Chemical Solution Deposition of Barium Titanate Thin Films with Ethylene Glycol as Solvent for Barium Acetate
Abstract
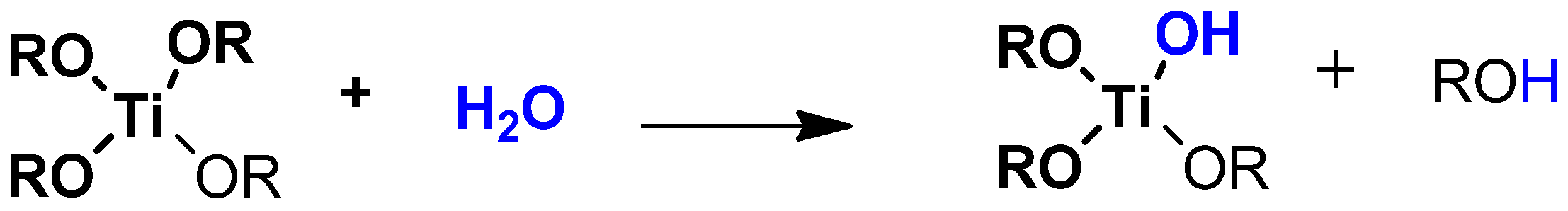
:1. Introduction
2. Results and Discussion
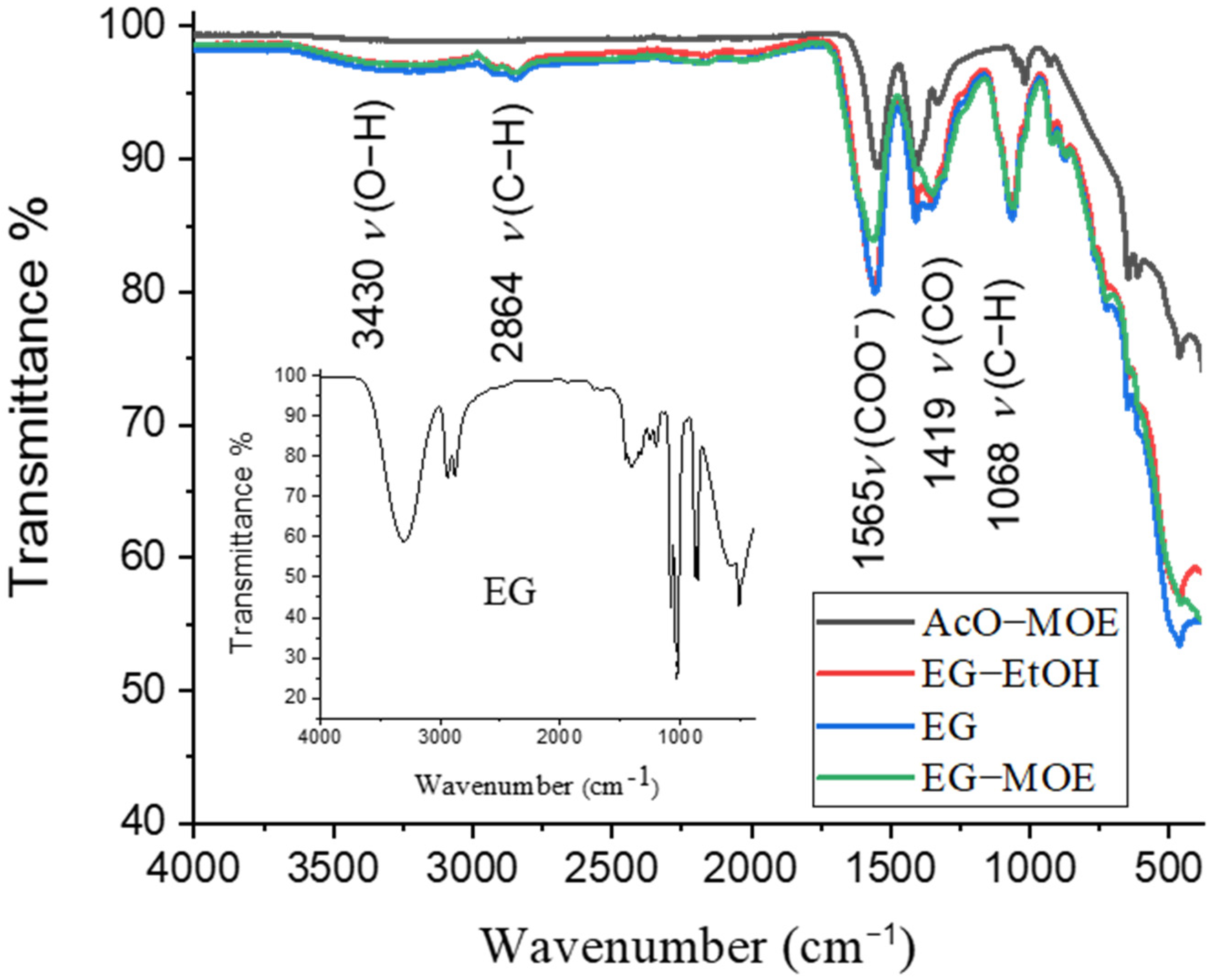
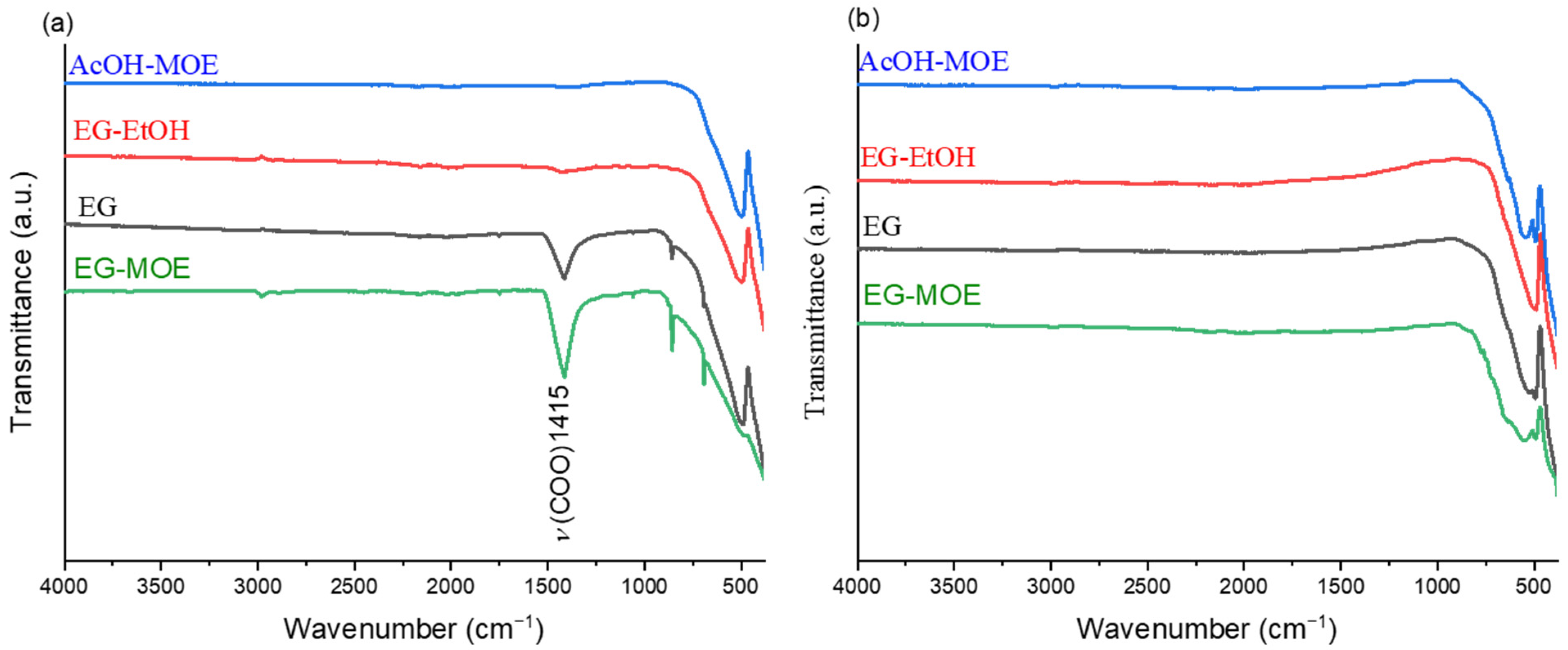
2.1. Thermal Decomposition of BT Xerogels
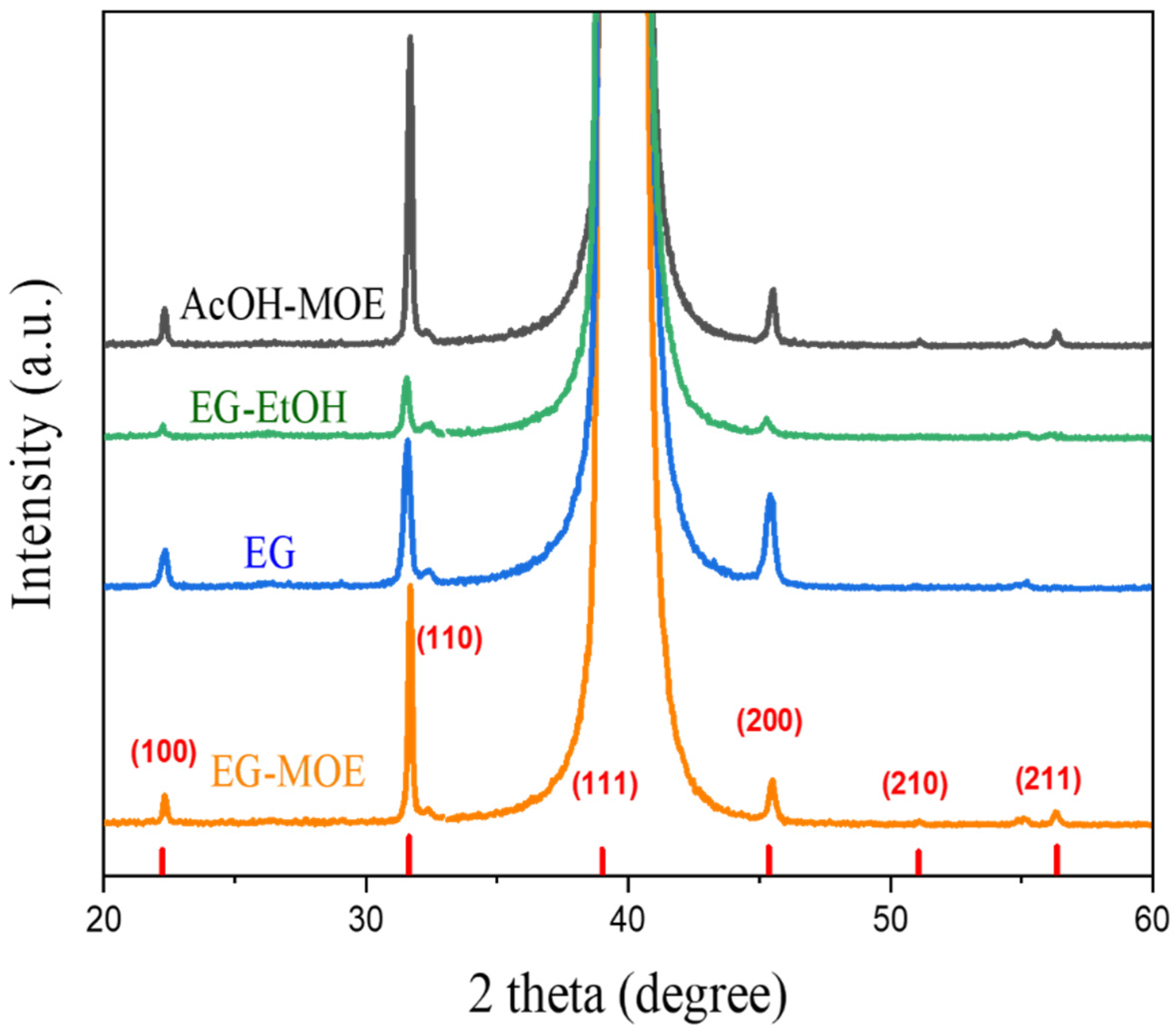
