient oxidation of secondary alcohols to ketones catalyzed by manganese complexes of N4 ligands with H2O2. Org. Lett. 2015, 17, 54–57. [Google Scholar] [CrossRef]
- Wang, X.W.; Wang, C.; Liu, Y.X.; Xiao, J.L. Acceptorless dehydrogenation and aerobic oxidation of alcohols with a reusable binuclear rhodium(II) catalyst in water. Green Chem. 2016, 18, 4605–4610. [Google Scholar] [CrossRef]
- Shibuya, M.; Pichierri, F.; Tomizawa, M.; Nagasawa, S.; Suzuki, I.; Iwabuchi, Y. Oxidation of nitroxyl radicals: Electrochemical and computational studies. Tetrahedron Lett. 2012, 53, 2070–2073. [Google Scholar] [CrossRef]
- Cao, Q.; Dornan, L.M.; Rogan, L.; Hughes, N.L.; Muldoon, M.J. Aerobic oxidation catalysis with stable radicals. Chem. Commun. 2014, 50, 4524–4543. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Xu, Z.; Shi, Y.; Cai, F.; Qiu, J.; Yang, G.; Hua, Z.; Chen, T. TEMPO-Functionalized Nanoreactors from Bottlebrush Copolymers for the Selective Oxidation of Alcohols in Water. J. Org. Chem. 2021, 86, 8027–8035. [Google Scholar] [CrossRef]
- Steves, J.E.; Stahl, S.S. Stable TEMPO and ABNO Catalyst Solutions for User-Friendly (bpy)Cu/Nitroxyl-Catalyzed Aerobic Alcohol Oxidation. J. Org. Chem. 2015, 80, 11184–11188. [Google Scholar] [CrossRef]
- Bothwell, J.M.; Krabbe, S.W.; Mohan, R.S. Applications of bismuth(III) compounds in organic synthesis. Chem. Soc. Rev. 2011, 40, 4649–4707. [Google Scholar] [CrossRef] [Green Version]
- Moon, H.W.; Cornella, J. Bismuth Redox Catalysis: An Emerging Main-Group Platform for Organic Synthesis. ACS Catal. 2022, 12, 1382–1393. [Google Scholar] [CrossRef]
- Mahire, V.N.; Mahulikar, P.P. Facile one-pot clean synthesis of benzimidazole motifs: Exploration on bismuth nitrate accelerated subtle catalysis. Chin. Chem. Lett. 2015, 26, 983–987. [Google Scholar] [CrossRef]
- Bisht, N.S.; Mehta, S.P.S.; Sahoo, N.G.; Dandapat, A. The room temperature synthesis of a CuO-Bi-BiOBr ternary Z-scheme photocatalyst for enhanced sunlight driven alcohol oxidation. Dalton Trans. 2021, 50, 5001–5010. [Google Scholar] [CrossRef]
- Terry, B.D.; DiMeglio, J.L.; Cousineau, J.P.; Bartlett, B.M. Nitrate Radical Facilitates Indirect Benzyl Alcohol Oxidation on Bismuth(III) Vanadate Photoelectrodes. ChemElectroChem 2020, 7, 3776–3782. [Google Scholar] [CrossRef]
- Powell, A.B.; Stahl, S.S. Aerobic oxidation of diverse primary alcohols to methyl esters with a readily accessible heterogeneous Pd/Bi/Te catalyst. Org. Lett. 2013, 15, 5072–5075. [Google Scholar] [CrossRef]
- Hu, Y.; Li, B. Efficient and selective palladium-catalyzed direct oxidative esterification of benzylic alcohols under aerobic conditions. Tetrahedron 2017, 73, 7301–7307. [Google Scholar] [CrossRef]
- Chakraborty, D.; Malik, P. Bismuth(III) Oxide Catalyzed Oxidation of Alcohols with tert-Butyl Hydroperoxide. Synthesis 2010, 2010, 3736–3740. [Google Scholar] [CrossRef]
- Lee, J.; Han, M.-k.; Kim, S.; Kim, S. Bismuth Tribromide Catalyzed Oxidation of Alcohols with Aqueous Hydrogen Peroxide. Synlett 2015, 26, 2434–2436. [Google Scholar] [CrossRef]
