Stability, Morphology, and Effects of In Vitro Digestion on the Antioxidant Properties of Polyphenol Inclusion Complexes with β-Cyclodextrin
Abstract
:1. Introduction
2. Results and Discussion
2.1. Confirmation of Inclusion Complexation Using Electrospray Ionization Tandem Mass Spectrometry (ESI-MS/MS)
2.2. Encapsulation Yield and Efficiency
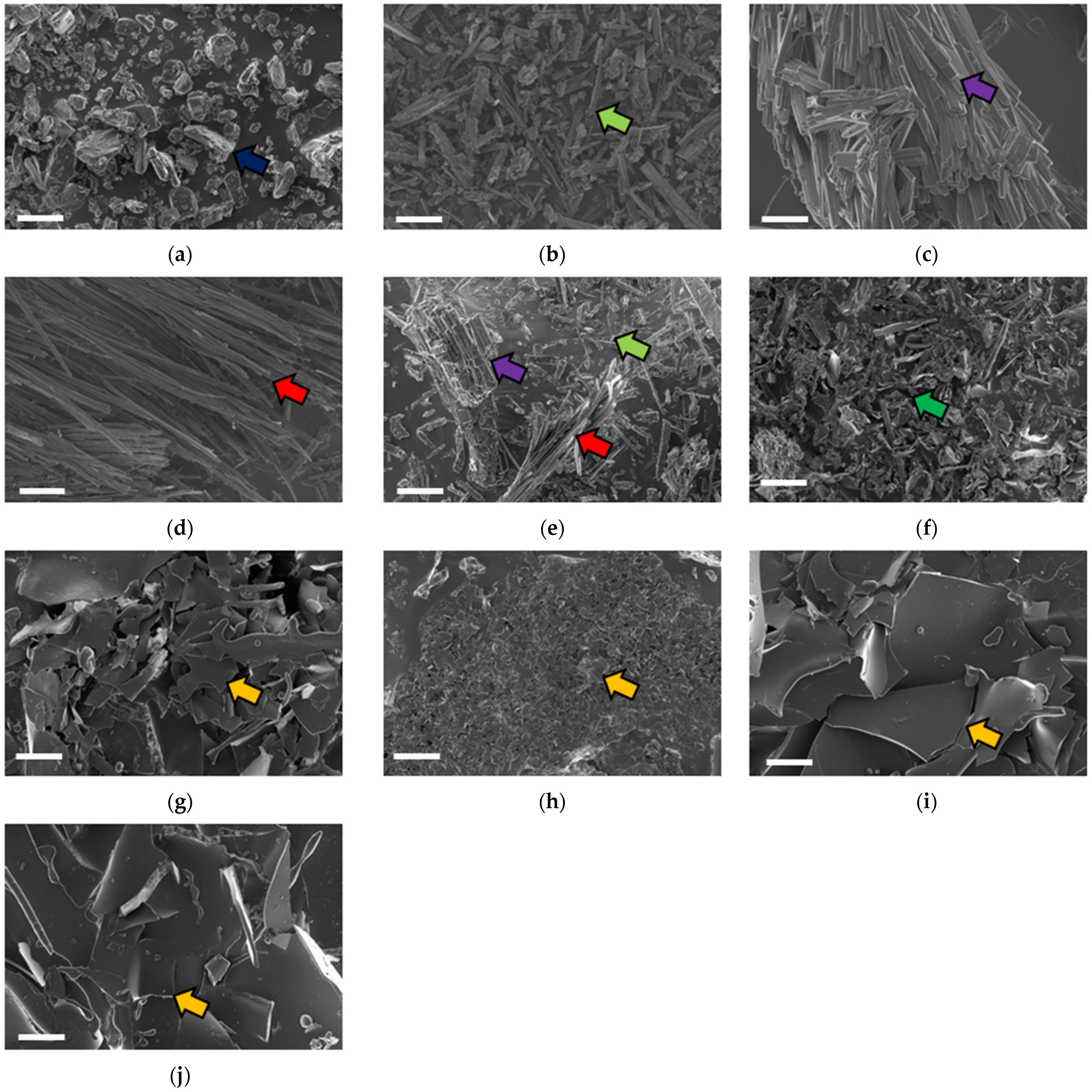
2.3. Surface Morphology
2.4. Thermal Stability
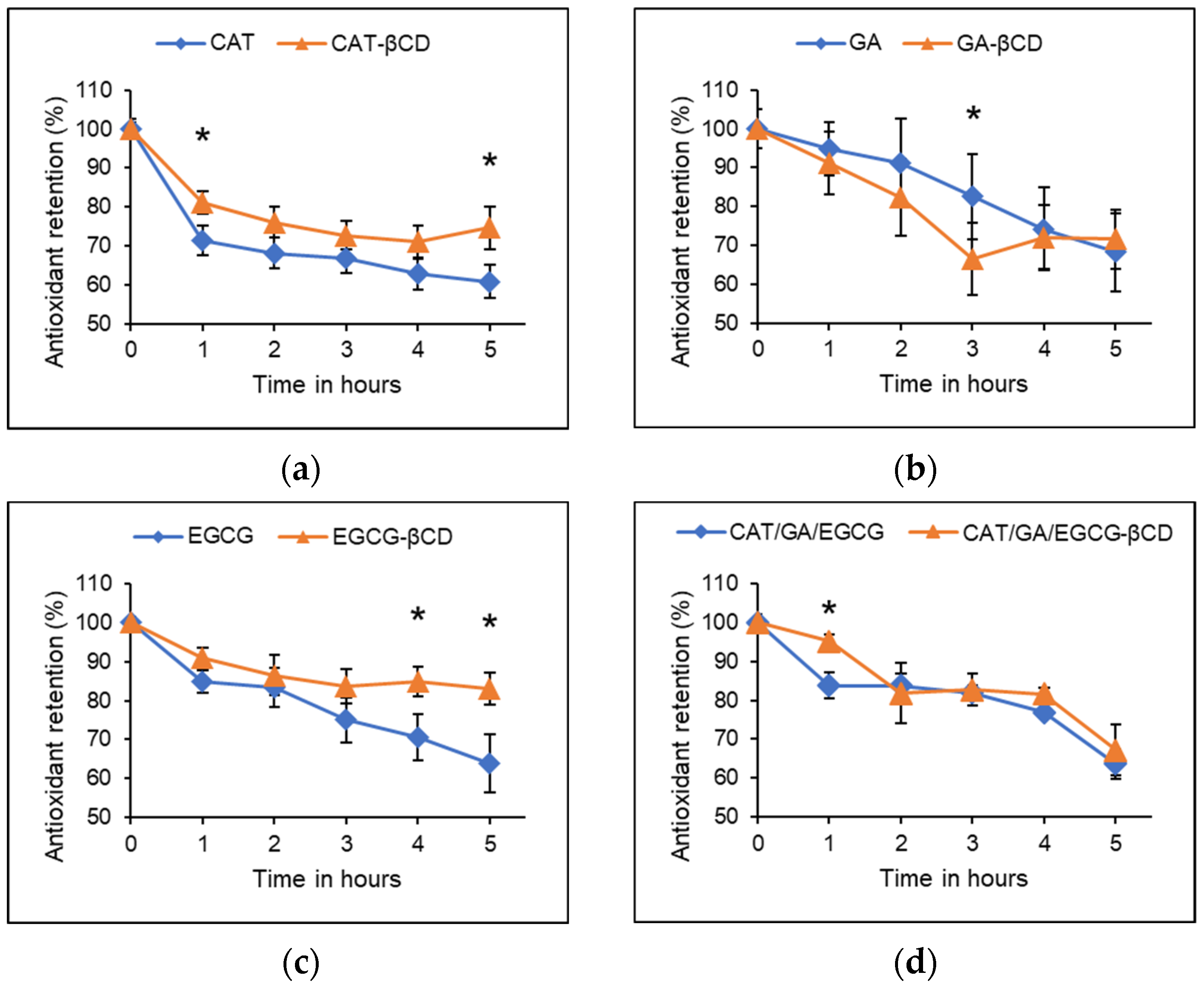
2.5. Storage Stability
2.6. Antioxidant Activity–Oxygen Radical Absorbance Capacity (ORAC)
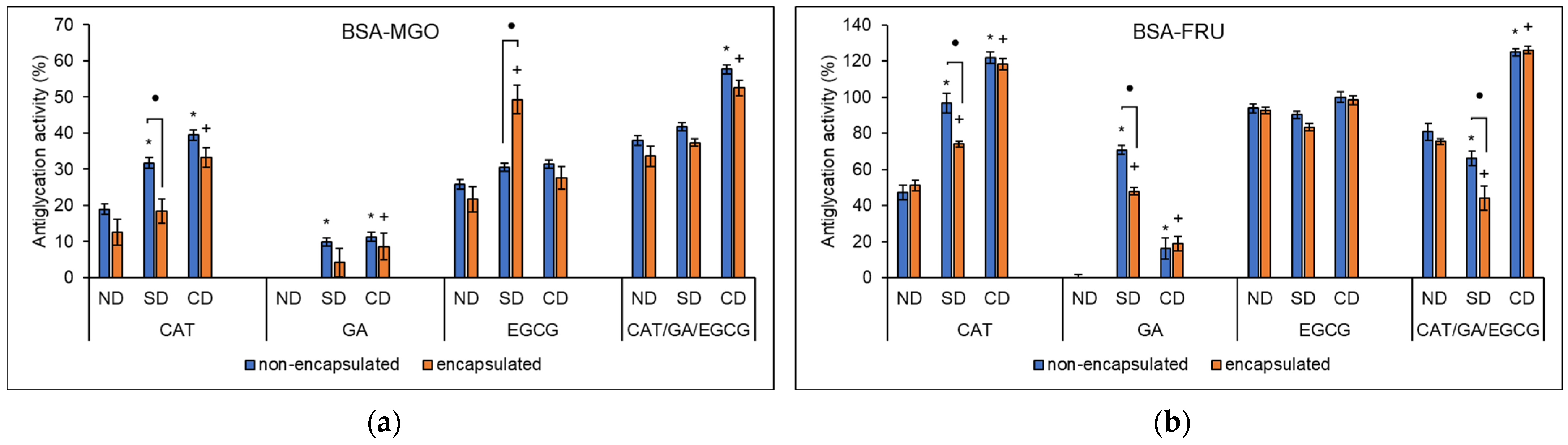
2.7. Antioxidant Activity–Advanced Glycation End-Products (AGEs)
2.8. Antioxidant Activity–Dichlorofluorescein Diacetate (DCFH-DA)
2.9. Cytotoxicity
3. Materials and Methods
3.1. Preparation of Inclusion Complexes
3.2. Confirmation of Inclusion Complexation Using Electrospray Ionization Tandem Mass Spectrometry
3.3. Encapsulation Yield and Efficiency
3.4. Morphological Characterization of the Inclusion Complexes
3.5. Thermal and Storage Stability of the Inclusion Complexes with Respect to the Antioxidant Retention
Antioxidant Determination
3.6. In Vitro Digestion of the Inclusion Complexes
3.6.1. Simple In Vitro Digestion
3.6.2. Complex In Vitro Digestion
3.7. Antioxidant Properties
3.7.1. Oxygen Radical Absorbance Capacity (ORAC) Assay
3.7.2. Advanced Glycation End-Products (AGEs) Assay
3.7.3. Dichlorofluorescein Diacetate (DCFH–DA) Assay
3.7.4. Cytotoxicity Assay
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural polyphenols: An overview. Int. J. Food Prop. 2017, 20, 1689–1699. [Google Scholar] [CrossRef] [Green Version]
- Scarano, A.; Butelli, E.; De Santis, S.; Cavalcanti, E.; Hill, L.; De Angelis, M.; Giovinazzo, G.; Chieppa, M.; Martin, C.; Santino, A. Combined dietary anthocyanins, flavonols, and stilbenoids alleviate inflammatory bowel disease symptoms in mice. Front. Nutr. 2018, 4, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro-Barquero, S.; Lamuela-Raventós, R.M.; Doménech, M.; Estruch, R. Relationship between Mediterranean dietary polyphenol intake and obesity. Nutrients 2018, 10, 1523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, S.; Thoo, Y.Y.; Young, D.J.; Siow, L.F. Stability and recovery of cyclodextrin encapsulated catechin in various food matrices. Food Chem. 2019, 275, 594–599. [Google Scholar] [CrossRef]
- Pasrija, D.; Ezhilarasi, P.N.; Indrani, D.; Anandharamakrishnan, C. Microencapsulation of green tea polyphenols and its effect on incorporated bread quality. LWT-Food Sci. Technol. 2015, 64, 289–296. [Google Scholar] [CrossRef]
- Larsen, J.R.; Dima, L.; Correll, C.U.; Manu, P. The pharmacological management of metabolic syndrome. Expert Rev. Clin. Pharmacol. 2018, 11, 397–410. [Google Scholar] [CrossRef]
- Owen, J.G.; Reisin, E. Anti-hypertensive drug treatment of patients with and the metabolic syndrome and obesity: A review of evidence, meta-analysis, post hoc and guidelines publications. Curr. Hypertens. Rep. 2015, 17, 46. [Google Scholar] [CrossRef]
- Nurgali, K.; Jagoe, R.T.; Abalo, R. Editorial: Adverse effects of cancer chemotherapy: Anything new to improve tolerance and reduce sequelae? Front Pharm. 2018, 9, 245. [Google Scholar] [CrossRef]
- Oliver, S.; Vittorio, O.; Cirillo, G.; Boyer, C. Enhancing the therapeutic effects of polyphenols with macromolecules. Polym. Chem. 2016, 7, 1529–1544. [Google Scholar] [CrossRef]
- Ho, S.; Thoo, Y.Y.; Young, D.J.; Siow, L.F. Cyclodextrin encapsulated catechin: Effect of pH, relative humidity and various food models on antioxidant stability. LWT Food Sci. Technol. 2017, 85, 232–239. [Google Scholar] [CrossRef]