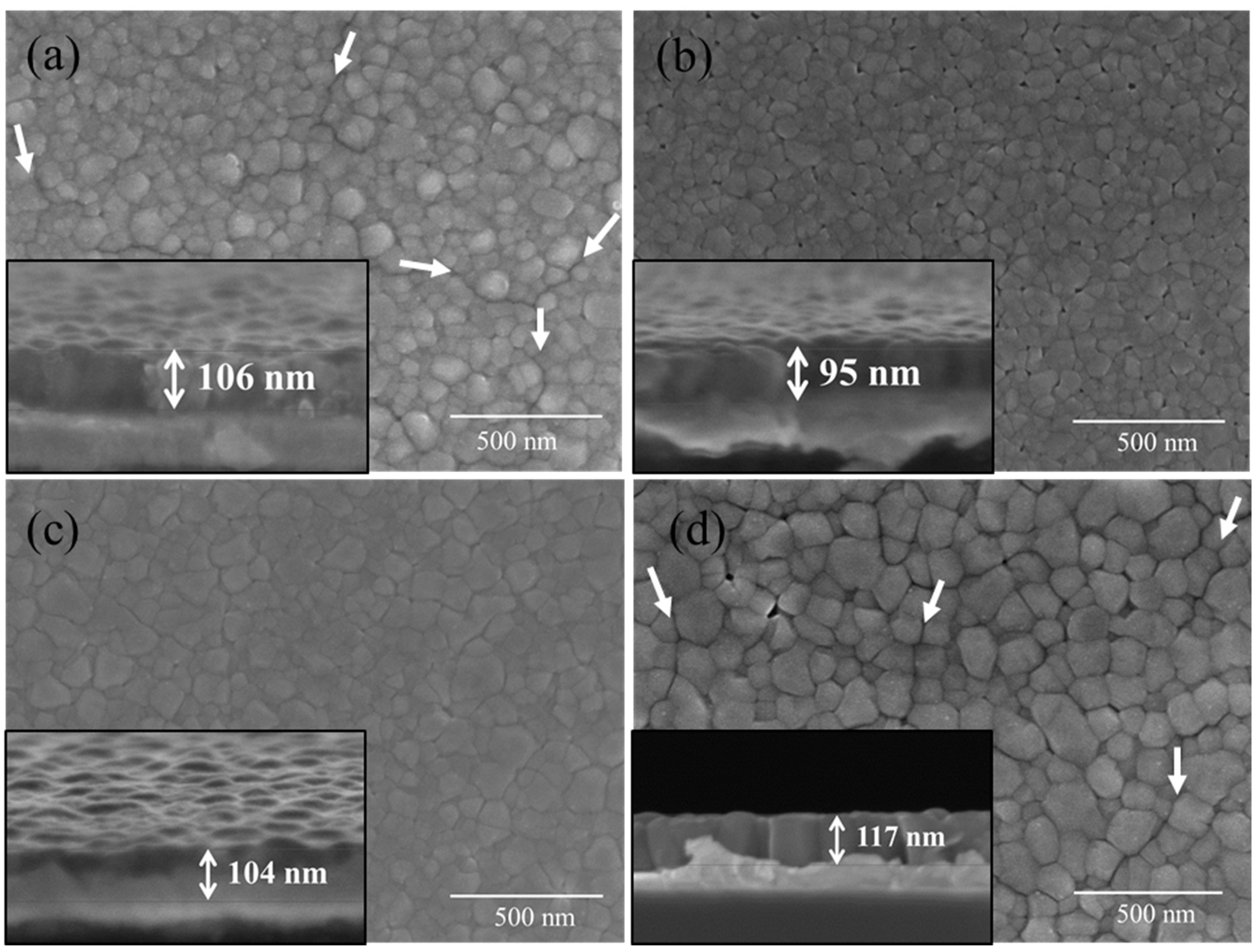
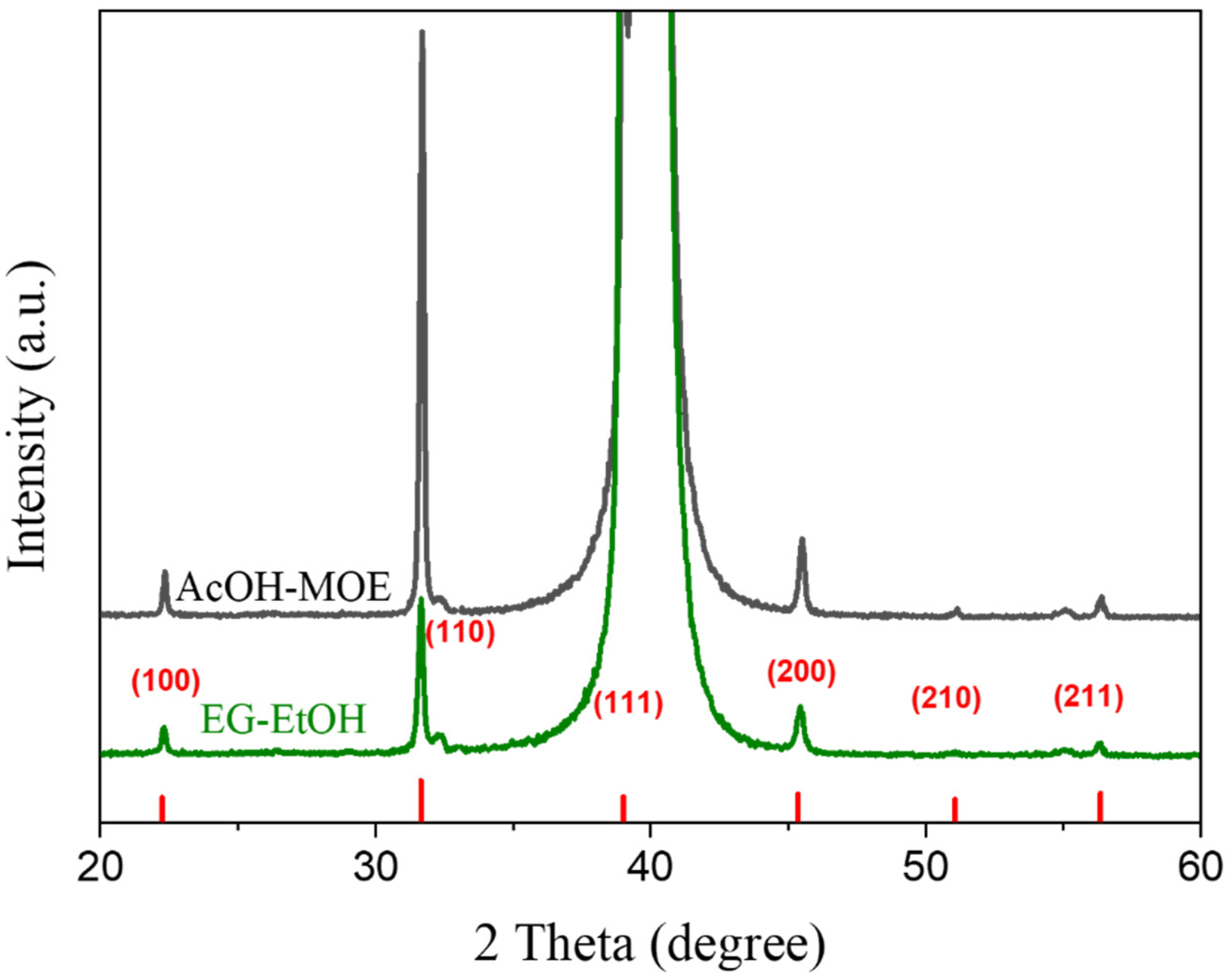
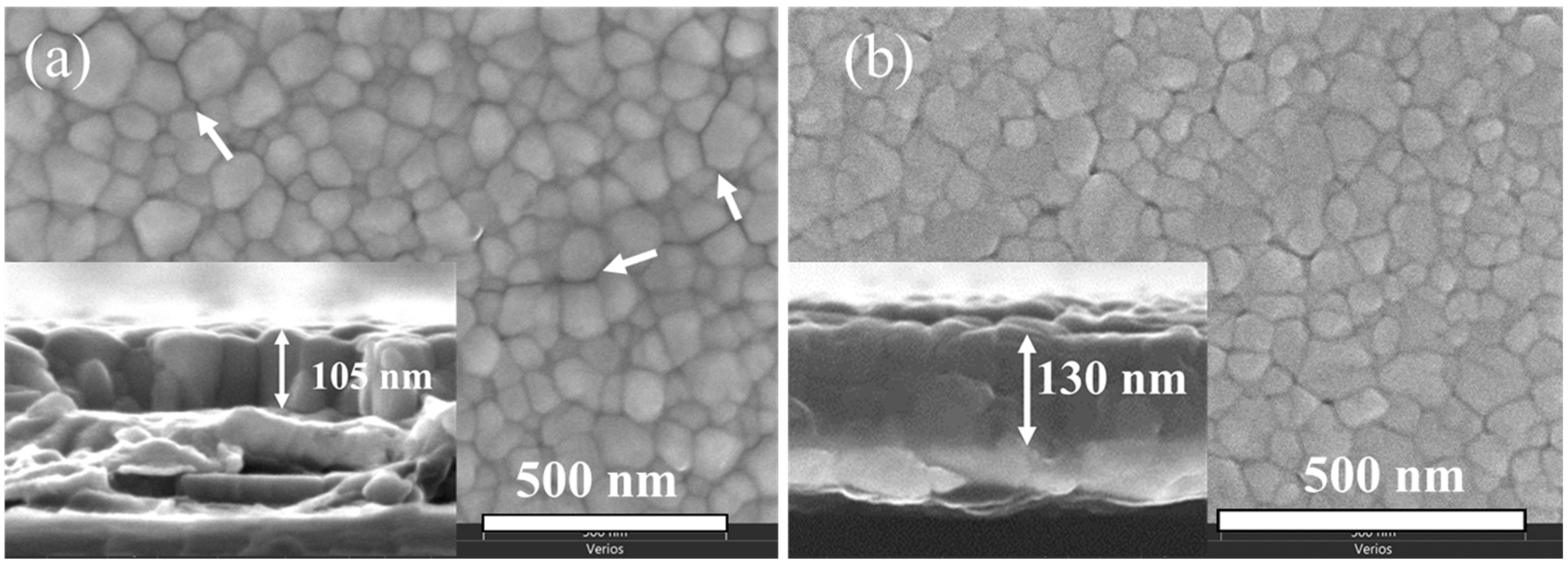
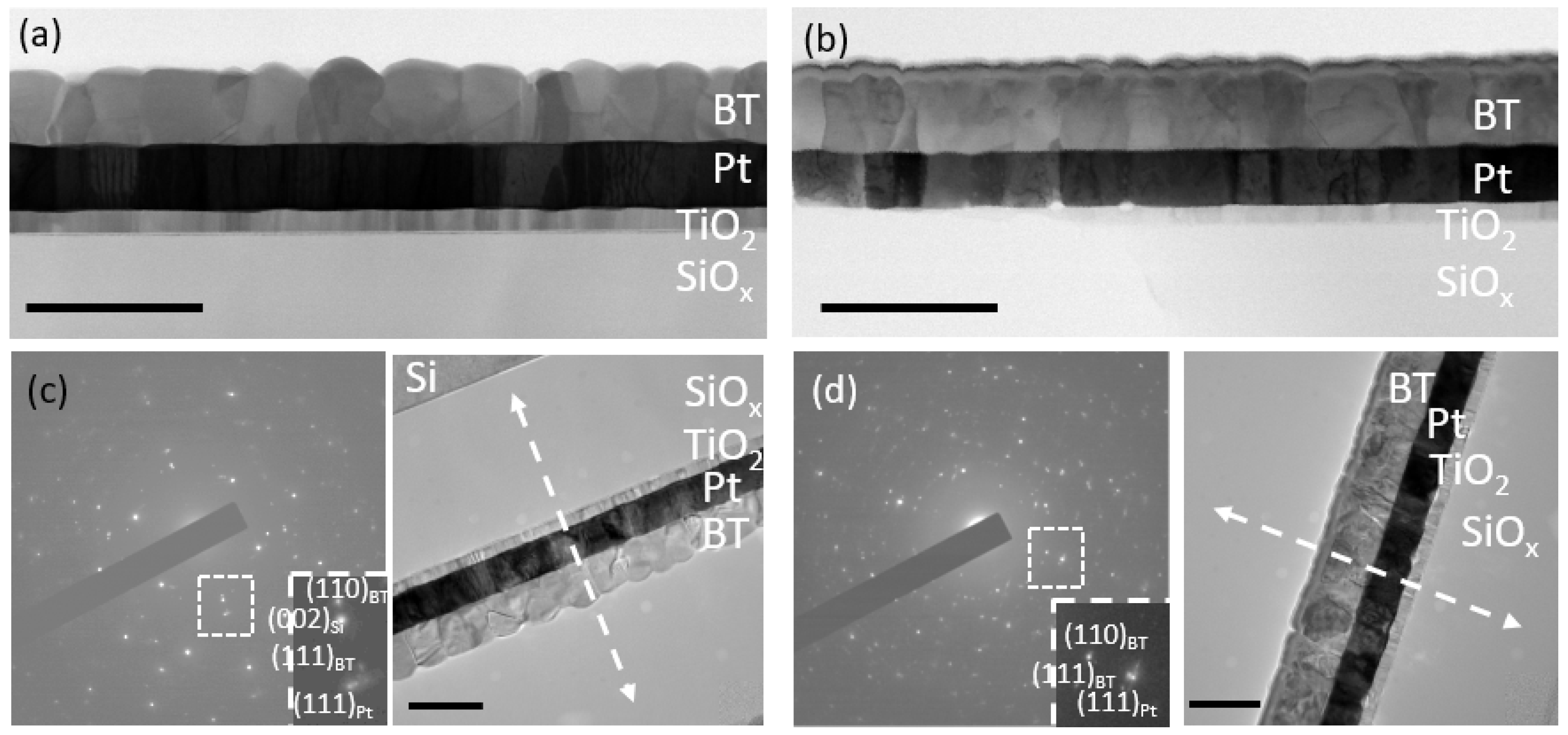
