Metabonomics Study of the Hematopoietic Effect of Medicinal Wine Maoji Jiu on a Blood Deficiency Rat Model by Ultra-High-Performance Liquid Chromatography Coupled to Quadrupole Time-of-Flight Mass Spectrometry and a Pattern Recognition Approach
Abstract
:1. Introduction
2. Results
2.1. Peripheral Blood Routine Analysis
2.2. Antioxidant Activity Analysis
2.3. The Stability of UHPLC–QTOF/MS System
2.4. Metabolic Profiling of Plasma
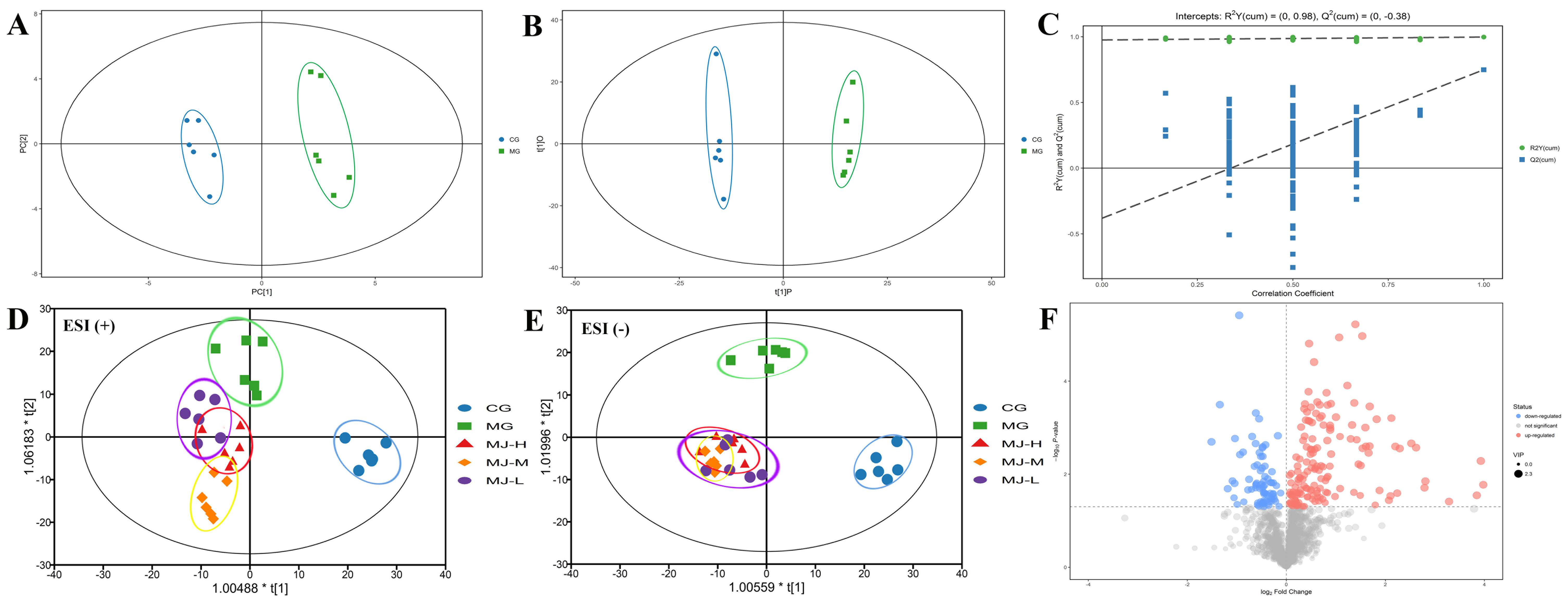
2.5. Multivariate Data Analysis
2.6. Identification and Quantization of Potential Biomarkers
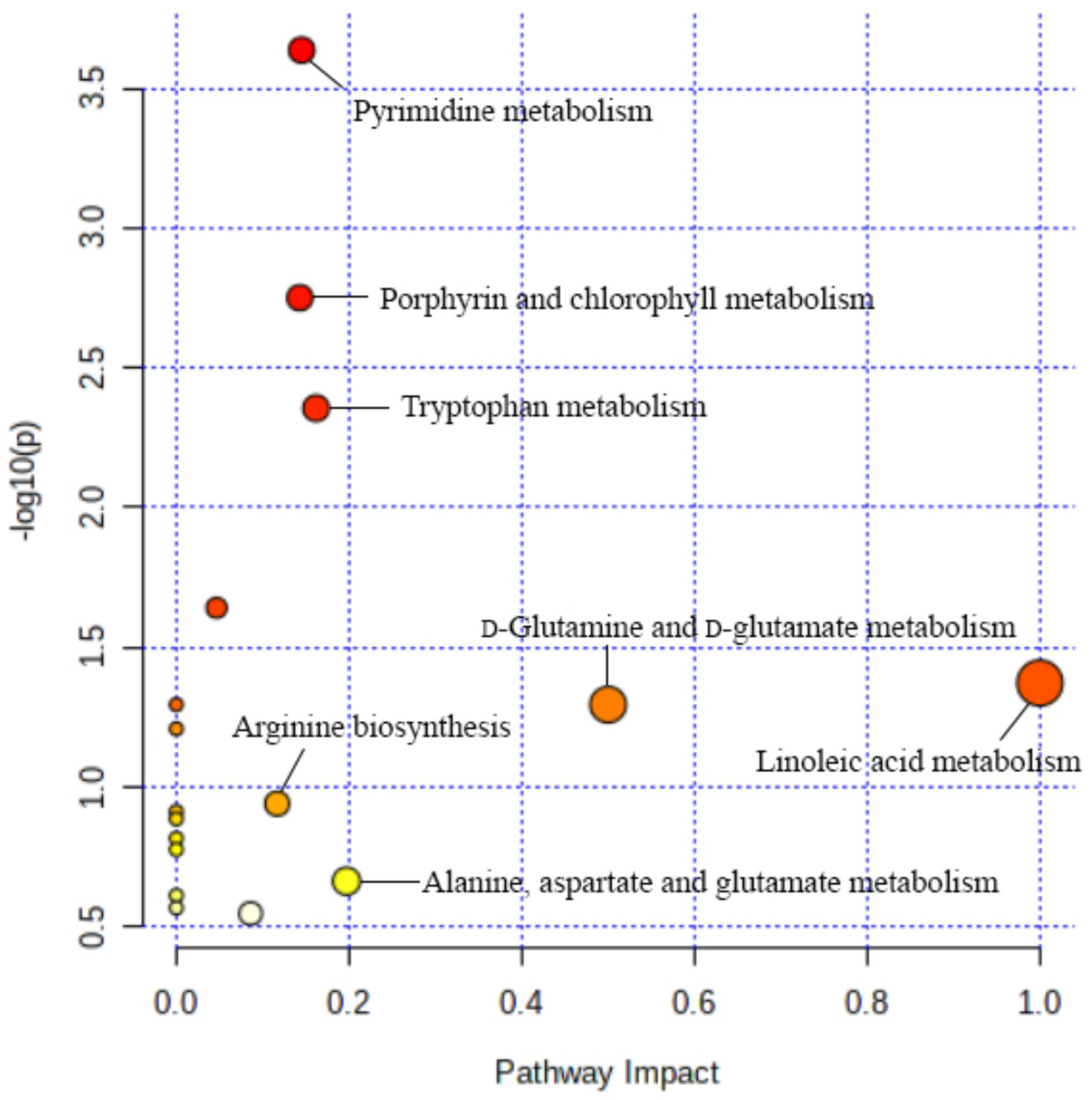
2.7. Biological Pathway Analysis
3. Discussion
4. Materials and Methods
4.1. Preparation of MJ
4.2. Blood Deficiency Model Building and Administration
4.3. Sample Preparation
4.4. UHPLC–QTOF/MS Conditions
4.5. Pattern Recognition Analysis and DATA Processing
4.6. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Marsh, J.C.; Bacigalupo, A.; Schrezenmeier, H.; Tichelli, A.; Risitano, A.M.; Passweg, J.R.; Killick, S.B.; Warren, A.J.; Foukaneli, T.; Aljurf, M.; et al. Prospective study of rabbit antithymocyte globulin and cyclosporine for aplastic anemia from the EBMT Severe Aplastic Anaemia Working Party. Blood 2012, 119, 5391–5396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheinberg, P.; Wu, C.O.; Nunez, O.; Scheinberg, P.; Boss, C.; Sloand, E.M.; Young, N.S. Treatment of severe aplastic anemia with a combination of horse antithymocyte globulin and cyclosporine, with or without sirolimus: A prospective randomized study. Haematologica 2009, 94, 348–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Editorial Committee of Chinese Pharmacopoeia. Pharmacopoeia of the People’s Republic of China; People’s Medical Publishing House: Beijing, China, 1977; pp. 717–718. [Google Scholar]
- Li, S.; Lin, H.; Qu, C.; Tang, Y.; Shen, J.; Li, W.; Yue, S.; Kai, J.; Shang, G.; Zhu, Z.; et al. Urine and plasma metabonomics coupled with UHPLC-QTOF/MS and multivariate data analysis on potential biomarkers in anemia and hematinic effects of herb pair Gui-Hong. J. Ethnopharmacol. 2015, 170, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Tang, Y.; Guo, J.; Huang, M.; Li, W.; Qian, D.; Duan, J. Enriching blood effect comparison in three kinds of blood deficiency model after oral administration of drug pair of Angelicae Sinensis Radix and Chuanxiong Rhizoma and each single herb. Chin. J. Chin. Mater. Med. 2011, 36, 1808–1814. [Google Scholar]
