Established Liposome-Coated IMB16-4 Polymeric Nanoparticles (LNPs) for Increasing Cellular Uptake and Anti-Fibrotic Effects In Vitro
Abstract
1. Introduction
2. Results and Discussion
2.1. The Morphology and Particle Size
2.2. Entrapment Efficiency (EE) and Drug Loading
2.3. Characterization of IMB16-4−LNPs
2.4. Stability Measurements
2.5. In Vitro Release
2.6. In Vitro Cellular Uptake
2.7. The Cytotoxicity
2.8. In Vitro Antifibrotic Effects
3. Materials and Methods
3.1. Materials
3.2. Preparation of IMB16-4−LNPs
3.3. The Content of IMB16-4−LNPs by HPLC
IMB16-4−LNPs
3.4. Physicochemical Characterization
3.5. Stability Measurements
3.6. In Vitro Drug Release Study
3.7. In Vitro Cytotoxicity
3.8. In Vitro Cellular Uptake
3.9. The Anti-Fibrotic Effects on LX-2 Cells
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Tsochatzis, E.A.; Bosch, J.; Burroughs, A.K. Liver cirrhosis. Lancet 2014, 383, 1749–1761. [Google Scholar] [CrossRef]
- Wang, F.-S.; Fan, J.-G.; Zhang, Z.; Gao, B.; Wang, H.-Y. The global burden of liver disease: The major impact of China. Hepatology 2014, 60, 2099–2108. [Google Scholar] [CrossRef] [PubMed]
- Roehlen, N.; Crouchet, E.; Baumert, T.F. Liver Fibrosis: Mechanistic Concepts and Therapeutic Perspectives. Cells 2020, 9, 875. [Google Scholar] [CrossRef] [PubMed]
- Kisseleva, T.; Brenner, D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat. Rev. Gastroenterol. Hepatol. 2020, 18, 151–166. [Google Scholar] [CrossRef]
