Pennelliiside D, a New Acyl Glucose from Solanum pennellii and Chemical Synthesis of Pennelliisides
Abstract
1. Introduction
2. Results and Discussion
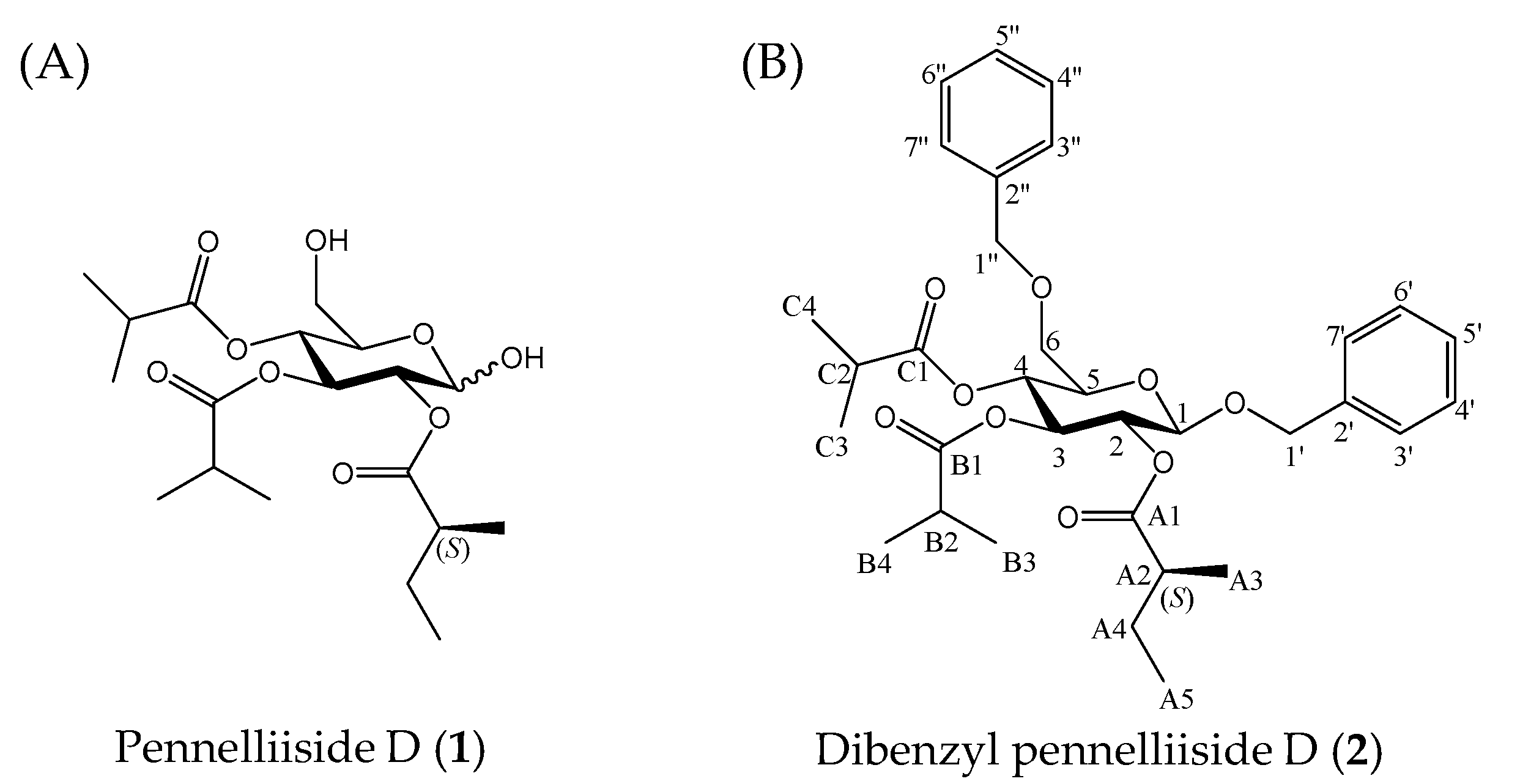
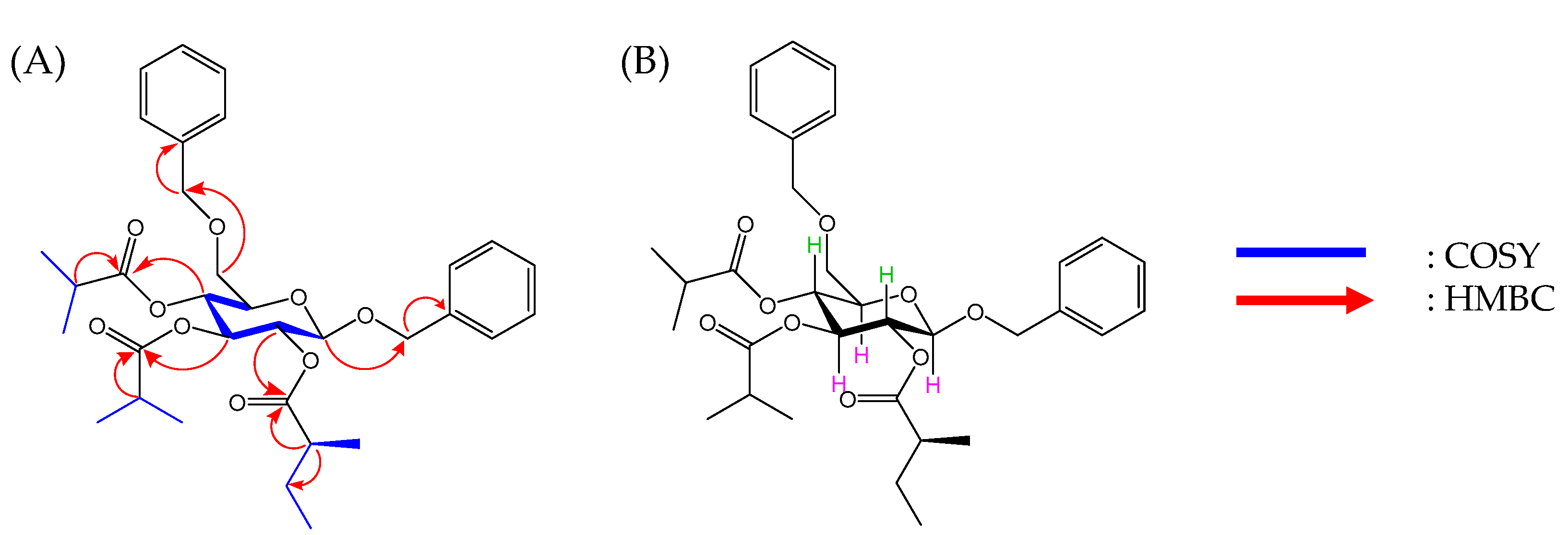
2.1. Isolation and Identification of Pennelliiside D (1)
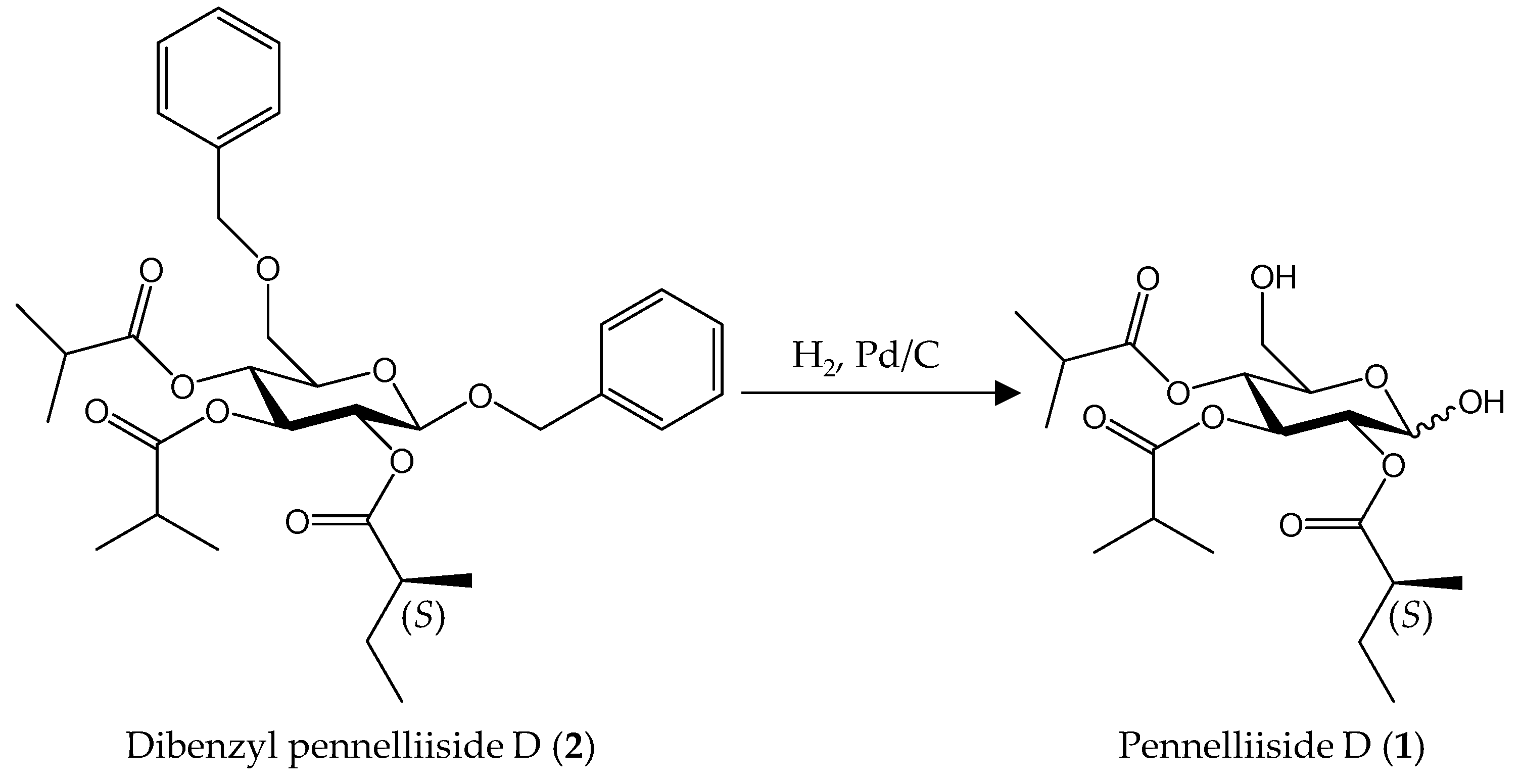
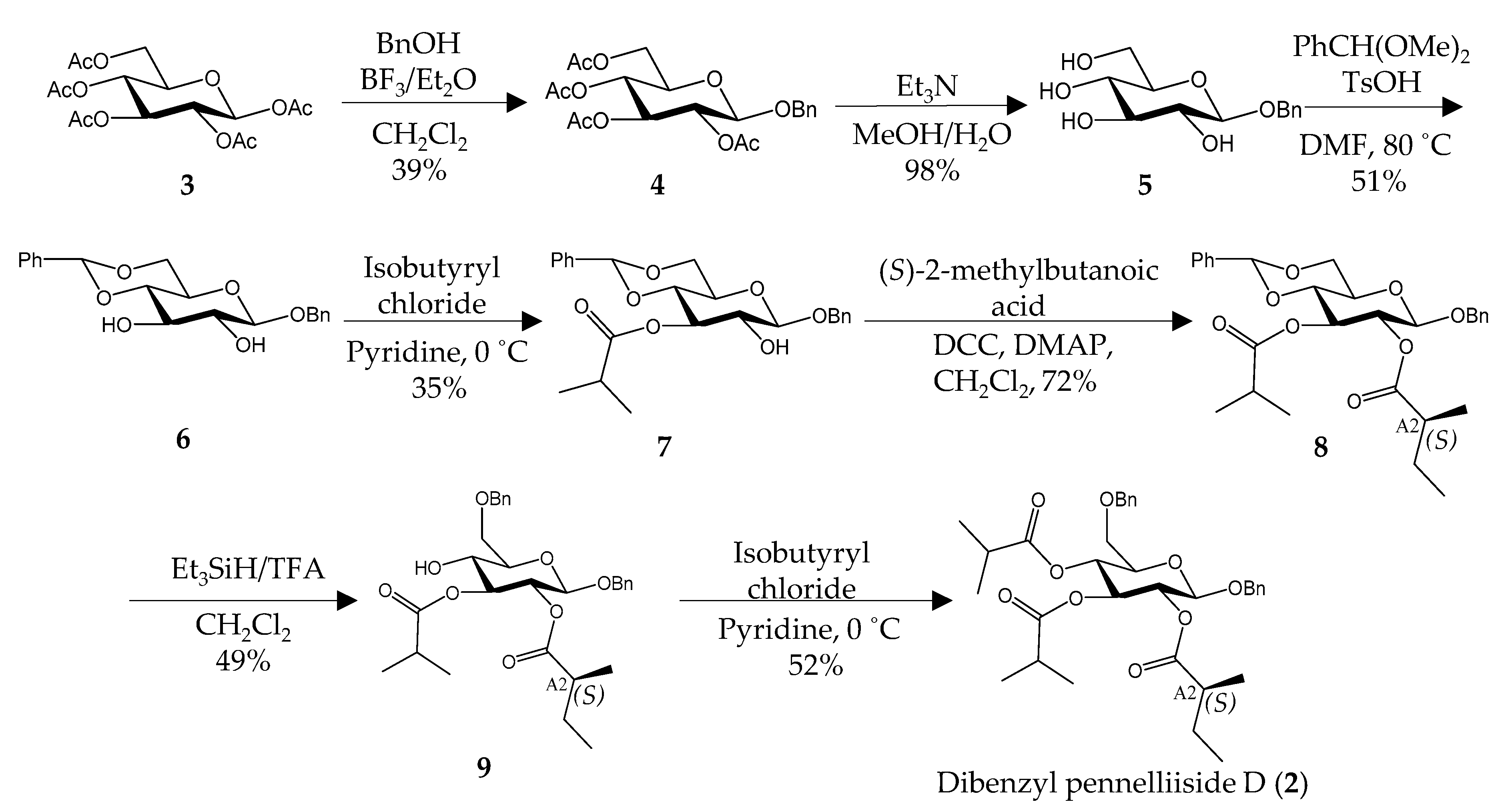
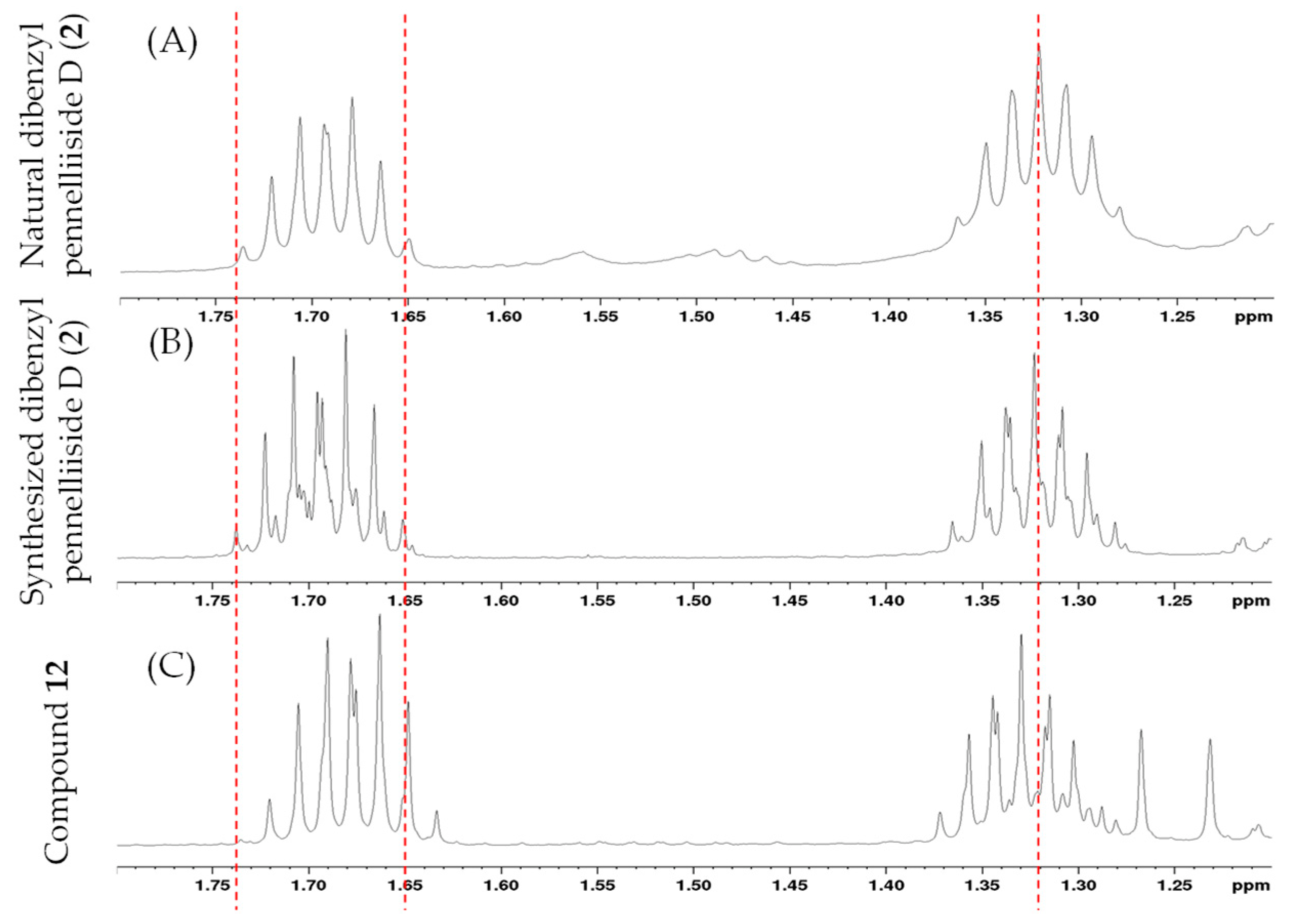
2.2. Synthesis of Pennelliiside D (1)
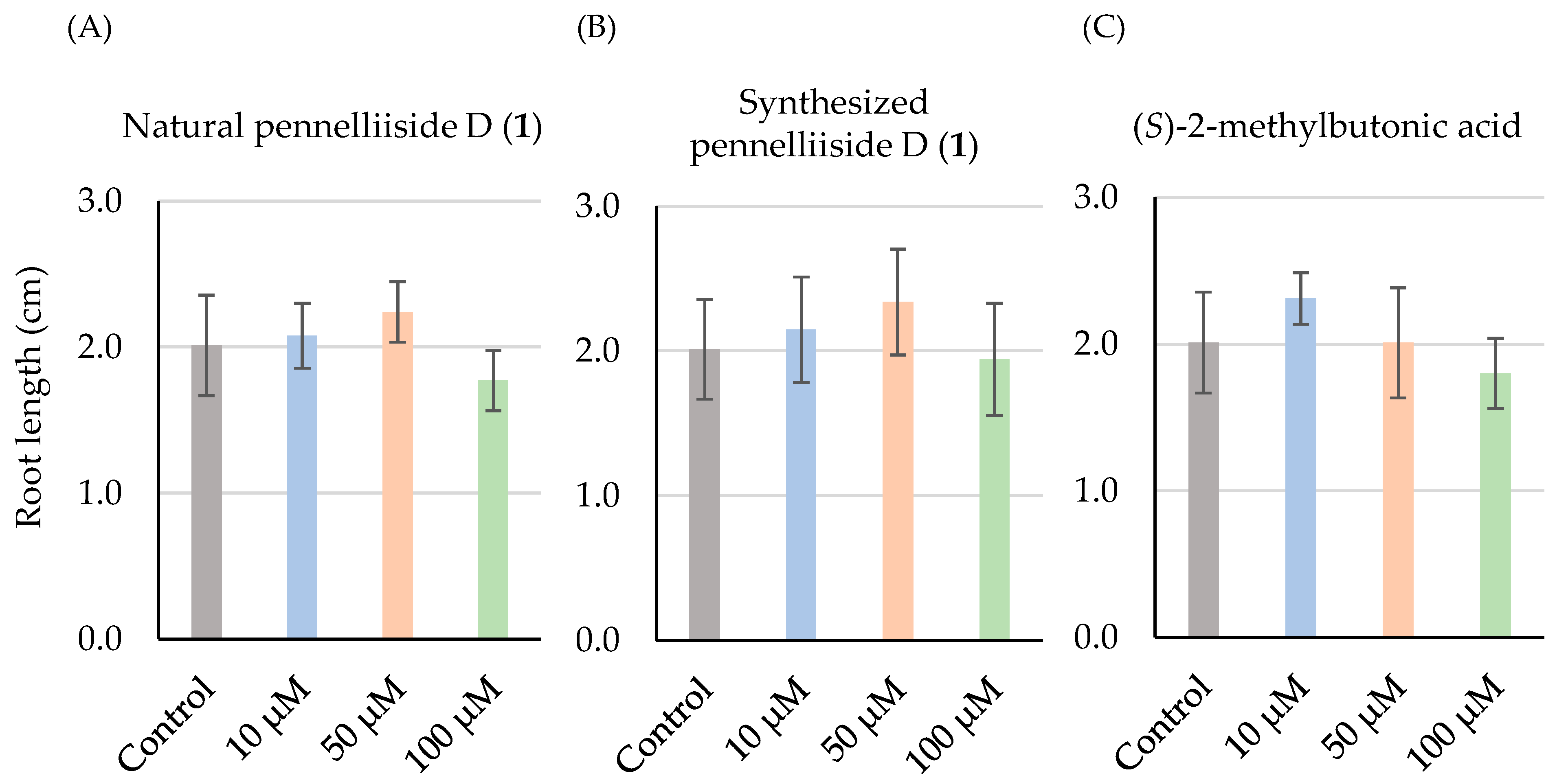
2.3. Root Growth-Inhibitory Activity of Pennelliiside D (1)
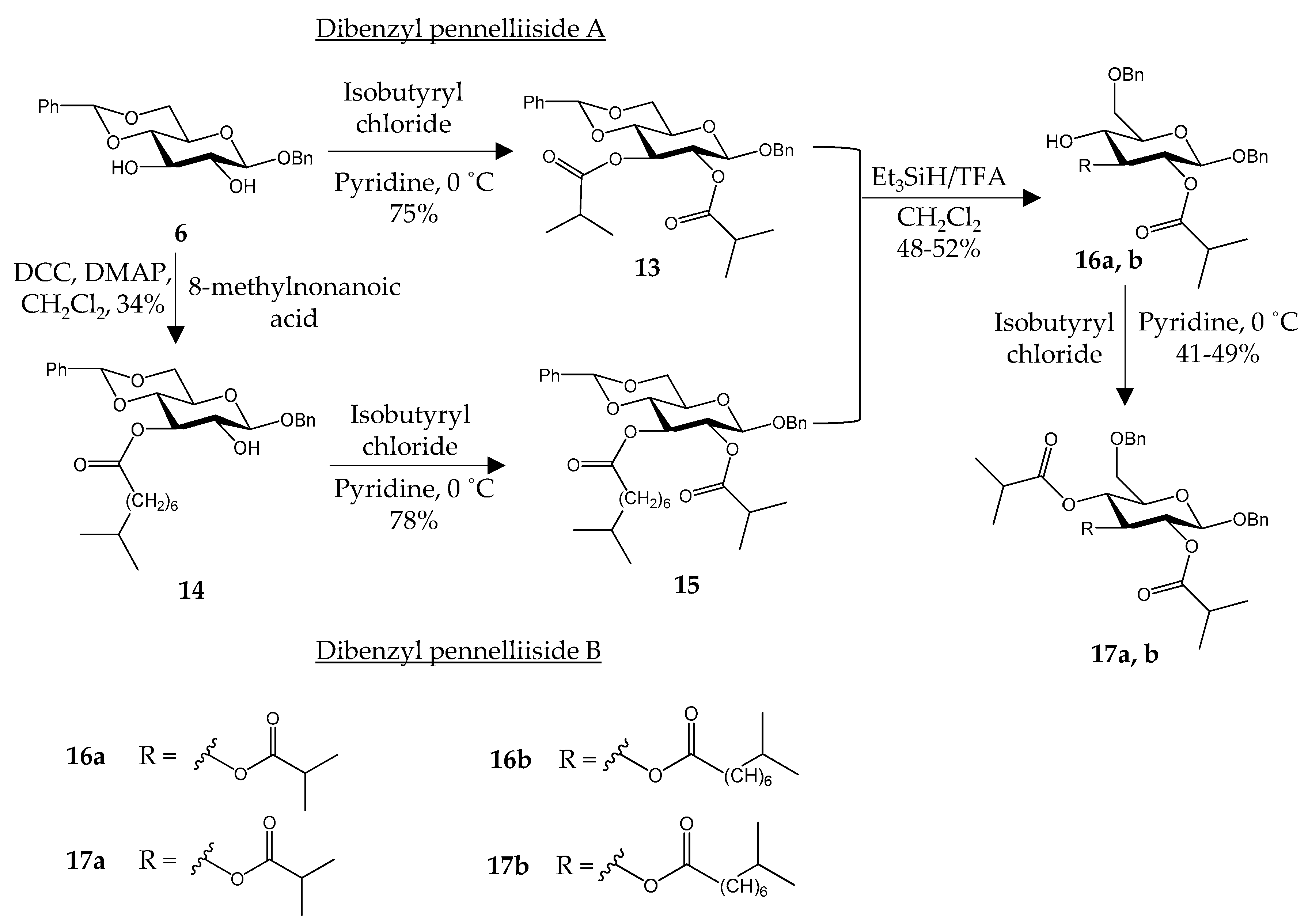
2.4. Synthesis of Dibenzyl Pennelliisides A and B
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Synthesis of Pennelliiside D (1)
3.4.1. Synthesis of 1-O-Benzyl-2,3,4,6-O-tetraacetyl-β-D-glucose (4)
3.4.2. Synthesis of 1-O-Benzyl-β-D-glucose (5)
3.4.3. Synthesis of 1-O-Benzyl-4,6-O-benzylidine-β-D-glucose (6)
3.4.4. Synthesis of 1-O-Benzyl-4,6-O-benzylidine-3-O-isobutyryl-β-D-glucose (7)
3.4.5. Synthesis of 1-O-Benzyl-4,6-O-benzylidine-3-O-isobutyryl-2-O-((S)-2-methylbutyryl)-β-D-glucose (8)
3.4.6. Synthesis of 1,6-O-Dibenzyl-3-O-isobutyryl-2-O-((S)-2-methylbutyryl)-β-D-glucose (9)
3.4.7. Synthesis of 1,6-O-Dibenzyl-3,4-O-diisobutyryl-2-O-((S)-2-methylbutyryl)-β-D-glucose (2)
3.4.8. Removal of Benzyl Ether
3.5. Synthesis of Pennelliisides A and B
3.5.1. Synthesis of 1-O-Benzyl-4,6-O-benzylidine-2,3-O-diisobutyryl-β-D-glucose (13)
3.5.2. Synthesis of 1-O-Benzyl-4,6-O-benzylidine-3-O-(8-methylnonanoyl)-β-D-glucose (14)