- Volf, I.; Ignat, I.; Neamtu, M.; Popa, V.I. Thermal stability, antioxidant activity, and photo-oxidation of natural polyphenols. Chem. Pap. 2014, 68, 121–129. [Google Scholar] [CrossRef]
- Li, N.; Taylor, L.S.; Ferruzzi, M.G.; Mauer, L.J. Kinetic study of catechin stability: Effects of pH, concentration, and temperature. J. Agric. Food Chem. 2012, 60, 12531–12539. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Taylor, L.S.; Ferruzzi, M.G.; Mauer, L.J. Color and chemical stability of tea polyphenol (−)-epigallocatechin-3-gallate in solution and solid states. Food Res. Int. 2013, 53, 909–921. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A double-edged sword in health benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef] [Green Version]
- Massounga Bora, A.F.; Ma, S.; Li, X.; Liu, L. Application of microencapsulation for the safe delivery of green tea polyphenols in food systems: Review and recent advances. Food Res. Int. 2018, 105, 241–249. [Google Scholar] [CrossRef]
- Ozkan, G.; Franco, P.; De Marco, I.; Xiao, J.; Capanoglu, E. A review of microencapsulation methods for food antioxidants: Principles, advantages, drawbacks and applications. Food Chem. 2019, 272, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Pinho, E.; Grootveld, M.; Soares, G.; Henriques, M. Cyclodextrins as encapsulation agents for plant bioactive compounds. Carbohydr. Polym. 2014, 101, 121–135. [Google Scholar] [CrossRef] [Green Version]
- Lu, Z.; Cheng, B.; Hu, Y.; Zhang, Y.; Zou, G. Complexation of resveratrol with cyclodextrins: Solubility and antioxidant activity. Food Chem. 2009, 113, 17–20. [Google Scholar] [CrossRef]
- Ho, S.; Thoo, Y.Y.; Young, D.J.; Siow, L.F. Inclusion complexation of catechin by β-cyclodextrins: Characterization and storage stability. LWT 2017, 86, 555–565. [Google Scholar] [CrossRef]
- Dima, C.; Assadpour, E.; Dima, S.; Jafari, S.M. Nutraceutical nanodelivery; an insight into the bioaccessibility/bioavailability of different bioactive compounds loaded within nanocarriers. Crit. Rev. Food Sci. Nutr. 2021, 61, 3031–3065. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.; Oniszczuk, A.; Oniszczuk, T.; Combrzyński, M.; Nowakowska, D.; Matwijczuk, A. Influence of in vitro digestion on composition, bioaccessibility and antioxidant activity of food polyphenols—a non-systematic review. Nutrients 2020, 12, 1401. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi Ekbatan, S.; Sleno, L.; Sabally, K.; Khairallah, J.; Azadi, B.; Rodes, L.; Prakash, S.; Donnelly, D.J.; Kubow, S. Biotransformation of polyphenols in a dynamic multistage gastrointestinal model. Food Chem. 2016, 204, 453–462. [Google Scholar] [CrossRef]
- Pimpão, R.C.; Ventura, M.R.; Ferreira, R.B.; Williamson, G.; Santos, C.N. Phenolic sulfates as new and highly abundant metabolites in human plasma after ingestion of a mixed berry fruit purée. Br. J. Nutr. 2015, 113, 454–463. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Liu, T.; Xu, J.; Li, J.; Chen, F.; Xiang, Z.; Huang, Y.; Zhang, D.; Hu, L.; Zhang, B.; et al. Interactions between β-cyclodextrin and tea catechins, and potential anti-osteoclastogenesis activity of the (−)-epigallocatechin-3-gallate–β-cyclodextrin complex. RSC Adv. 2019, 9, 28006–28018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, P.; Dinda, A.K.; Chaudhury, S.; Dasgupta, S. β-cyclodextrin encapsulated polyphenols as effective antioxidants. Biopolymers 2018, 109, e23084. [Google Scholar] [CrossRef]
- Grgić, J.; Šelo, G.; Planinić, M.; Tišma, M.; Bucić-Kojić, A. Role of the encapsulation in bioavailability of phenolic compounds. Antioxidants 2020, 9, 923. [Google Scholar] [CrossRef] [PubMed]