- Tsuchida, T.; Friedman, S.L. Mechanisms of hepatic stellate cell activation. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 397–411. [Google Scholar] [CrossRef]
- Nakano, Y.; Kamiya, A.; Sumiyoshi, H.; Tsuruya, K.; Kagawa, T.; Inagaki, Y. A Deactivation Factor of Fibrogenic Hepatic Stellate Cells Induces Regression of Liver Fibrosis in Mice. Hepatology 2020, 71, 1437–1452. [Google Scholar] [CrossRef]
- El-Mezayen, N.S.; El-Hadidy, W.F.; El-Refaie, W.M.; Shalaby, T.I.; Khattab, M.M.; El-Khatib, A.S. Oral vitamin-A-coupled valsartan nanomedicine: High hepatic stellate cell receptors accessibility and prolonged enterohepatic residence. J. Control. Release 2018, 283, 32–44. [Google Scholar] [CrossRef]
- Ramakrishna, G.; Rastogi, A.; Trehanpati, N.; Sen, B.; Khosla, R.; Sarin, S.K. From Cirrhosis to Hepatocellular Carcinoma: New Molecular Insights on Inflammation and Cellular Senescence. Liver Cancer 2013, 2, 367–383. [Google Scholar] [CrossRef]
- Campana, L.; Iredale, J.P. Regression of Liver Fibrosis. Semin. Liver Dis. 2017, 37, 001–010. [Google Scholar] [CrossRef]
- Lo, R.C.; Kim, H. Histopathological evaluation of liver fibrosis and cirrhosis regression. Clin. Mol. Hepatol. 2017, 23, 302–307. [Google Scholar] [CrossRef]
- Taimr, P. Fibrogenesis, hepatic stellate cells and the immune system. Immunol. Dis. Liver Gut 2004, 135, 36–41. [Google Scholar]