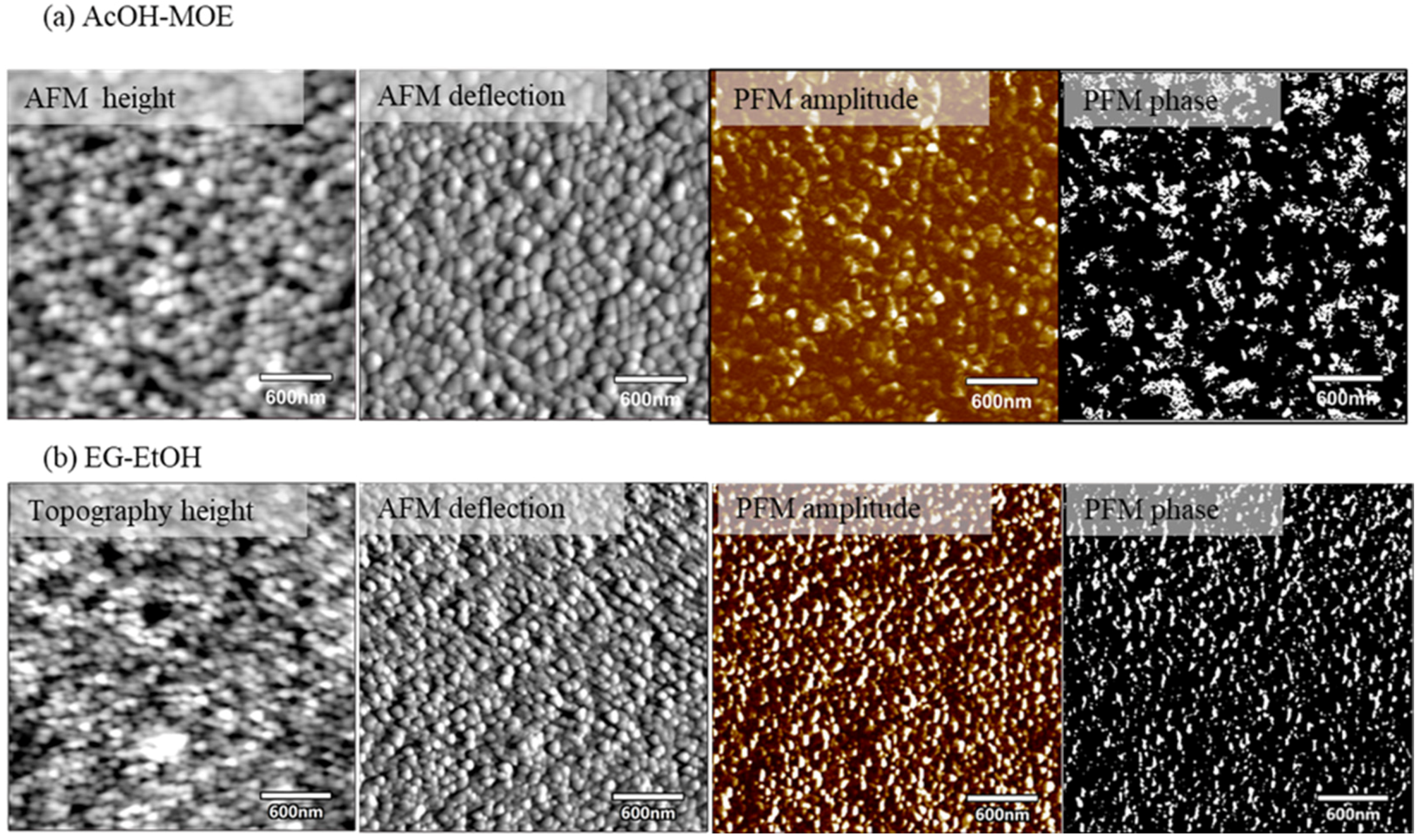
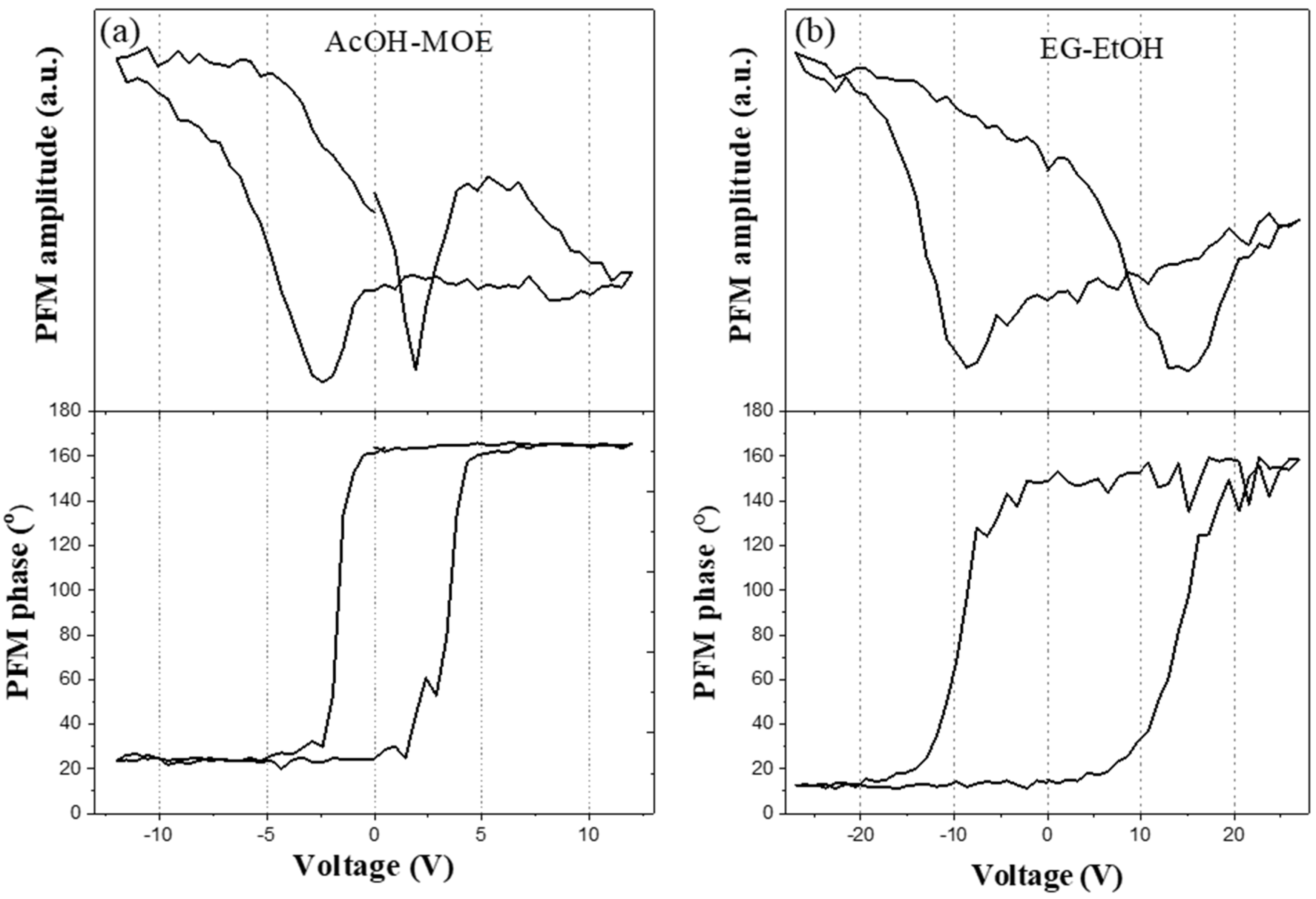
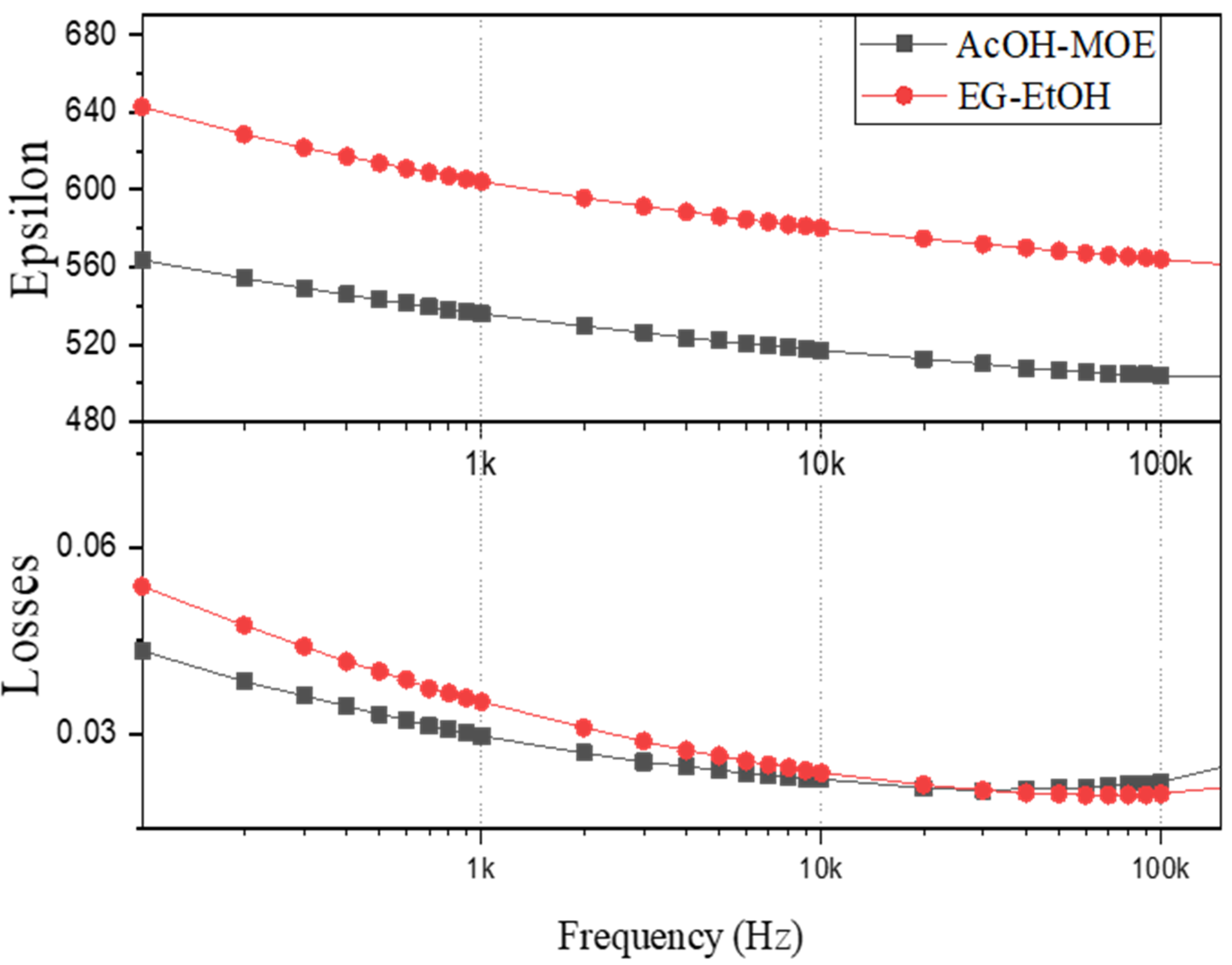
2.2. Phase Composition, Microstructure and Properties of BT Thin Films
- -
- The final temperature of the thermal decomposition of the xerogels was in the case of EG and EG-MOE gels at about 1100 °C to ensure complete removal of carbonaceous residues, while for the EG-EtOH gel the thermal decomposition was concluded at a much lower temperature, about 700 °C, cf. Figure 2 and Figure 3.