- Żyżelewicz, D.; Oracz, J.; Kaczmarska, M.; Budryn, G.; Grzelczyk, J. Preparation and characterization of inclusion complex of (+)-catechin with β-cyclodextrin. Food Res. Int. 2018, 113, 263–268. [Google Scholar] [CrossRef]
- Budryn, G.; Nebesny, E.; Pałecz, B.; Rachwał-Rosiak, D.; Hodurek, P.; Miśkiewicz, K.; Oracz, J.; Żyżelewicz, D. Inclusion complexes of β-cyclodextrin with chlorogenic acids (CHAs) from crude and purified aqueous extracts of green Robusta coffee beans (Coffea canephora L.). Food Res. Int. 2014, 61, 202–213. [Google Scholar] [CrossRef]
- da Rosa, C.G.; Borges, C.D.; Zambiazi, R.C.; Nunes, M.R.; Benvenutti, E.V.; da Luz, S.R.; D’Avila, R.F.; Rutz, J.K. Microencapsulation of gallic acid in chitosan, β-cyclodextrin and xanthan. Ind. Crops Prod. 2013, 46, 138–146. [Google Scholar] [CrossRef]
- Barman, S.; Barman, B.K.; Roy, M.N. Preparation, characterization and binding behaviors of host-guest inclusion complexes of metoclopramide hydrochloride with α- and β-cyclodextrin molecules. J. Mol. Struct. 2018, 1155, 503–512. [Google Scholar] [CrossRef]
- Folch-Cano, C.; Guerrero, J.; Speisky, H.; Jullian, C.; Olea-Azar, C. NMR and molecular fluorescence spectroscopic study of the structure and thermodynamic parameters of EGCG/β-cyclodextrin inclusion complexes with potential antioxidant activity. J. Incl. Phenom. Macrocycl. Chem. 2014, 78, 287–298. [Google Scholar] [CrossRef]
- Wilkowska, A.; Ambroziak, W.; Czyżowska, A.; Adamiec, J. Effect of microencapsulation by spray drying and freeze drying technique on the antioxidant properties of blueberry (Vaccinium myrtillus) juice polyphenolic compounds. Pol. J. Food Nutr. Sci. 2016, 66, 11–16. [Google Scholar] [CrossRef] [Green Version]
- Malapert, A.; Reboul, E.; Tourbin, M.; Dangles, O.; Thiéry, A.; Ziarelli, F.; Tomao, V. Characterization of hydroxytyrosol-β-cyclodextrin complexes in solution and in the solid state, a potential bioactive ingredient. LWT 2019, 102, 317–323. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.; Yang, J.; Wang, Q.; Ren, L.; Zhou, J. Physicochemical properties of catechin/β-cyclodextrin inclusion complex obtained via co-precipitation. CyTA J. Food 2019, 17, 544–551. [Google Scholar] [CrossRef] [Green Version]
- Olga, G.; Styliani, C.; Ioannis, R.G. Coencapsulation of ferulic and gallic acid in hp-b-cyclodextrin. Food Chem. 2015, 185, 33–40. [Google Scholar] [CrossRef]
- Ishizu, T.; Hirata, C.; Yamamoto, H.; Harano, K. Structure and intramolecular flexibility of β-cyclodextrin complex with (−)-epigallocatechin gallate in aqueous solvent. Magn. Reson. Chem. 2006, 44, 776–783. [Google Scholar] [CrossRef]
- Huang, M.; Liang, C.; Tan, C.; Huang, S.; Ying, R.; Wang, Y.; Wang, Z.; Zhang, Y. Liposome co-encapsulation as a strategy for the delivery of curcumin and resveratrol. Food Funct. 2019, 10, 6447–6458. [Google Scholar] [CrossRef]
- Liu, X.; Wang, P.; Zou, Y.X.; Luo, Z.G.; Tamer, T.M. Co-encapsulation of Vitamin C and β-Carotene in liposomes: Storage stability, antioxidant activity, and in vitro gastrointestinal digestion. Food Res. Int. 2020, 136, 109587. [Google Scholar] [CrossRef]
- Shutava, T.G.; Balkundi, S.S.; Vangala, P.; Steffan, J.J.; Bigelow, R.L.; Cardelli, J.A.; O’Neal, D.P.; Lvov, Y.M. Layer-by-layer-coated gelatin nanoparticles as a vehicle for delivery of natural polyphenols. ACS Nano 2009, 3, 1877–1885. [Google Scholar] [CrossRef]
- Cao, Y.; Teng, J.; Selbo, J. Amorphous solid dispersion of epigallocatechin gallate for enhanced physical stability and controlled release. Pharmaceuticals 2017, 10, 88. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Mascaraque, L.G.; Soler, C.; Lopez-Rubio, A. Stability and bioaccessibility of EGCG within edible micro-hydrogels. Chitosan vs. gelatin, a comparative study. Food Hydrocoll. 2016, 61, 128–138. [Google Scholar] [CrossRef] [Green Version]