- Kisseleva, T.; Brenner, D.A. Role of hepatic stellate cells in fibrogenesis and the reversal of fibrosis. J. Gastroenterol. Hepatol. 2007, 22, S73–S78. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Jackson, E.K. Strong antiproliferative effects of baicalein in cultured rat hepatic stellate cells. Eur. J. Pharmacol. 1999, 378, 129–135. [Google Scholar] [CrossRef]
- Benyon, R.C.; Iredale, J.P.; Goddard, S.; Winwood, P.J.; Arthur, M.J. Expression of tissue inhibitor of metalloproteinases 1 and 2 is increased in fibrotic human liver. Gastroenterology 1996, 110, 821–831. [Google Scholar] [CrossRef] [PubMed]
- Iredale, J.P.; Benyon, R.C.; Pickering, J.; McCullen, M.; Northrop, M.; Pawley, S.; Hovell, C.; Arthur, M.J. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J. Clin. Investig. 1998, 102, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Murphy, F.; Waung, J.; Collins, J.; Arthur, M.J.; Nagase, H.; Mann, D.; Benyon, R.C.; Iredale, J.P. N-Cadherin cleavage during activated hepatic stellate cell apoptosis is inhibited by tissue inhibitor of metalloproteinase-1. Gut 2004, 3 (Suppl. 1), S8. [Google Scholar] [CrossRef][Green Version]
- Murphy, F.R.; Issa, R.; Zhou, X.; Ratnarajah, S.; Nagase, H.; Arthur, M.J.; Benyon, C.; Iredale, J.P. Inhibition of apoptosis of activated hepatic stellate cells by tissue inhibitor of metalloproteinase-1 is mediated via effects on matrix metalloproteinase inhibition: Implications for reversibility of liver fibrosis. J. Biol. Chem. 2002, 277, 11069–11076. [Google Scholar] [CrossRef]