- -
- Intergranular cracks developed in the EG-MOE film while the EG and EG-EtOH films were crack-free.
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Liu, W.; Ren, X. Large piezoelectric effect in Pb-free ceramics. Phys. Rev. Lett. 2009, 103, 257602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, J.; Ke, X.; Acosta, M.; Glaum, J.; Ren, X. High piezoelectricity by multiphase coexisting point: Barium titanate derivatives. MRS Bull. 2018, 43, 595–599. [Google Scholar] [CrossRef] [Green Version]
- Acosta, M.; Novak, N.; Rojas, V.; Patel, S.; Vaish, R.; Koruza, J.; Rödel, J.J.A.P. BaTiO3-based piezoelectrics: Fundamentals, current status, and perspectives. Appl. Phys. Rev. 2017, 4, 041305. [Google Scholar] [CrossRef] [Green Version]
- Krupanidhi, S.B.; Peng, C.J. Studies on structural and electrical properties of barium strontium titanate thin films developed by metallo-organic decomposition. Thin Solid Films 1997, 305, 144–156. [Google Scholar] [CrossRef]
- Bao, P.; Jackson, T.J.; Wang, X.; Lancaster, M.J. Barium strontium titanate thin film varactors for room-temperature microwave device applications. J. Phys. D Appl. Phys. 2008, 41, 063001. [Google Scholar] [CrossRef]
- Pečnik, T.; Glinšek, S.; Kmet, B.; Malič, B. Combined effects of thickness, grain size, and residual stress on the dielectric properties of Ba0.5Sr0.5TiO3 thin films. J. Alloys Compd. 2015, 646, 766–772. [Google Scholar] [CrossRef]
- Hasenkox, U.; Hoffmann, S.; Waser, R. Influence of precursor chemistry on the formation of MTiO3 (M = Ba, Sr) ceramic thin films. J. Sol-Gel Sci. Technol. 1998, 12, 67–79. [Google Scholar] [CrossRef]
- Schwartz, R.W.; Clem, P.G.; Voigt, J.A.; Byhoff, E.R.; Van Stry, M.; Headley, T.J.; Missert, N.A. Control of Microstructure and Orientation in Solution-Deposited BaTiO3 and SrTiO3 Thin Films. J. Am. Cer. Soc. 1999, 82, 2359–2367. [Google Scholar] [CrossRef]
- Edmondson, B.I.; Kwon, S.; Lam, C.H.; Ortmann, J.E.; Demkov, A.A.; Kim, M.J.; Ekerdt, J.G. Epitaxial, electro-optically active barium titanate thin films on silicon by chemical solution deposition. J. Am. Cer. Soc. 2020, 103, 1209–1218. [Google Scholar] [CrossRef]
- Raeder, T.M.; Bakken, K.; Glaum, J.; Einarsrud, M.A.; Grande, T. Enhanced in-plane ferroelectricity in BaTiO3 thin films fabricated by aqueous chemical solution deposition. AIP Adv. 2018, 8, 105228. [Google Scholar] [CrossRef] [Green Version]
- Bakken, K.; Blichfeld, A.B.; Chernyshov, D.; Grande, T.; Glaum, J.; Einarsrud, M.A. Mechanisms for texture in BaTiO3 thin films from aqueous chemical solution deposition. J. Sol-Gel Sci. Technol. 2020, 95, 562–572. [Google Scholar] [CrossRef]
- Schneller, T. Simple Alkoxide Based Precursor Systems. In Chemical Solution Deposition of Functional Oxide Thin Films; Schneller, T., Waser, R., Kosec, M., Payne, D., Eds.; Springer: Vienna, Austria, 2013; pp. 3–28. [Google Scholar]
- Shneller, T.; Griesche, D. Carboxylate Based Precursor Systems. In Chemical Solution Deposition of Functional Oxide Thin Films; Schneller, T., Waser, R., Kosec, M., Payne, D., Eds.; Springer: Vienna, Austria, 2013; pp. 29–50. [Google Scholar]
- Chi, Q.G.; Zhu, H.F.; Xu, J.C.; Wang, X.; Lin, J.Q.; Sun, Z.; Lei, Q.Q. Microstructures and electrical properties of 0.5(Ba0.7Ca0.3)TiO3−0.5Ba(Zr0.2Ti0.8)O3 thin films prepared by a sol–gel route. Cer. Int. 2013, 39, 8195–8198. [Google Scholar] [CrossRef]
- Huang, L.; Dai, Y.; Wu, Y.; Pei, X.; Chen, W. Enhanced ferroelectric and piezoelectric properties of (1-x)BaZr0.2Ti0.8O3−xBa0.7Ca0.3TiO3 thin films by sol–gel process. Appl. Surf. Sci. 2016, 388, 35–39. [Google Scholar] [CrossRef]
- Ihlefeld, J.F.; Maria, J.P.; Borland, W. Dielectric and microstructural properties of barium titanate zirconate thin films on copper substrates. J. Mater. Res. 2005, 20, 2838–2844. [Google Scholar] [CrossRef]
- Laaziz, I.; Larbot, A.; Julbe, A.; Guizard, C.; Cot, L. Hydrolysis of mixed titanium and zirconium alkoxides by an esterification reaction. J. Solid State Chem. 1992, 98, 393–403. [Google Scholar] [CrossRef]
- Bradley, D.C.; Mehrotra, R.C.; Gaur, D.P. Physical Properties of Metal Alkoxides. In Metal Alkoxides; Academic Press: London, UK; New York, NY, USA; San Franscisco, CA, USA, 1978; pp. 159–164. [Google Scholar]
- Imhoff, L.; Barolin, S.; Pellegri, N.; Stachiotti, M.G. Chelate route for the synthesis of PbZrxTi1−xO3 thin films. J. Sol-Gel Sci. Technol. 2017, 83, 375–381. [Google Scholar] [CrossRef]