- Li, W.; Tang, Y.; Guo, J.; Shang, E.; Qian, Y.; Wang, L.; Zhang, L.; Liu, P.; Su, S.; Qian, D.; et al. Comparative metabolomics analysis on hematopoietic functions of herb pair Gui-Xiong by ultra-high-performance liquid chromatography coupled to quadrupole time-of-flight mass spectrometry and pattern recognition approach. J. Chromatogr. A 2014, 1346, 49–56. [Google Scholar] [CrossRef]
- Zhang, J.J.; Huang, Y.F.; Wang, L.L.; Li, W.; Wang, J.X.; Wang, C.; Qu, S.S. Comparative study on effects of blood enriching on mouse model of blood deficiency syndrome induced by compound method of bleeding, starved feeding and exhausting of Paeoniae Radix Alba and Paeoniae Radix Rubra, paeoniflorin and albiflorin. Chin. J. Chin. Mater. Med. 2013, 38, 3358–3362. [Google Scholar]
- Zeng, F.; Xu, Y.; Jiang, S.; Zhang, Y.; Zhao, C.; Li, L. Study on blood enriching effect of Centropus sinensis dried body alcohol extract on blood-deficiency model mice. China Pharm. 2016, 27, 4373–4375. [Google Scholar]
- Nicholson, J.K.; Lindon, J.C.; Holmes, E. ‘Metabonomics’: Understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica 1999, 29, 1181–1189. [Google Scholar] [CrossRef]
- Lao, Y.M.; Jiang, J.G.; Yan, L. Application of metabonomic analytical techniques in the modernization and toxicology research of traditional Chinese medicine. Br. J. Pharmacol. 2009, 157, 1128–1141. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Yang, B.; Sun, H.; Zhang, A. Pattern recognition approaches and computational systems tools for ultra performance liquid chromatography-mass spectrometry-based comprehensive metabolomic profiling and pathways analysis of biological data sets. Anal. Chem. 2012, 84, 428–439. [Google Scholar] [CrossRef]
- Jiang, M.; Lu, C.; Zhang, C.; Yang, J.; Tan, Y.; Lu, A.; Chan, K. Syndrome differentiation in modern research of traditional Chinese medicine. J. Ethnopharmacol. 2012, 140, 634–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebrahimi, F.; Ibrahim, B.; Teh, C.H.; Murugaiyah, V.; Chan, K.L. Urinary NMR-based metabolomic analysis of rats possessing variable sperm count following orally administered Eurycoma longifolia extracts of different quassinoid levels. J. Ethnopharmacol. 