- Ikejima, K.; Okumura, K.; Kon, K.; Takei, Y.; Sato, N. Role of adipocytokines in hepatic fibrogenesis. J. Gastroenterol. Hepatol. 2007, 22 (Suppl. 1), S87–S92. [Google Scholar] [CrossRef]
- Derynck, R.; Zhang, Y.E. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 2003, 425, 577–584. [Google Scholar] [CrossRef]
- Yi, C.; Shen, Z.; Stemmer-Rachamimov, A.; Dawany, N.; Troutman, S.; Showe, L.C.; Liu, Q.; Shimono, A.; Sudol, M.; Holmgren, L.; et al. The p130 Isoform of Angiomotin Is Required for Yap-Mediated Hepatic Epithelial Cell Proliferation and Tumorigenesis. Sci. Signal. 2013, 6, ra77. [Google Scholar] [CrossRef]
- Sun, Y.-M.; Chen, S.-Y.; You, H. Regression of liver fibrosis: Evidence and challenges. Chin. Med. J. 2020, 133, 1696–1702. [Google Scholar] [CrossRef] [PubMed]
- Koyama, Y.; Brenner, D.A. New therapies for hepatic fibrosis. Clin. Res. Hepatol. Gastroenterol. 2015, 39 (Suppl. 1), S75–S79. [Google Scholar] [CrossRef] [PubMed]
- Traber, P.G.; Chou, H.; Zomer, E.; Hong, F.; Klyosov, A.; Fiel, M.-I.; Friedman, S.L. Regression of Fibrosis and Reversal of Cirrhosis in Rats by Galectin Inhibitors in Thioacetamide-Induced Liver Disease. PLoS ONE 2013, 8, e75361. [Google Scholar] [CrossRef] [PubMed]