- López de Dicastillo, C.; Piña, C.; Garrido, L.; Arancibia, C.; Galotto, M.J. Enhancing thermal stability and bioaccesibility of açaí fruit polyphenols through electrohydrodynamic encapsulation into zein electrosprayed particles. Antioxidants 2019, 8, 464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López de Dicastillo, C.; López-Carballo, G.; Gavara, R.; Muriel Galet, V.; Guarda, A.; Galotto, M.J. Improving polyphenolic thermal stability of Aristotelia Chilensis fruit extract by encapsulation within electrospun cyclodextrin capsules. J. Food Process. Preserv. 2019, 43, e14044. [Google Scholar] [CrossRef]
- Vincente, A.R.; Manganaris, G.A.; Ortiz, C.M.; Sozzi, G.O.; Crisosto, C.H. Nutritional quality of fruits and vegetables. In Postharvest Handling, 3rd ed.; Florkowski, W.J., Shewfelt, R.L., Brueckner, B., Prussia, S.E., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 69–122. [Google Scholar]
- Choi, Y.-R.; Chang, Y.H. Microencapsulation of gallic acid through the complex of whey protein concentrate-pectic polysaccharide extracted from Ulmus davidiana. Food Hydrocoll. 2018, 85, 222–228. [Google Scholar] [CrossRef]
- Gómez-Mascaraque, L.G.; Lagarón, J.M.; López-Rubio, A. Electrosprayed gelatin submicroparticles as edible carriers for the encapsulation of polyphenols of interest in functional foods. Food Hydrocoll. 2015, 49, 42–52. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; Liu, B.; Wang, F.; Huang, D.; Zhong, F.; Li, Y. Characterization and in vitro digestion properties of cassava starch and epigallocatechin-3-gallate (EGCG) blend. LWT 2021, 137, 110398. [Google Scholar] [CrossRef]
- Chaaban, H.; Ioannou, I.; Paris, C.; Charbonnel, C.; Ghoul, M. The photostability of flavanones, flavonols and flavones and evolution of their antioxidant activity. J. Photochem. Photobiol. A Chem. 2017, 336, 131–139. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Dávalos, A.; Gómez-Cordovés, C.; Bartolomé, B. Extending applicability of the oxygen radical absorbance capacity (ORAC−Fluorescein) assay. J. Agric. Food Chem. 2004, 52, 48–54. [Google Scholar] [CrossRef]
- Zulueta, A.; Esteve, M.J.; Frígola, A. ORAC and TEAC assays comparison to measure the antioxidant capacity of food products. Food Chem. 2009, 114, 310–316. [Google Scholar] [CrossRef]
- Roy, M.K.; Koide, M.; Rao, T.P.; Okubo, T.; Ogasawara, Y.; Juneja, L.R. ORAC and DPPH assay comparison to assess antioxidant capacity of tea infusions: Relationship between total polyphenol and individual catechin content. Int. J. Food Sci. Nutr. 2010, 61, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.Q.; Xiao, J.; Fan, H.X.; Yu, Y.; He, R.R.; Feng, X.L.; Kurihara, H.; So, K.F.; Yao, X.S.; Gao, H. Polyphenols from wolfberry and their bioactivities. Food Chem. 2017, 214, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Folch-Cano, C.; Jullian, C.; Speisky, H.; Olea-Azar, C. Antioxidant activity of inclusion complexes of tea catechins with β-cyclodextrins by ORAC assays. Food Res. Int. 2010, 43, 2039–2044. [Google Scholar] [CrossRef]
- Hu, Q.; Wang, T.; Zhou, M.; Xue, J.; Luo, Y. In vitro antioxidant-activity evaluation of gallic-acid-grafted chitosan conjugate synthesized by free-radical-induced grafting method. J. Agric. Food Chem. 2016, 64, 5893–5900. [Google Scholar] [CrossRef]
- Serem, J.C. Identification and characterization of bioactivity of simulated gastrointestinal digested indigenous southern African honey samples. Ph.D. Thesis, University of Pretoria, Pretoria, South Africa, 2018. [Google Scholar]
- Shi, M.; Ying, D.Y.; Hlaing, M.M.; Ye, J.H.; Sanguansri, L.; Augustin, M.A. Development of broccoli by-products as carriers for delivering EGCG. Food Chem. 2019, 301, 125301. [Google Scholar] [CrossRef]