- Ueno, M.; Ohmura, S.D.; Wada, M.; Miyoshi, N. Aerobic oxidation of alcohols using bismuth bromide as a catalyst. Tetrahedron Lett. 2019, 60, 570–573. [Google Scholar] [CrossRef]
- Mukhopadhyay, C.; Datta, A. Bismuth(III) nitrate pentahydrate: A stoichiometric reagent for microwave induced mild and highly efficient aerial oxidation of aromatic aldehydes under solvent-free conditions. Catal. Commun. 2008, 9, 2588–2592. [Google Scholar] [CrossRef]
- Baruah, D.; Hussain, F.L.; Suri, M.; Saikia, U.P.; Sengupta, P.; Dutta, D.K.; Konwar, D. Bi(NO3)3·5H2O and cellulose mediated Cu-NPs—A highly efficient and novel catalytic system for aerobic oxidation of alcohols to carbonyls and synthesis of DFF from HMF. Catal. Commun. 2016, 77, 9–12. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, L.; Li, B. NHPI/tert-butyl nitrite: A highly efficient metal-free catalytic system for aerobic oxidation of alcohols to carbonyl compounds using molecular oxygen as the terminal oxidant. Catal. Commun. 2016, 83, 82–87. [Google Scholar] [CrossRef]
- Hu, Y.; Li, S.; Li, H.; Li, Y.; Li, J.; Duanmu, C.; Li, B. Copper-catalyzed tandem oxidative synthesis of quinazolinones from 2-aminobenzonitriles and benzyl alcohols. Org. Chem. Front. 2019, 6, 2744–2748. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, L.; Li, B. Iron nitrate/TEMPO-catalyzed aerobic oxidative synthesis of quinazolinones from alcohols and 2-aminobenzamides with air as the oxidant. RSC Adv. 2016, 6, 65196–65204. [Google Scholar] [CrossRef]
- Hu, Y.; Xia, J.; Li, J.; Li, H.; Li, Y.; Li, S.; Duanmu, C.; Li, B.; Wang, X. Direct oxidative esterification of alcohols catalyzed by a nitrogen-doped carbon black-supported PdBi bimetallic catalyst under ambient conditions. J. Mater. Sci. 2021, 56, 7308–7320. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, L.; Li, B. Practical CuCl/DABCO/4-HO-TEMPO-catalyzed oxidative synthesis of nitriles from alcohols with air as oxidant. Chin. Chem. Lett. 2018, 29, 464–466. [Google Scholar] [CrossRef]
- Rogan, L.; Hughes, N.L.; Cao, Q.; Dornan, L.M.; Muldoon, M.J. Copper(I)/ketoABNO catalysed aerobic alcohol oxidation. Catal. Sci. Technol. 2014, 4, 1720–1725. [Google Scholar] [CrossRef]
- Karimi, B.; Farhangi, E.; Vali, H.; Vahdati, S. SBA-15-Functionalized 3-Oxo-ABNO as Recyclable Catalyst for Aerobic Oxidation of Alcohols under Metal-Free Conditions. ChemSusChem 2014, 7, 2735–2741. [Google Scholar] [CrossRef]
- Ma, J.; Hong, C.; Wan, Y.; Li, M.; Hu, X.; Mo, W.; Hu, B.; Sun, N.; Jin, L.; Shen, Z. Aerobic oxidation of secondary alcohols in water with ABNO/tert-butyl nitrite/KPF6 catalytic system. Tetrahedron Lett. 2017, 58, 652–657. [Google Scholar] [CrossRef]
- Kim, S.; Kim, Y.; Jin, H.; Park, M.H.; Kim, Y.; Lee, K.M.; Kim, M. Europium-Catalyzed Aerobic Oxidation of Alcohols to Aldehydes/Ketones and Photoluminescence Tracking. Adv. Synth. Catal. 2019, 361, 1259–1264. [Google Scholar] [CrossRef]