3.5.3. Synthesis of 1-O-Benzyl-4,6-O-benzylidine-2-O-isobutyryl-3-O-(8-methylnonanoyl)-β-D-glucose (15)
3.5.4. Synthesis of 16a, b and 17a, b
3.6. Root Growth-Inhibitory Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Leong, B.J.; Lybrand, D.B.; Lou, Y.R.; Fan, P.; Schilmiller, A.L.; Last, R.L. Evolution of Metabolic Novelty: A Trichome-Expressed Invertase Creates Specialized Metabolic Diversity in Wild Tomato. Sci. Adv. 2019, 5, eaaw3754. [Google Scholar] [CrossRef] [PubMed]
- Slocombe, S.P.; Schauvinhold, I.; McQuinn, R.P.; Besser, K.; Welsby, N.A.; Harper, A.; Aziz, N.; Li, Y.; Larson, T.R.; Giovannoni, J.; et al. Transcriptomic and Reverse Genetic Analyses of Branched-Chain Fatty Acid and Acyl Sugar Production in Solanum pennellii and Nicotiana benthamiana. Plant Physiol. 2008, 148, 1830–1846. [Google Scholar] [CrossRef] [PubMed]
- Kroumova, A.B.; Wagner, G.J. Different Elongation Pathways in the Biosynthesis of Acyl Groups of Trichome Exudate Sugar Esters from Various Solanaceous Plants. Planta 2003, 216, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Glas, J.J.; Schimmel, B.C.J.; Alba, J.M.; Escobar-Bravo, R.; Schuurink, R.C.; Kant, M.R. Plant Glandular Trichomes as Targets for Breeding or Engineering of Resistance to Herbivores. Int. J. Mol. Sci. 2012, 13, 17077–17103. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.; Scossa, F.; Bolger, M.E.; Lanz, C.; Maumus, F.; Tohge, T.; Quesneville, H.; Alseekh, S.; Sørensen, I.; Lichtenstein, G.; et al. The Genome of the Stress-Tolerant Wild Tomato Species Solanum pennellii. Nat. Genet. 2014, 46, 1034–1038. [Google Scholar] [CrossRef]