- Oliveira, A.; Pintado, M. In vitro evaluation of the effects of protein–polyphenol–polysaccharide interactions on (+)-catechin and cyanidin-3-glucoside bioaccessibility. Food Funct. 2015, 6, 3444–3453. [Google Scholar] [CrossRef]
- de Morais, F.P.R.; Pessato, T.B.; Rodrigues, E.; Peixoto Mallmann, L.; Mariutti, L.R.B.; Netto, F.M. Whey protein and phenolic compound complexation: Effects on antioxidant capacity before and after in vitro digestion. Food Res. Int. 2020, 133, 109104. [Google Scholar] [CrossRef]
- Freeman, B.L.; Eggett, D.L.; Parker, T.L. Synergistic and antagonistic interactions of phenolic compounds found in navel oranges. J. Food Sci. 2010, 75, 570–576. [Google Scholar] [CrossRef]
- Carbonneau, M.A.; Cisse, M.; Mora-Soumille, N.; Dairi, S.; Rosa, M.; Michel, F.; Lauret, C.; Cristol, J.P.; Dangles, O. Antioxidant properties of 3-deoxyanthocyanidins and polyphenolic extracts from Côte d’Ivoire’s red and white sorghums assessed by ORAC and in vitro LDL oxidisability tests. Food Chem. 2014, 145, 701–709. [Google Scholar] [CrossRef]
- Saadeh, R.; Al-Jabari, M.; Abdoh, A.; Al-Bawab, A. Stability study of green tea natural extract in aqueous solutions and its chemical kinetics. Dirasat. Pure Sci. 2009, 36, 62–75. [Google Scholar]
- Jeon, S.Y.; Oh, S.; Kim, E.; Imm, J.Y. α-Glucosidase inhibiton and antiglycation activity of laccase-catalyzed catechin polymers. J. Agric. Food Chem. 2013, 61, 4577–4584. [Google Scholar] [CrossRef] [PubMed]
- de Lima-Júnior, J.P.; Franco, R.R.; Saraiva, A.L.; Moraes, I.B.; Espindola, F.S. Anacardium humile St. Hil as a novel source of antioxidant, antiglycation and α-amylase inhibitors molecules with potential for management of oxidative stress and diabetes. J. Ethnopharmacol. 2021, 268, 113667. [Google Scholar] [CrossRef]
- Park, J.J.; Lee, W.Y. Anti-glycation effects of brown algae extracts and its phenolic compounds. Food Biosci. 2021, 41, 101042. [Google Scholar] [CrossRef]
- Spagnuolo, L.; Della Posta, S.; Fanali, C.; Dugo, L.; De Gara, L. Antioxidant and antiglycation effects of polyphenol compounds extracted from hazelnut skin on advanced glycation end-products (AGEs) formation. Antioxidants 2021, 10, 424. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.R.; Jung, W.; Kim, M.K.; Chong, Y. Identification of (−)-epigallocatechin (EGC) as a methylglyoxal (MGO)-trapping agent and thereby as an inhibitor of advanced glycation end product (AGE) formation. Appl. Biol. Chem. 2018, 61, 587–591. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Zhang, G.; Hu, M.; Pan, J.; Li, A.; Zhang, Y. Molecular characteristics of gallocatechin gallate affecting protein glycation. Food Hydrocoll. 2020, 105, 105782. [Google Scholar] [CrossRef]
- Wu, C.H.; Yen, G.C. Inhibitory effect of naturally occurring flavonoids on the formation of advanced glycation endproducts. J. Agric. Food Chem. 2005, 53, 3167–3173. [Google Scholar] [CrossRef]
- Gharib, A.; Faezizadeh, Z.; Godarzee, M. Treatment of diabetes in the mouse model by delphinidin and cyanidin hydrochloride in free and liposomal forms. Planta Med. 2013, 79, 1599–1604. [Google Scholar] [CrossRef]
- Lavelli, V.; Sri Harsha, P.S.C. Microencapsulation of grape skin phenolics for pH controlled release of antiglycation agents. Food Res. Int. 2019, 119, 822–828. [Google Scholar] [CrossRef]
- Yao, Y.; Cheng, X.; Wang, L.; Wang, S.; Ren, G. Biological potential of sixteen legumes in China. Int. J. Mol. Sci. 2011, 12, 7048–7058. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, A.C.F.; Martins, I.M.; Moreira, D.K.T.; Macedo, G.A. Use of agro-industrial residues as potent antioxidant, antiglycation agents, and α-amylase and pancreatic lipase inhibitory activity. J. Food Process. Preserv. 2020, 44, e14397. [Google Scholar] [CrossRef]