2016, 182, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lin, H.; Tang, Y.; Li, W.; Shen, J.; Kai, J.; Yue, S.; Shang, G.; Zhu, Z.; Shang, E.; et al. Comparative metabolomics analysis on invigorating blood circulation for herb pair Gui-Hong by ultra-high-performance liquid chromatography coupled to quadrupole time-of-flight mass spectrometry and pattern recognition approach. J. Pharm. Biomed. Anal. 2015, 107, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, X.; Wang, H.; Xiao, H.; Jing, F.; Tang, L.; Li, D.; Zhang, Y.; Wu, H.; Yang, H. Metabolomics study on the effects of Buchang Naoxintong capsules for treating cerebral ischemia in rats using UPLC-Q/TOF-MS. J. Ethnopharmacol. 2016, 180, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.H.; Li, S.Q.; Zou, G.A.; Yu, C.Y.; Sun, Y.G.; Zhang, H.W.; Gu, Y.; Zou, Z.M. Urinary metabonomics study of anti-depressive effect of Chaihu-Shu-Gan-San on an experimental model of depression induced by chronic variable stress in rats. J. Pharm. Biomed. Anal. 2011, 55, 533–539. [Google Scholar] [CrossRef]
- Gu, Q.; David, F.; Lynen, F.; Rumpel, K.; Dugardeyn, J.; Van Der Straeten, D.; Xu, G.; Sandra, P. Evaluation of automated sample preparation, retention time locked gas chromatography-mass spectrometry and data analysis methods for the metabolomic study of Arabidopsis species. J. Chromatogr. A 2011, 1218, 3247–3254. [Google Scholar] [CrossRef]
- Hong, Z.; Lin, Z.; Liu, Y.; Tan, G.; Lou, Z.; Zhu, Z.; Chai, Y.; Fan, G.; Zhang, J.; Zhang, L. Innovative microwave-assisted oximation and silylation procedures for metabolomic analysis of plasma samples using gas chromatography-mass spectrometry. J. Chromatogr. A 2012, 1254, 14–22. [Google Scholar] [CrossRef]
- Trost, L.B.; Bergfeld, W.F.; Calogeras, E. The diagnosis and treatment of iron deficiency and its potential relationship to hair loss. J. Am. Acad. Dermatol. 2006, 54, 824–844. [Google Scholar] [CrossRef]
- Shi, X.; Tang, Y.; Zhu, H.; Li, W.; Li, Z.; Li, W.; Duan, J.A. Comparative tissue distribution profiles of five major bio-active components in normal and blood deficiency rats after oral administration of Danggui Buxue Decoction by UPLC-TQ/MS. J. Pharm. Biomed. Anal. 2014, 88, 207–215. [Google Scholar] [CrossRef]
- Barreda, D.R.; Hanington, P.C.; Belosevic, M. Regulation of myeloid development and function by colony stimulating factors. Dev. Com. Immunol. 2004, 28, 509–554. [Google Scholar] [CrossRef]