- Aflitos, S.; Schijlen, E.; De Jong, H.; De Ridder, D.; Smit, S.; Finkers, R.; Wang, J.; Zhang, G.; Li, N.; Mao, L.; et al. Exploring Genetic Variation in the Tomato (Solanum Section Lycopersicon) Clade by Whole-Genome Sequencing; The 100 Tomato Genome Sequencing Consortium. Plant J. 2014, 80, 136–148. [Google Scholar] [CrossRef]
- Chitwood, D.H.; Kumar, R.; Headland, L.R.; Ranjan, A.; Covington, M.F.; Ichihashi, Y.; Fulop, D.; Jiménez-Gómez, J.M.; Peng, J.; Maloof, J.N.; et al. A Quantitative Genetic Basis for Leaf Morphology in a Set of Precisely Defined Tomato Introgression Lines. Plant Cell 2013, 25, 2465–2481. [Google Scholar] [CrossRef]
- Liu, Z.; Alseekh, S.; Brotman, Y.; Zheng, Y.; Fei, Z.; Tieman, D.M.; Giovannoni, J.J.; Fernie, A.R.; Klee, H.J. Identification of a Solanum pennellii Chromosome 4 Fruit Flavor and Nutritional Quality-Associated Metabolite QTL. Front. Plant Sci. 2016, 7, 1671. [Google Scholar] [CrossRef]
- Schilmiller, A.; Shi, F.; Kim, J.; Charbonneau, A.L.; Holmes, D.; Daniel Jones, A.; Last, R.L. Mass Spectrometry Screening Reveals Widespread Diversity in Trichome Specialized Metabolites of Tomato Chromosomal Substitution Lines. Plant J. 2010, 62, 391–403. [Google Scholar] [CrossRef]
- Schilmiller, A.L.; Charbonneau, A.L.; Last, R.L. Identification of a BAHD Acetyltransferase That Produces Protective Acyl Sugars in Tomato Trichomes. Proc. Natl. Acad. Sci. USA 2012, 109, 16377–16382. [Google Scholar] [CrossRef]