- Franco, R.R.; Ribeiro Zabisky, L.F.; Pires de Lima Júnior, J.; Mota Alves, V.H.; Justino, A.B.; Saraiva, A.L.; Goulart, L.R.; Espindola, F.S. Antidiabetic effects of Syzygium cumini leaves: A non-hemolytic plant with potential against process of oxidation, glycation, inflammation and digestive enzymes catalysis. J. Ethnopharmacol. 2020, 261, 113132. [Google Scholar] [CrossRef] [PubMed]
- Spínola, V.; Llorent-Martínez, E.J.; Castilho, P.C. Inhibition of α-amylase, α-glucosidase and pancreatic lipase by phenolic compounds of Rumex maderensis (Madeira sorrel). Influence of simulated gastrointestinal digestion on hyperglycaemia-related damage linked with aldose reductase activity and protein glycation. Lwt 2020, 118, 108727. [Google Scholar] [CrossRef]
- Spínola, V.; Pinto, J.; Llorent-Martínez, E.J.; Castilho, P.C. Changes in the phenolic compositions of Elaeagnus umbellata and Sambucus lanceolata after in vitro gastrointestinal digestion and evaluation of their potential anti-diabetic properties. Food Res. Int. 2019, 122, 283–294. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, G.; Hu, X.; Pan, J.; Liao, Y.; Ding, H. Inhibitory effect of epicatechin gallate on protein glycation. Food Res. Int. 2019, 122, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.S.; Liou, S.Y.; Wu, H.C.; Tsai, F.J.; Tsai, C.H.; Huang, C.Y.; Chang, Y.L. Efficacy of epigallocatechin-3-gallate and Amla (Emblica officinalis) extract for the treatment of diabetic-uremic patients. J. Med. Food 2011, 14, 718–723. [Google Scholar] [CrossRef]
- Ou, J.; Huang, J.; Wang, M.; Ou, S. Effect of rosmarinic acid and carnosic acid on AGEs formation in vitro. Food Chem. 2017, 221, 1057–1061. [Google Scholar] [CrossRef]
- Schalkwijk, C.G. Vascular AGE-ing by methylglyoxal: The past, the present and the future. Diabetologia 2015, 58, 1715–1719. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.; Sheng, B.; Li, H.; Wu, Y.; Xu, D.; Li, C. Glycation from α-dicarbonyl compounds has different effects on the heat-induced aggregation of bovine serum albumin and β-casein. Food Chem. 2021, 340, 128108. [Google Scholar] [CrossRef]
- Cannizzaro, L.; Rossoni, G.; Savi, F.; Altomare, A.; Marinello, C.; Saethang, T.; Carini, M.; Payne, D.M.; Pisitkun, T.; Aldini, G.; et al. Regulatory landscape of AGE-RAGE-oxidative stress axis and its modulation by PPARγ activation in high fructose diet-induced metabolic syndrome. Nutr. Metab. 2017, 14, 5. [Google Scholar] [CrossRef] [Green Version]
- Kellett, M.E.; Greenspan, P.; Pegg, R.B. Modification of the cellular antioxidant activity (CAA) assay to study phenolic antioxidants in a Caco-2 cell line. Food Chem. 2018, 244, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Mateos, R.; Goya, L.; Amigo-Benavent, M.; Sarriá, B.; Bravo, L. A phenolic extract from grape by-products and its main hydroxybenzoic acids protect Caco-2 cells against pro-oxidant induced toxicity. Food Chem. Toxicol. 2016, 88, 65–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, H.; Liu, D.; Yu, X.; Sun, H.; Li, Y. A Caco-2 cell-based quantitative antioxidant activity assay for antioxidants. Food Chem. 2015, 175, 601–608. [Google Scholar] [CrossRef]
- Sessa, M.; Tsao, R.; Liu, R.; Ferrari, G.; Donsì, F. Evaluation of the stability and antioxidant activity of nanoencapsulated resveratrol during in vitro digestion. J. Agric. Food Chem. 2011, 59, 12352–12360. [Google Scholar] [CrossRef] [PubMed]