- Colino, C.I.; Lanao, J.M.; Gutierrez-Millan, C. Targeting of Hepatic Macrophages by Therapeutic Nanoparticles. Front. Immunol. 2020, 11, 218. [Google Scholar] [CrossRef] [PubMed]
- Koyama, Y.; Xu, J.; Liu, X.; Brenner, D.A. New Developments on the Treatment of Liver Fibrosis. Dig. Dis. 2016, 34, 589–596. [Google Scholar] [CrossRef]
- Tsuchida, T. Mechanisms of hepatic stellate cell activation as a therapeutic target for the treatment of non-alcoholic steatohepatitis. Nihon Yakurigaku Zasshi 2019, 154, 203–209. [Google Scholar] [CrossRef]
- Niu, X.; Wang, X.; Niu, B.; Wang, Y.; He, H.; Li, G. New IMB16-4 Nanoparticles Improved Oral Bioavailability and Enhanced Anti-Hepatic Fibrosis on Rats. Pharmaceuticals 2022, 15, 85. [Google Scholar] [CrossRef]
- Niu, X.; Wang, X.; Niu, B.; Li, G.; Yang, X.; Wang, Y.; Li, G. Novel IMB16-4 Compound Loaded into Silica Nanoparticles Exhibits Enhanced Oral Bioavailability and Increased Anti-Liver Fibrosis In Vitro. Molecules 2021, 26, 1545. [Google Scholar] [CrossRef]
- Garg, N.K.; Tandel, N.; Jadon, R.; Tyagi, R.K.; Katare, O.P. Lipid–polymer hybrid nanocarrier-mediated cancer therapeutics: Current status and future directions. Drug Discov. Today 2018, 23, 1610–1621. [Google Scholar] [CrossRef]
- Hadinoto, K.; Sundaresan, A.; Cheow, W.S. Lipid–polymer hybrid nanoparticles as a new generation therapeutic delivery platform: A review. Eur. J. Pharm. Biopharm. 2013, 85, 427–443. [Google Scholar] [CrossRef]