- Goffreda, J.C.; Mutschler, M.A.; Avé, D.A.; Tingey, W.M.; Steffens, J.C. Aphid Deterrence by Glucose Esters in Glandular Trichome Exudate of the Wild Tomato, Lycopersicon pennellii. J. Chem. Ecol. 1989, 15, 2135–2147. [Google Scholar] [CrossRef]
- Luu, V.T.; Weinhold, A.; Ullah, C.; Dressel, S.; Schoettner, M.; Gase, K.; Gaquerel, E.; Xu, S.; Baldwin, I.T. O-Acyl Sugars Protect a Wild Tobacco from Both Native Fungal Pathogens and a Specialist Herbivore. Plant Physiol. 2017, 174, 370–386. [Google Scholar] [CrossRef] [PubMed]
- Kroumova, A.B.M.; Zaitlin, D.; Wagner, G.J. Natural Variability in Acyl Moieties of Sugar Esters Produced by Certain Tobacco and Other Solanaceae Species. Phytochemistry 2016, 130, 218–227. [Google Scholar] [CrossRef]
- Nakashima, T.; Nambu, Y.; Inoue, Y.; Masimbula, R.; Matsuura, H. Pennelliisides A-C, 2,3,4-Trisubstituted Acyl Glucoses Isolated from Solanum pennellii. J. Nat. Prod. 2020, 83, 2337–2346. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.; Leong, B.J.; Last, R.L. Tip of the Trichome: Evolution of Acylsugar Metabolic Diversity in Solanaceae. Curr. Opin. Plant Biol. 2019, 49, 8–16. [Google Scholar] [CrossRef]
- Schilmiller, A.L.; Last, R.L.; Pichersky, E. Harnessing Plant Trichome Biochemistry for the Production of Useful Compounds. Plant J. 2008, 54, 702–711. [Google Scholar] [CrossRef] [PubMed]
- Lybrand, D.B.; Anthony, T.M.; Jones, A.D.; Last, R.L. An Integrated Analytical Approach Reveals Trichome Acylsugar Metabolite Diversity in the Wild Tomato Solanum pennellii. Metabolites 2020, 10, 401. [Google Scholar] [CrossRef]