- Thiengkaew, P.; Thanitwatthanasak, S.; Srisala, S.; Jittorntrum, B.; Chunhabundit, R.; Chitprasert, P. Response surface optimization of microfluidic formulations of nanobilosomes for enhancement of aqueous solubility, digestive stability, and cellular antioxidant activity of mangiferin. Food Chem. 2021, 351, 129315. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Huang, H.; Wen, Z.; Fan, Y. Fabrication of chitosan-gallic acid conjugate for improvement of physicochemical stability of β-carotene nanoemulsion: Impact of MW of chitosan. Food Chem. 2021, 362, 130218. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, V.S.S.; Poejo, J.; Matias, A.A.; Rodríguez-Rojo, S.; Cocero, M.J.; Duarte, C.M.M. Using different natural origin carriers for development of epigallocatechin gallate (EGCG) solid formulations with improved antioxidant activity by PGSS-drying. RSC Adv. 2016, 6, 67599–67609. [Google Scholar] [CrossRef]
- Liang, R.; Chen, L.; Yokoyama, W.; Williams, P.A.; Zhong, F. Niosomes consisting of tween-60 and cholesterol improve the chemical stability and antioxidant activity of (−)-epigallocatechin gallate under intestinal tract conditions. J. Agric. Food Chem. 2016, 64, 9180–9188. [Google Scholar] [CrossRef]
- Phan, M.A.T.; Bucknall, M.; Arcot, J. Effect of different anthocyanidin glucosides on lutein uptake by Caco-2 cells, and their combined activities on anti-oxidation and anti-inflammation in vitro and ex vivo. Molecules 2018, 23, 2035. [Google Scholar] [CrossRef] [Green Version]
- Phan, M.A.T.; Bucknall, M.; Arcot, J. Interactive effects of β-carotene and anthocyanins on cellular uptake, antioxidant activity and anti-inflammatory activity in vitro and ex vivo. J. Funct. Foods 2018, 45, 129–137. [Google Scholar] [CrossRef]
- Phan, M.A.T.; Bucknall, M.P.; Arcot, J. Interferences of anthocyanins with the uptake of lycopene in Caco-2 cells, and their interactive effects on anti-oxidation and anti-inflammation in vitro and ex vivo. Food Chem. 2019, 276, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Elisia, I.; Kitts, D.D. Anthocyanins inhibit peroxyl radical-induced apoptosis in Caco-2 cells. Mol. Cell. Biochem. 2008, 312, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Bellion, P.; Digles, J.; Will, F.; Dietrich, H.; Baum, M.; Eisenbrand, G.; Janzowski, C. Polyphenolic apple extracts: Effects of raw material and production method on antioxidant effectiveness and reduction of DNA damage in Caco-2 cells. J. Agric. Food Chem. 2010, 58, 6636–6642. [Google Scholar] [CrossRef] [PubMed]
- Zakłos-Szyda, M.; Pawlik, N.; Polka, D.; Nowak, A.; Koziołkiewicz, M.; Podsędek, A. Viburnum opulus fruit phenolic compounds as cytoprotective agents able to decrease free fatty acids and glucose uptake by Caco-2 cells. Antioxidants 2019, 8, 262. [Google Scholar] [CrossRef] [Green Version]
- Moreno, T.; de Paz, E.; Navarro, I.; Rodríguez-Rojo, S.; Matías, A.; Duarte, C.; Sanz-Buenhombre, M.; Cocero, M.J. Spray drying formulation of polyphenols-rich grape marc extract: Evaluation of operating conditions and different natural carriers. Food Bioprocess Technol. 2016, 9, 2046–2058. [Google Scholar] [CrossRef]
- Sun, Y.; Chi, J.; Ye, X.; Wang, S.; Liang, J.; Yue, P.; Xiao, H.; Gao, X. Nanoliposomes as delivery system for anthocyanins: Physicochemical characterization, cellular uptake, and antioxidant properties. Lwt 2021, 139, 110554. [Google Scholar] [CrossRef]
- Ferreira-Santos, P.; Ibarz, R.; Fernandes, J.M.; Pinheiro, A.C.; Botelho, C.; Rocha, C.M.R.; Teixeira, J.A.; Martín-Belloso, O. Encapsulated pine bark polyphenolic extract during gastrointestinal digestion: Bioaccessibility, bioactivity and oxidative stress prevention. Foods 2021, 10, 328. [Google Scholar] [CrossRef]