- Ji, P.; Wei, Y.; Hua, Y.; Zhang, X.; Yao, W.; Ma, Q.; Yuan, Z.; Wen, Y.; Yang, C. A novel approach using metabolomics coupled with hematological and biochemical parameters to explain the enriching-blood effect and mechanism of unprocessed Angelica sinensis and its 4 kinds of processed products. J. Ethnopharmacol. 2018, 211, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Di, Z.; Wang, Y.; An, M.; Hu, J.; Jin, Y.; Zhang, Z.; Zhang, Y. Study on anti-fatigue, anti-oxidative and hemostatic effects of small molecule Asini Corii Colla. Chin. Pharmacol. Bull. 2019, 35, 203–208. [Google Scholar]
- Kaikkonen, J.E.; Kresanov, P.; Ahotupa, M.; Jula, A.; Mikkilä, V.; Viikari, J.S.; Kähönen, M.; Lehtimäki, T.; Raitakari, O.T. High serum n6 fatty acid proportion is associated with lowered LDL oxidation and inflammation: The Cardiovascular Risk in Young Finns Study. Free Radic. Res. 2014, 48, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Nowak, J.Z. Oxidative stress, polyunsaturated fatty acids-derived oxidation products and bisretinoids as potential inducers of CNS diseases: Focus on age-related macular degeneration. Pharmacol. Rep. 2013, 65, 288–304. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, A.; He, W.; He, P.; Xu, B.; Xia, T.; Chen, X.; Yang, K. Effects of fluoride on the expression of NCAM, oxidative stress, and apoptosis in primary cultured hippocampal neurons. Toxicology 2007, 236, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Fan, W.B.; Cong, Y.; Xu, H.D.; Li, C.J.; Meng, J.; Bao, N.R.; Zhao, J.N. Linoleic acid induces red blood cells and hemoglobin damage via oxidative mechanism. Int. J. Clin. Exp. Pathol. 2015, 8, 5044–5052. [Google Scholar]
- Ganor, Y.; Levite, M. The neurotransmitter glutamate and human T cells: Glutamate receptors and glutamate-induced direct and potent effects on normal human T cells, cancerous human leukemia and lymphoma T cells, and autoimmune human T cells. J. Neural. Transm. 2014, 121, 983–1006. [Google Scholar] [CrossRef]
- Mayor, D.; Tymianski, M. Neurotransmitters in the mediation of cerebral ischemic injury. Neuropharmacology 2018, 134 Pt B, 178–188. [Google Scholar] [CrossRef]
- Sibon, D.; Coman, T.; Rossignol, J.; Lamarque, M.; Kosmider, O.; Bayard, E.; Fouquet, G.; Rignault, R.; Topçu, S.; Bonneau, P.; et al. Enhanced Renewal of Erythroid Progenitors in Myelodysplastic Anemia by Peripheral Serotonin. Cell Rep. 2019, 26, 3246–3256.e4. [Google Scholar] [CrossRef] [Green Version]