- Yamada, K.; Fujita, H.; Kunishima, M. A Novel Acid-Catalyzed O-Benzylating Reagent with the Smallest Unit of Imidate Structure. Org. Lett. 2012, 14, 5026–5029. [Google Scholar] [CrossRef]
- Yamada, K.; Yoshida, S.; Fujita, H.; Kitamura, M.; Kunishima, M. O-Benzylation of Carboxylic Acids Using 2,4,6-Tris(benzyloxy)-1,3,5-triazine (TriBOT) under Acidic or Thermal Conditions. Eur. J. Org. Chem. 2015, 2015, 7997–8002. [Google Scholar] [CrossRef]
- Corona-Castañeda, B.; Chérigo, L.; Fragoso-Serrano, M.; Gibbons, S.; Pereda-Miranda, R. Modulators of Antibiotic Activity from Ipomoea murucoides. Phytochemistry 2013, 95, 277–283. [Google Scholar] [CrossRef]
- Rosas-Ramírez, D.; Escalante-Sánchez, E.; Pereda-Miranda, R. Batatins III-VI, Glycolipid Ester-Type Dimers from Ipomoea batatas. Phytochemistry 2011, 72, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Degenstein, J.C.; Murria, P.; Easton, M.; Sheng, H.; Hurt, M.; Dow, A.R.; Gao, J.; Nash, J.J.; Agrawal, R.; Delgass, W.N.; et al. Fast Pyrolysis of 13c-Labeled Cellobioses: Gaining Insights into the Mechanisms of Fast Pyrolysis of Carbohydrates. J. Org. Chem. 2015, 80, 1909–1914. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.K.; Harrison, H.F.; Chortyk, O.T. Effects of Various Synthetic Sucrose Esters on Weed Seed Germination and Crop Growth: Structure-Activity and Dose-Response Relationships. J. Agric. Food Chem. 1997, 45, 4833–4837. [Google Scholar] [CrossRef]
| Position | Type | Natural Dibenzyl Pennelliiside D (2) | |
|---|---|---|---|
| δC | δH (J in Hz) | ||