- Al-Jamal, W.T.; Kostarelos, K. Liposome–nanoparticle hybrids for multimodal diagnostic and therapeutic applications. Nanomedicine 2007, 2, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Mandal, B.; Bhattacharjee, H.; Mittal, N.; Sah, H.; Balabathula, P.; Thoma, L.A.; Wood, G.C. Core–shell-type lipid–polymer hybrid nanoparticles as a drug delivery platform. Nanomedicine 2013, 9, 474–491. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Li, X.; Guo, Y.; Zhang, Z. Lipid-enveloped hybrid nanoparticles for drug delivery. Nanoscale 2013, 5, 860–872. [Google Scholar] [CrossRef] [PubMed]
- Raemdonck, K.; Braeckmans, K.; Demeester, J.; De Smedt, S.C. Merging the best of both worlds: Hybrid lipid-enveloped matrix nanocomposites in drug delivery. Chem. Soc. Rev. 2014, 43, 444–472. [Google Scholar] [CrossRef]
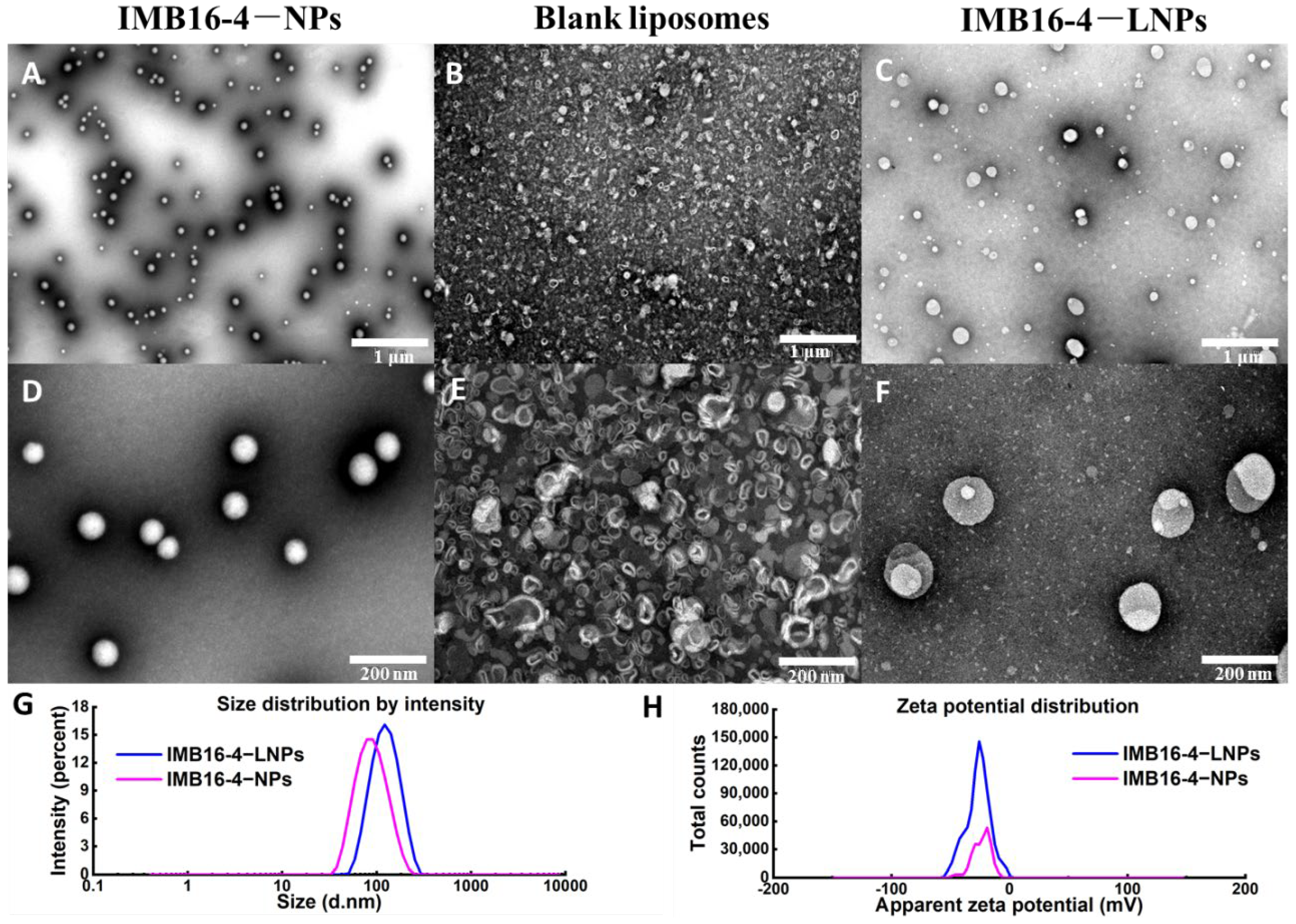
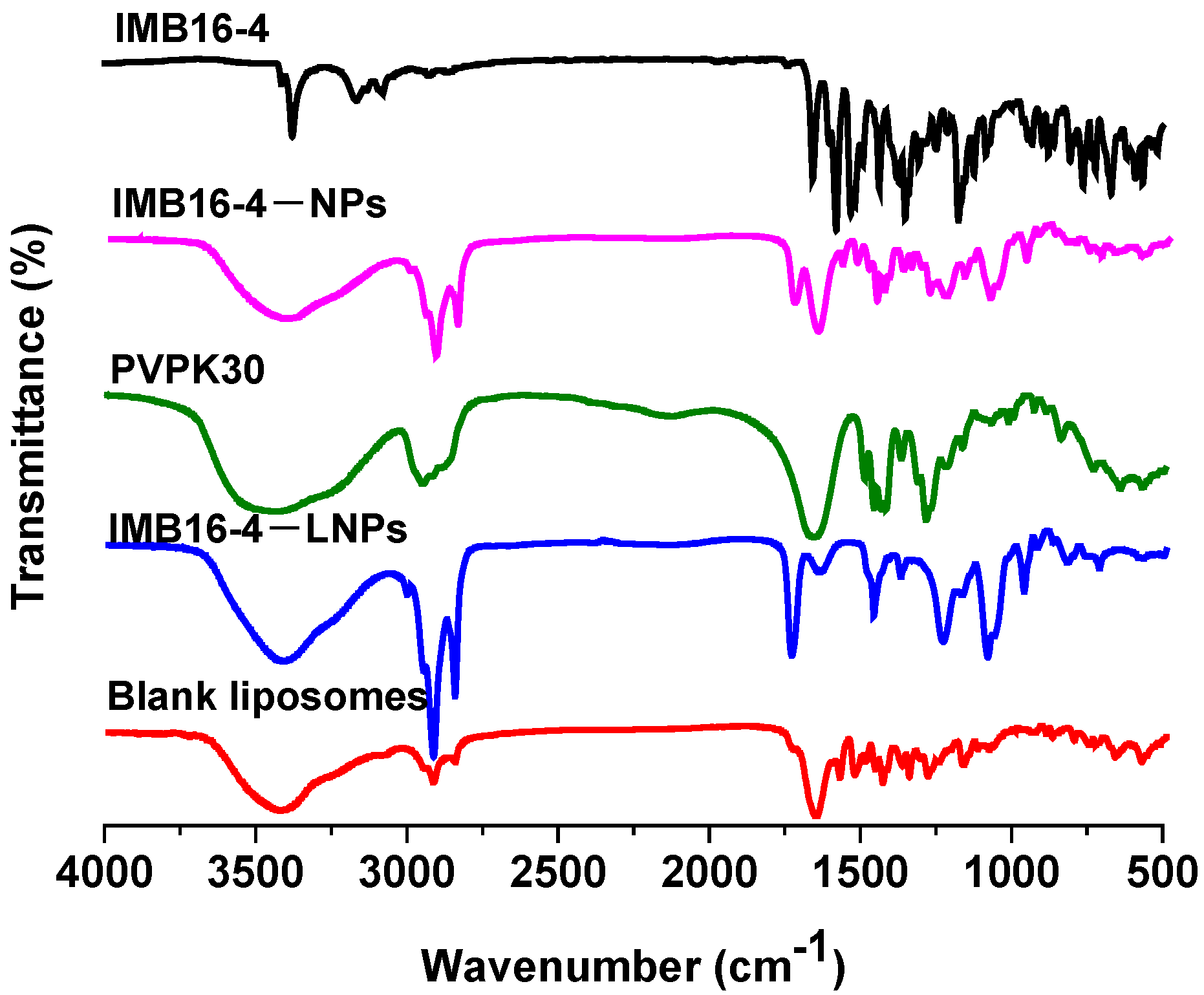


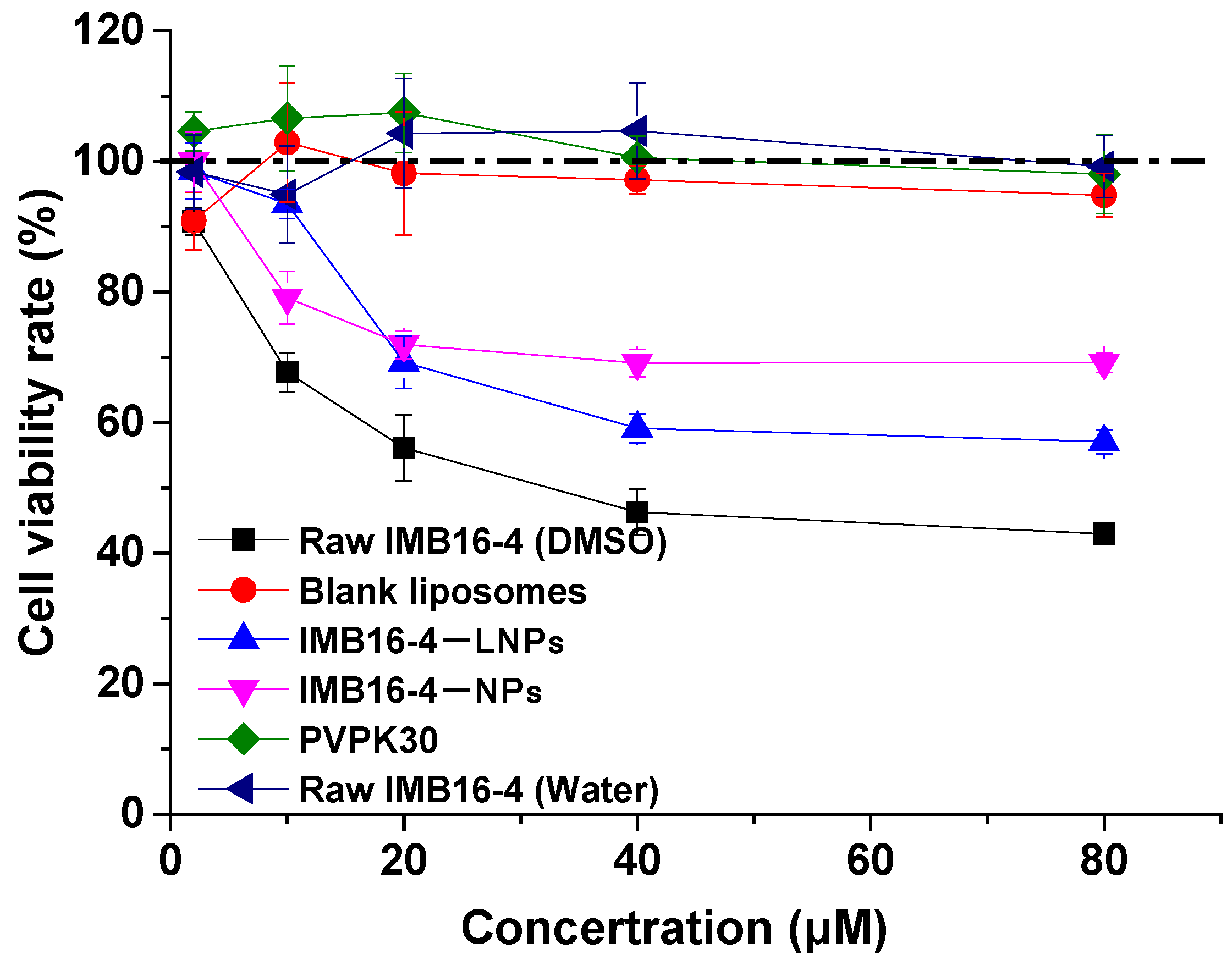
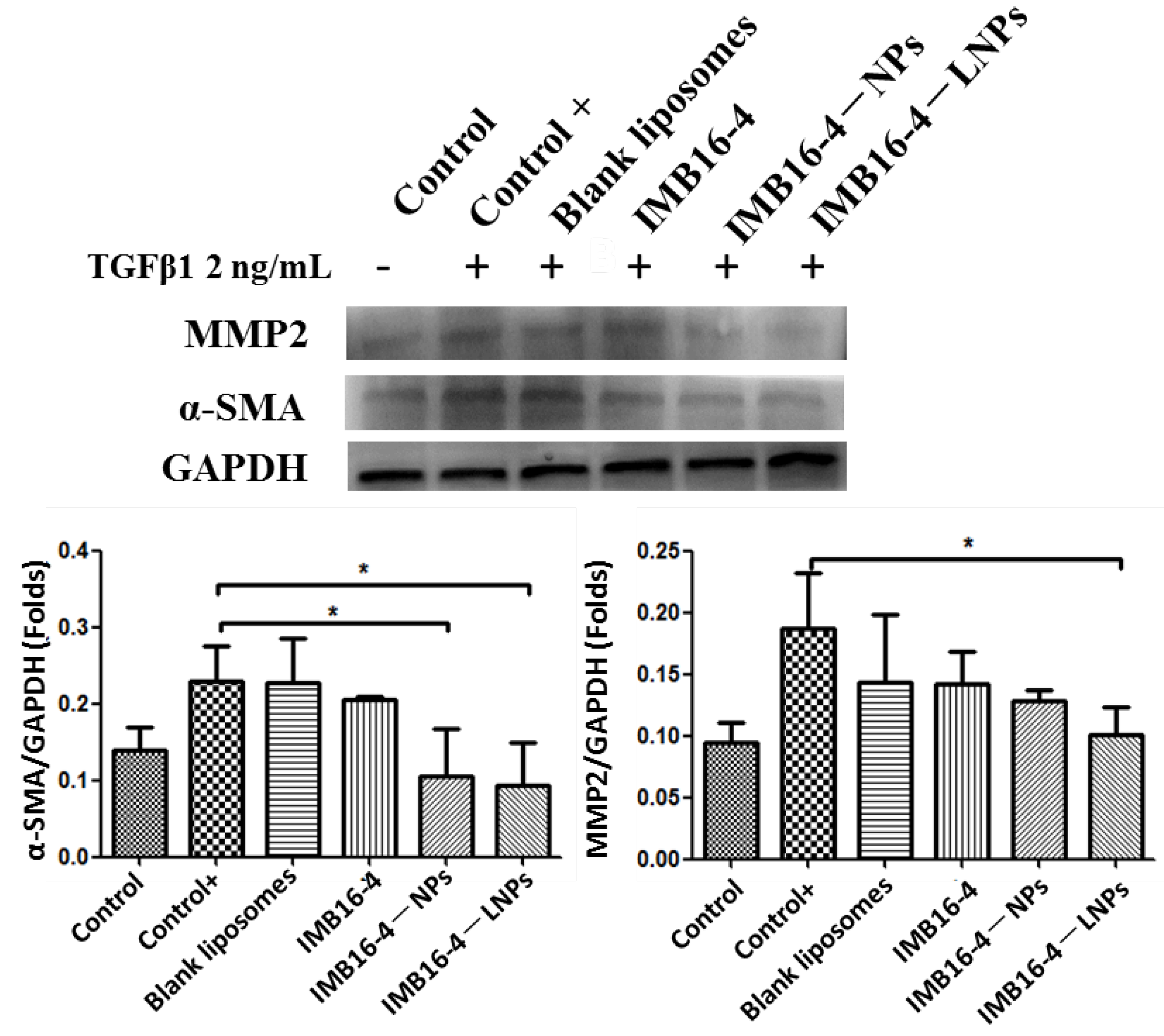
| Sample | Particle Size (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|
| IMB16-4−NPs | 83.8 ± 35.0 | 0.148 | −23.9 |
| IMB16-4−LNPs | 119.0 ± 43.1 | 0.183 | −26.6 |
| Date (Days) | Particle Size (nm) | PDI | ZP | The Content Percentage (%) |
|---|---|---|---|---|
| 0 | 117.7 ± 52.5 | 0.267 | −26.0 | 100.0 |
| 10 | 122.3 ± 72.6 | 0.217 | −26.0 | 104.7 |
| 20 | 121.7 ± 73.8 | 0.252 | −29.9 | 97.0 |