- Dale, W.E.; Dang, Y.; Brown, O.R. Tryptophan metabolism through the kynurenine pathway in rat brain and liver slices. Free Radic. Biol. Med. 2000, 29, 191–198. [Google Scholar] [CrossRef]
- Tang, J.; Chen, R.; Liao, L. Simultaneous determination of kynurenine and tryptophan in human serum by HPLC. China Pharm. 2012, 23, 3595–3597. [Google Scholar]
- Li, W.X.; Huang, M.Y.; Tang, Y.P.; Guo, J.M.; Shang, E.X.; Wang, L.Y.; Qian, D.W.; Duan, J.A. Metabolomic study of the action mechanism of nourishing blood effect of fo-shou-san on blood deficiency mice. Acta. Pharm. Sin. 2013, 48, 1301–1306. [Google Scholar]
- Sachar, M.; Anderson, K.E.; Ma, X. Protoporphyrin IX: The Good, the Bad, and the Ugly. J. Pharmacol. Exp. Ther. 2016, 356, 267–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dailey, H.A.; Meissner, P.N. Erythroid heme biosynthesis and its disorders. Cold Spring Harb. Perspect. Med. 2013, 3, a011676. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Fei, Q.Q.; Wang, J.; Zhu, F.X.; Chen, Y.; Tang, D.Q.; Chen, B. Study on blood enrichment mechanism of steamed notoginseng based on metabolomics method. Chin. J. Chin. Mater. Med. 2019, 44, 2139–2148. [Google Scholar]
- Löffler, M.; Fairbanks, L.D.; Zameitat, E.; Marinaki, A.M.; Simmonds, H.A. Pyrimidine pathways in health and disease. Trends Mol. Med. 2005, 11, 430–437. [Google Scholar] [CrossRef]
- Paglia, D.E.; Valentine, W.N. Characteristics of a pyrimidine-specific 5′-nucleotidase in human erythrocytes. J. Biol. Chem. 1975, 250, 7973–7979. [Google Scholar] [CrossRef]


| ESI Mode | Control Group | Model Group | MJ-H | MJ-M | MJ-L | |
|---|---|---|---|---|---|---|
| x-Axis | y-Axis | |||||
| + | 23.32 | −4.23 | 32.62 ± 5.03 | 27.40 ± 2.77 * | 32.05 ± 2.53 | 33.45 ± 2.85 |
| − | 23.39 | −6.81 | 34.80 ± 2.69 | 30.08 ± 3.46 * | 32.63 ± 0.98 | 28.93 ± 4.07 * |
| No. | ESI Mode | tR (min) | m/z | VIP | p | HMDB ID | Metabolites | Trend in Model Group a | Trend in MJ-H Group b | Trend in MJ-M Group b | Trend in MJ-L Group b |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | + | 2.53 | 113.0342 | 2.22 | 0.0025 | 00300 | Uracil | ↑ ** | ↓ ** | ↓ ** | ↓ * |
| 2 | + | 3.71 | 266.0743 | 2.22 | 0.0011 | 00089 | Cytidine | ↑ ** | ↓ * | ↓ * | ↓ |
| 3 | + | 5.04 | 124.0387 | 1.96 | 0.0157 | 02243 | Picolinic acid | ↓ ** | ↑ * | ↑ | ↑ |
| 4 | + | 6.21 | 148.0602 | 1.40 | 0.0489 | 00148 | L-Glutamate | ↑ * | ↓ * | ↓ | ↓ |