- Livage, C.; Safari, A.; Klein, L.C. Glycol-based sol-gel process for the fabrication of ferroelectric PZT thin films. J. Sol-Gel Sci. Technol. 1994, 2, 605–609. [Google Scholar] [CrossRef]
- Budd, K.D.; Payne, D.A. Preparation of strontium titanate ceramics and internal boundary layer capacitors by the Pechini method. In Proceedings of the Better Ceramics Trough Chemistry, Albuquerque, NM, USA, February 1984. [Google Scholar]
- Bradley, D.C.; Mehrotra, R.C.; Gaur, D.P. Synthesis of Metal Alkoxides. In Metal Alkoxides; Academic Press: London,UK; New York, NY, USA; San Franscisco, CA, USA, 1978; p. 191. [Google Scholar]
- Ashiri, R. Detailed FT-IR spectroscopy characterization and thermal analysis of synthesis of barium titanate nanoscale particles through a newly developed process. Vib. Spectrosc. 2013, 66, 24–29. [Google Scholar] [CrossRef]
- Malič, B.; Kosec, M.; Smolej, K.; Stavber, S. Effect of precursor type on the microstructure of PbTiO3 thin films. J. Eur. Ceram. Soc. 1999, 19, 1345–1348. [Google Scholar] [CrossRef]
- Malič, B.; Calzada, M.L.; Cilensek, J.; Pardo, L.; Kosec, M. Thermal analysis study of diol based precursors for chemical solution deposition of 0·7Pb(Mg1/3Nb2/3)O3-0·3PbTiO3 thin films. Adv. Appl. Cer. 2010, 109, 147–151. [Google Scholar] [CrossRef]
- Malič, B.; Kupec, A.; Kosec, M. Thermal Analysis. In Chemical Solution Deposition of Functional Oxide Thin Films; Schneller, T., Waser, R., Kosec, M., Payne, D., Eds.; Springer: Vienna, Austria, 2013; pp. 163–179. [Google Scholar]
- Hoffmann, S.; Hasenkox, U.; Waser, R.; Jia, C.L.; Urban, K. Chemical Solution Deposited BaTi03 AND SrTi03 Thin Films with Columnar Microstructure. MRS Online Proc. Libr. 1997, 474, 9. [Google Scholar] [CrossRef]
- Pečnik, T.; Benčan, A.; Glinšek, S.; Malič, B. Tailoring the microstructure and dielectric properties of Ba0.5Sr0.5TiO3 thin films by solution-based processing in the frame of the Microstructural Zone Model. J. All. Comp. 2018, 743, 812–818. [Google Scholar] [CrossRef]
- Ihlefeld, J.F.; Borland, W.J.; Maria, J.P. Enhanced dielectric and crystalline properties in ferroelectric barium titanate thin films. Adv. Funct. Mater. 2007, 17, 1199–1203. [Google Scholar] [CrossRef]
- Ihlefeld, J.F.; Vodnick, A.M.; Baker, S.P.; Borland, W.J.; Maria, J.P. Extrinsic scaling effects on the dielectric response of ferroelectric thin films. J. Appl. Phys. 2008, 103, 074112. [Google Scholar] [CrossRef]
- Kuwabara, M.; Takahashi, S.; Kuroda, T. Preparation of ferroelectric BaTiO3 thin films on polycrystalline BaPbO3 substrates by sol-gel processing and their electrical properties. Appl. Phys. Lett. 1993, 62, 3372–3374. [Google Scholar] [CrossRef]
- Bajac, B.; Vukmirović, J.; Tripković, Đ.; Đurđić, E.; Stanojev, J.; Cvejić, Ž.; Srdić, V.V. Structural characterization and dielectric properties of BaTiO3 thin films obtained by spin coating. Proc. Appl. Cer. 2014, 8, 219–224. [Google Scholar] [CrossRef]
- Basantakumar Sharma, H.; Sarma, H.N.K.; Mansingh, A. Ferroelectric and dielectric properties of sol-gel processed barium titanate ceramics and thin films. J. Mater. Sci. 1999, 34, 1385–1390. [Google Scholar] [CrossRef]
- Uršič, H.; Prah, U. Investigations of ferroelectric polycrystalline bulks and thick films using piezoresponse force microscopy. Proc. R. Soc. A Math. Phys. Eng. Sci. 2019, 475, 20180782. [Google Scholar] [CrossRef]
- ORCA—Conductive AFM, Asylum Research. Available online: https://mundylab.umd.edu/wp-content/uploads/ORCASupportNote_rev1.pdf (accessed on 25 April 2022).






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konsago, S.W.; Žiberna, K.; Kmet, B.; Benčan, A.; Uršič, H.; Malič, B. Chemical Solution Deposition of Barium Titanate Thin Films with Ethylene Glycol as Solvent for Barium Acetate. Molecules 2022, 27, 3753. https://doi.org/10.3390/molecules27123753
Konsago SW, Žiberna K, Kmet B, Benčan A, Uršič H, Malič B. Chemical Solution Deposition of Barium Titanate Thin Films with Ethylene Glycol as Solvent for Barium Acetate. Molecules. 2022; 27(12):3753. https://doi.org/10.3390/molecules27123753
Chicago/Turabian StyleKonsago, Sabi William, Katarina Žiberna, Brigita Kmet, Andreja Benčan, Hana Uršič, and Barbara Malič. 2022. "Chemical Solution Deposition of Barium Titanate Thin Films with Ethylene Glycol as Solvent for Barium Acetate" Molecules 27, no. 12: 3753. https://doi.org/10.3390/molecules27123753