| 30 | 122.5 ± 73.4 | 0.218 | −25.0 | 102.2 |
| 40 | 113.1 ± 43.9 | 0.233 | −28.4 | 95.4 |
| 50 | 119.2 ± 64.7 | 0.221 | −25.0 | 98.0 |
| 60 | 123.2 ± 45.6 | 0.241 | −26.7 | 101.7 |
| Concentration (μM) | IMB16-4−NPs (mg/g Prot) | IMB16-4−LNPs (mg/g Prot) |
|---|---|---|
| 1 | 0 ± 0 | 3.60 ± 0.89 ** |
| 2 | 1.48 ± 0.35 | 6.68 ± 2.85 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, X.; Meng, Y.; Wang, Y.; Li, G. Established Liposome-Coated IMB16-4 Polymeric Nanoparticles (LNPs) for Increasing Cellular Uptake and Anti-Fibrotic Effects In Vitro. Molecules 2022, 27, 3738. https://doi.org/10.3390/molecules27123738
Niu X, Meng Y, Wang Y, Li G. Established Liposome-Coated IMB16-4 Polymeric Nanoparticles (LNPs) for Increasing Cellular Uptake and Anti-Fibrotic Effects In Vitro. Molecules. 2022; 27(12):3738. https://doi.org/10.3390/molecules27123738
Chicago/Turabian StyleNiu, Xia, Yanan Meng, Yucheng Wang, and Guiling Li. 2022. "Established Liposome-Coated IMB16-4 Polymeric Nanoparticles (LNPs) for Increasing Cellular Uptake and Anti-Fibrotic Effects In Vitro" Molecules 27, no. 12: 3738. https://doi.org/10.3390/molecules27123738
APA StyleNiu, X., Meng, Y., Wang, Y., & Li, G. (2022). Established Liposome-Coated IMB16-4 Polymeric Nanoparticles (LNPs) for Increasing Cellular Uptake and Anti-Fibrotic Effects In Vitro. Molecules, 27(12), 3738. https://doi.org/10.3390/molecules27123738