| 1 | CH | 100.3 | 4.38, d (7.6) |
| 2 | CH | 71.9 | 5.46, m |
| 3 | CH | 73.5 | 5.48, m |
| 4 | CH | 70.1 | 5.30, dd (10.7, 9.5) |
| 5 | CH | 74.3 | 3.42, m |
| 6 | CH2 | 69.9 | 3.47, m |
| 1a′ | CH2 | 70.7 | 4.75, d (12.2) |
| 1b′ | 4.45, d (12.2) | ||
| 2′ | C | 138.0 | |
| 3′ | CH | 128.1–128.6 | 7.26, t (7.4) |
| 4′ | CH | 128.1–128.6 | 7.12–7.19, m |
| 5′ | CH | 128.1–128.6 | 7.08, t (7.3) |
| 6′ | CH | 128.1–128.6 | 7.12–7.19, m |
| 7′ | CH | 128.1–128.6 | 7.26, t (7.4) |
| 1 | C | 174.7 | |
| A2 | CH | 41.86 | 2.28, m |
| A3 | CH3 | 17.2 | 1.08, d (7.0) |
| A4 | CH2 | 27.2 | 1.32, 1.69, m, m |
| A5 | CH3 | 12.2 | 0.81, t (7.4) |
| B1 | C | 176.2 | |
| B2 | CH | 34.6 | 2.41, m |
| B3 | CH3 | 19.2–19.5 | 1.08, d (7.0) |
| B4 | CH3 | 19.2–19.5 | 1.08, d (7.0) |
| C1 | C | 175.3 | |
| C2 | CH | 34.5 | 2.31, m |
| C3 | CH3 | 19.2–19.5 | 1.02, d (7.0) |
| C4 | CH3 | 19.2–19.5 | 0.98, d (7.0) |
| 1a″ | CH2 | 73.9 | 4.33, d (5.5) |
| 1b″ | 4.33, d (5.5) | ||
| 2″ | C | 139.0 | |
| 3″ | CH | 128.1–128.7 | 7.26, t (7.4) |
| 4″ | CH | 128.1–128.7 | 7.12–7.19, m |
| 5″ | CH | 128.1–128.7 | 7.08, t (7.3) |
| 6″ | CH | 128.1–128.7 | 7.12–7.19, m |
| 7″ | CH | 128.1–128.7 | 7.26, t (7.4) |
| Position | α Anomer | β Anomer | |||
|---|---|---|---|---|---|
| Type | δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 1 | CH | 90.4 | 5.48, d (3.6) | 96.1 | 4.76, d (6.9) |
| 2 | CH | 71.4 | 4.85, dd (6.8, 3.6) | 73.5 | 4.91, dd (7.7, 6.89) |
| 3 | CH | 69.0 | 5.65, dd (10.9, 9.9) | 71.4 | 5.41, dd (10.4, 9.6) |