| 5 | + | 7.85 | 613.5462 | 2.04 | 0.0003 | 03337 | Glutathione disulfide | ↓ ** | ↑ * | ↑ | ↑ |
| 6 | − | 0.75 | 279.2280 | 1.79 | 0.0066 | 00673 | Linoleic acid | ↑ ** | ↓ * | ↓ * | ↓ |
| 7 | − | 1.00 | 164.0327 | 1.49 | 0.0093 | 04089 | Formylanthranilic acid | ↓ ** | ↑ ** | ↑ * | ↑ |
| 8 | − | 1.27 | 583.2440 | 1.20 | 0.0307 | 00054 | Bilirubin | ↑ ** | ↓ * | ↓ * | ↓ |
| 9 | − | 2.57 | 265.0383 | 1.56 | 0.0197 | 00296 | Uridine | ↑ ** | ↓ * | ↓ | ↓ |
| 10 | − | 2.84 | 561.2397 | 1.93 | 0.0013 | 00241 | Protoporphyrin | ↑ ** | ↓ ** | ↓ * | ↓ * |
| 11 | − | 3.13 | 190.0476 | 1.67 | 0.0022 | 00763 | 5-Hydroxyindoleacetate | ↓ ** | ↑ ** | ↑ ** | ↑ * |
| 12 | − | 3.62 | 155.0065 | 1.34 | 0.0368 | 00226 | Orotate | ↑ ** | ↓ ** | ↓ * | ↓ |
| 13 | − | 3.98 | 203.0791 | 1.66 | 0.0036 | 00929 | L-Tryptophan | ↓ ** | ↑ * | ↑ | ↑ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, F.; Xu, Y.; Li, Y.; Yan, Z.; Li, L. Metabonomics Study of the Hematopoietic Effect of Medicinal Wine Maoji Jiu on a Blood Deficiency Rat Model by Ultra-High-Performance Liquid Chromatography Coupled to Quadrupole Time-of-Flight Mass Spectrometry and a Pattern Recognition Approach. Molecules 2022, 27, 3791. https://doi.org/10.3390/molecules27123791
Zeng F, Xu Y, Li Y, Yan Z, Li L. Metabonomics Study of the Hematopoietic Effect of Medicinal Wine Maoji Jiu on a Blood Deficiency Rat Model by Ultra-High-Performance Liquid Chromatography Coupled to Quadrupole Time-of-Flight Mass Spectrometry and a Pattern Recognition Approach. Molecules. 2022; 27(12):3791. https://doi.org/10.3390/molecules27123791
Chicago/Turabian StyleZeng, Fanqiang, Yongli Xu, Yilian Li, Zhigang Yan, and Li Li. 2022. "Metabonomics Study of the Hematopoietic Effect of Medicinal Wine Maoji Jiu on a Blood Deficiency Rat Model by Ultra-High-Performance Liquid Chromatography Coupled to Quadrupole Time-of-Flight Mass Spectrometry and a Pattern Recognition Approach" Molecules 27, no. 12: 3791. https://doi.org/10.3390/molecules27123791
APA StyleZeng, F., Xu, Y., Li, Y., Yan, Z., & Li, L. (2022). Metabonomics Study of the Hematopoietic Effect of Medicinal Wine Maoji Jiu on a Blood Deficiency Rat Model by Ultra-High-Performance Liquid Chromatography Coupled to Quadrupole Time-of-Flight Mass Spectrometry and a Pattern Recognition Approach. Molecules, 27(12), 3791. https://doi.org/10.3390/molecules27123791