- Aggen, D.H.; Arnold, J.N.; Hayes, P.D.; Smoter, N.; Mohan, R.S. Bismuth compounds in organic synthesis. Bismuth nitrate catalyzed chemoselective synthesis of acylals from aromatic aldehydes. Tetrahedron 2004, 60, 3675–3679. [Google Scholar] [CrossRef] [Green Version]
- Ma, S.M.; Liu, J.X.; Li, S.H.; Chen, B.; Cheng, J.J.; Kuang, J.Q.; Liu, Y.; Wan, B.Q.; Wang, Y.L.; Ye, J.T.; et al. Development of a General and Practical Iron Nitrate/TEMPO-Catalyzed Aerobic Oxidation of Alcohols to Aldehydes/Ketones: Catalysis with Table Salt. Adv. Synth. Catal. 2011, 353, 1005–1017. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, L.; Li, B. Fe(NO3)3/2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ): An efficient catalyst system for selective oxidation of alcohols under aerobic conditions. Catal. Commun. 2018, 103, 42–46. [Google Scholar] [CrossRef]




 | |||||
| Entry | Bi-Catalyst (mol%) | Nitroxyl Radical (mol%) | Solvent | Time (h) | Yield (%) b |
| 1 | - | TEMPO (3) | CH3CN | 3 | trace |
| 2 | Bi(NO3)3 (5) | - | CH3CN | 3 | trace |
| 3 | Bi2(SO4)3 (5) | TEMPO (3) | CH3CN | 3 | 10 |
| 4 | Bi2O3 (5) | TEMPO (3) | CH3CN | 3 | 23 |
| 5 | BiBr3 (5) | TEMPO (3) | CH3CN | 3 | trace |
| 6 | Bi(OTf)3 (5) | TEMPO (3) | CH3CN | 3 | 15 |
| 7 | Bi(NO3)3 (5) | TEMPO (3) | CH3CN | 3 | 89 |
| 8 | Bi(NO3)3 (5) | 4-HO-TEMPO (3) | CH3CN | 3 | 86 |
| 9 c | Bi(NO3)3 (5) | ACT (3) | CH3CN | 3 | 92 |
| 10 | Bi(NO3)3 (5) | Keto-ABNO (3) | CH3CN | 3 | 95 |
| 11 | Bi(NO3)3 (10) | Keto-ABNO (5) | CH3CN | 2 | 98 |
| 12 | Bi(NO3)3 (15) | Keto-ABNO (10) | CH3CN | 2 | 98 |
| 13 | Bi(NO3)3 (10) | Keto-ABNO (5) | CH3CN | 1 | 93 |
| 14 | Bi(NO3)3 (10) | Keto-ABNO (5) | Ethanol | 2 | 86 |
| 15 | Bi(NO3)3 (10) | Keto-ABNO (5) | DMSO | 2 | 75 |
| 16 | Bi(NO3)3 (10) | Keto-ABNO (5) | DCE | 2 | 94 |
| 17 | Bi(NO3)3 (10) | Keto-ABNO (5) | H2O | 2 | 45 |
| 18 d | Bi(NO3)3 (10) | Keto-ABNO (5) | CH3CN | 2 | 98 |
| 19 e | Bi(NO3)3 (10) | Keto-ABNO (5) | CH3CN | 2 | 15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Chen, L.; Shen, G.; Li, J.; Li, S.; Li, H.; Li, Y. A Highly Efficient Bismuth Nitrate/Keto-ABNO Catalyst System for Aerobic Oxidation of Alcohols to Carbonyl Compounds under Mild Conditions. Molecules 2022, 27, 3727. https://doi.org/10.3390/molecules27123727
Hu Y, Chen L, Shen G, Li J, Li S, Li H, Li Y. A Highly Efficient Bismuth Nitrate/Keto-ABNO Catalyst System for Aerobic Oxidation of Alcohols to Carbonyl Compounds under Mild Conditions. Molecules. 2022; 27(12):3727. https://doi.org/10.3390/molecules27123727
Chicago/Turabian StyleHu, Yongke, Lei Chen, Gulou Shen, Jin Li, Shaozhong Li, Huaju Li, and Yanxing Li. 2022. "A Highly Efficient Bismuth Nitrate/Keto-ABNO Catalyst System for Aerobic Oxidation of Alcohols to Carbonyl Compounds under Mild Conditions" Molecules 27, no. 12: 3727. https://doi.org/10.3390/molecules27123727
APA StyleHu, Y., Chen, L., Shen, G., Li, J., Li, S., Li, H., & Li, Y. (2022). A Highly Efficient Bismuth Nitrate/Keto-ABNO Catalyst System for Aerobic Oxidation of Alcohols to Carbonyl Compounds under Mild Conditions. Molecules, 27(12), 3727. https://doi.org/10.3390/molecules27123727