| 4 | CH | 68.8 | 5.01, dd (10.9, 9.7) | 68.8 | 5.09, dd (10.4, 8.1) |
| 5 | CH | 69.7 | 4.06, m | 74.7 | 3.59, m |
| 6 | CH2 | 61.3 | 3.71, 3.55, m | 61.3 | 3.75, 3.59, m |
| A1 | C | 176.6 | 176.6 | ||
| A2 | CH | 41.1 | 2.38, m | 41.1 | 2.42, m |
| A3 | CH3 | 16.4–19.4 | 1.03–1.17, m | 16.4–19.4 | 1.07–1.21, m |
| A4 | CH2 | 26.7 | 1.41, 1.62, m | 26.7 | 1.45, 1.66, m |
| A5 | CH3 | 11.7 | 0.85, m | 11.7 | 0.90, m |
| B1 | C | 176.0 | 176.0 | ||
| B2 | CH | 34.2 | 2.50, m | 34.2 | 2.50, m |
| B3 | CH3 | 16.4–19.4 | 1.03–1.17, m | 16.4–19.4 | 1.07–1.21, m |
| B4 | CH3 | 16.4–19.4 | 1.03–1.17, m | 16.4–19.4 | 1.07–1.21, m |
| C1 | C | 176.9 | 176.9 | ||
| C2 | CH | 34.2 | 2.56, m | 34.2 | 2.56, m |
| C3 | CH3 | 16.4–19.4 | 1.03–1.17, m | 16.4–19.4 | 1.07–1.21, m |
| C4 | CH3 | 16.4–19.4 | 1.03–1.17, m | 16.4–19.4 | 1.07–1.21, m |
| Position. | Type | α Anomer | β Anomer | ||
|---|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | ||
| 1 | CH | 90.4 | 5.48, d (3.6) | 96.1 | 4.72, d (6.9) |
| 2 | CH | 71.4 | 4.85, dd (6.8, 3.6) | 73.5 | 4.87, dd (7.7, 6.89) |
| 3 | CH | 69.0 | 5.65, dd (10.9, 9.9) | 71.4 | 5.37, dd (10.4, 9.6) |
| 4 | CH | 68.8 | 5.01, dd (10.9, 9.7) | 68.8 | 5.05, dd (10.4, 8.1) |
| 5 | CH | 69.7 | 4.06, m | 74.7 | 3.55, m |
| 6 | CH2 | 61.3 | 3.71, 3.55, m | 61.3 | 3.71, 3.55, m |
| A1 | C | 176.6 | 176.6 | ||
| A2 | CH | 41.1 | 2.24–2.42, m | 41.1 | 2.24–2.42, m |
| A3 | CH3 | 16.4–19.4 | 1.03-1.17, m | 16.4–19.4 | 1.03-1.17, m |
| A4 | CH2 | 26.7 | 1.41, 1.62, m | 26.7 | 1.41, 1.62, m |
| A5 | CH3 | 11.7 | 0.85, m | 11.7 | 0.85, m |
| B1 | C | 176.0 | 176.0 | ||
| B2 | CH | 34.2 | 2.50, m | 34.2 | 2.50, m |
| B3 | CH3 | 16.4–19.4 | 1.03–1.17, m | 16.4–19.4 | 1.03–1.17, m |
| B4 | CH3 | 16.4–19.4 | 1.03–1.17, m | 16.4–19.4 | 1.03–1.17, m |
| C1 | C | 176.9 | 176.9 | ||
| C2 | CH | 34.2 | 2.56, m | 34.2 | 2.52, m |
| C3 | CH3 | 16.4–19.4 | 1.03–1.17, m | 16.4–19.4 | 1.03–1.17, m |
| C4 | CH3 | 16.4–19.4 | 1.03–1.17, m | 16.4–19.4 | 1.03–1.17, m |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masimbula, R.; Kobayashi, H.; Nakashima, T.; Nambu, Y.; Kitaoka, N.; Matsuura, H. Pennelliiside D, a New Acyl Glucose from Solanum pennellii and Chemical Synthesis of Pennelliisides. Molecules 2022, 27, 3728. https://doi.org/10.3390/molecules27123728
Masimbula R, Kobayashi H, Nakashima T, Nambu Y, Kitaoka N, Matsuura H. Pennelliiside D, a New Acyl Glucose from Solanum pennellii and Chemical Synthesis of Pennelliisides. Molecules. 2022; 27(12):3728. https://doi.org/10.3390/molecules27123728
Chicago/Turabian StyleMasimbula, Rishni, Hiroto Kobayashi, Tenki Nakashima, Yurika Nambu, Naoki Kitaoka, and Hideyuki Matsuura. 2022. "Pennelliiside D, a New Acyl Glucose from Solanum pennellii and Chemical Synthesis of Pennelliisides" Molecules 27, no. 12: 3728. https://doi.org/10.3390/molecules27123728
APA StyleMasimbula, R., Kobayashi, H., Nakashima, T., Nambu, Y., Kitaoka, N., & Matsuura, H. (2022). Pennelliiside D, a New Acyl Glucose from Solanum pennellii and Chemical Synthesis of Pennelliisides. Molecules, 27(12), 3728. https://doi.org/10.3390/molecules27123728