- Ozdemir, N.; Pola, C.C.; Teixeira, B.N.; Hill, L.E.; Bayrak, A.; Gomes, C.L. Preparation of black pepper oleoresin inclusion complexes based on beta-cyclodextrin for antioxidant and antimicrobial delivery applications using kneading and freeze drying methods: A comparative study. LWT 2018, 91, 439–445. [Google Scholar] [CrossRef]
- Liu, F.; Antoniou, J.; Li, Y.; Majeed, H.; Liang, R.; Ma, Y.; Ma, J.; Zhong, F. Chitosan/sulfobutylether-β-cyclodextrin nanoparticles as a potential approach for tea polyphenol encapsulation. Food Hydrocoll. 2016, 57, 291–300. [Google Scholar] [CrossRef]
- Serem, J.C.; Bester, M.J. Physicochemical properties, antioxidant activity and cellular protective effects of honeys from southern Africa. Food Chem. 2012, 133, 1544–1550. [Google Scholar] [CrossRef]
- Mangolim, C.S.; Moriwaki, C.; Nogueira, A.C.; Sato, F.; Baesso, M.L.; Neto, A.M.; Matioli, G. Curcumin–β-cyclodextrin inclusion complex: Stability, solubility, characterisation by FT-IR, FT-Raman, X-ray diffraction and photoacoustic spectroscopy, and food application. Food Chem. 2014, 153, 361–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daglia, M.; Ferrari, D.; Collina, S.; Curti, V. Influence of in vitro simulated gastroduodenal digestion on methylglyoxal concentration of Manuka (Lectospermum scoparium) honey. J. Agric. Food Chem. 2013, 61, 2140–2145. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carriere, F.; Boutrou, R.; Corredig, M.; Dupont, D. A standardised static in vitro digestion method suitable for food–an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuchiya, M.; Scita, G.; Freisleben, H.J.; Kagan, V.E.; Packer, L. Antioxidant radical-scavenging activity of carotenoids and retinoids compared to α-tocopherol. In Methods in Enzymology; Packer, L., Ed.; Academic Press: Cambridge, MA, USA, 1992; Volume 213, pp. 460–472. [Google Scholar]
- Moyo, S.M.; Serem, J.C.; Bester, M.J.; Mavumengwana, V.; Kayitesi, E. The impact of boiling and in vitro human digestion of Solanum nigrum complex (Black nightshade) on phenolic compounds bioactivity and bioaccessibility. Food Res. Int. 2020, 137, 109720. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Roche, L.; González, K.; Mesta, F.; Couder, B.; Tavarez, Z.; Zavala, R.; Hernandez, I.; Garrido, G.; Rodeiro, I.; Vanden Berghe, W. Polyphenolic fraction obtained from Thalassia testudinum marine plant and thalassiolin B exert cytotoxic effects in colorectal cancer cells and arrest tumor progression in a xenograft mouse model. Front Pharm. 2020, 11, 1939. [Google Scholar] [CrossRef] [PubMed]


| Compound (MW (g/mol)) | Q1 Parent Ion (m/z) | Q3 Product Ion (m/z) | DP (V) | CE (eV) | CXP (V) |
|---|---|---|---|---|---|
| CAT (290.27) | 291.4 | 139.1 | 65 | 25 | 10 |
| 123.1 | 65 | 25 | 10 | ||
| GA (170.12) | 169.1 | 125.1 | −30 | −21 | −8 |
| 79.1 | −30 | −33 | −13 | ||
| EGCG (458.37) | 459.5 | 289.4 | 54 | 12 | 10 |
| 139.3 | 54 | 35 | 10 | ||
| EGCG (458.37) | 457.5 | 169.1 | −51 | −61 | −10 |
| 125.1 | −51 | −25 | −10 | ||
| βCD (1134.98) | 1136.2 | 325.4 | 63 | 41 | 18 |
| 487.5 | 63 | 31 | 10 | ||
| βCD (1134.98) | 1134.4 | 1014.4 | −180 | −62.5 | −14 |
| 852.3 | −180 | −62.5 | −10 | ||
| CAT + βCD (1425.25) | 1424.5 | 1134.4 | −70 | −55 | −10 |
| 289.1 | −70 | −70 | −10 | ||
| GA + βCD (1305.10) | 1304.5 | 169.1 | −65 | −82 | −10 −10 −10 |
| 1134.5 | −65 | −28 | |||
| 1219.4 | −65 | −52 | |||
| EGCG + βCD (1593.35) | 1594.5 | 325.1 | 62 | 65 | 10 |
| 487.0 | 62 | 55 | 10 | ||
| 289.1 | 62 | 80 | 10 |
| Inclusion Complexes | Encapsulation Yield (%) | Encapsulation Efficiency (%) |
|---|---|---|
| CAT+βCD | 91.27 ± 2.90 a | 96.62 ± 0.61 cd |
| GA+βCD | 93.36 ± 5.45 a | 95.65 ± 1.34 d |
| EGCG+βCD | 92.49 ± 3.25 a | 98.16 ± 0.56 cd |
| CAT/GA+βCD | 98.96 ± 8.89 a | 98.99 ± 1.63 bc |
| CAT/EGCG+βCD | 93.37 ± 5.84 a | 101.48 ± 1.41 b |
| GA/EGCG+βCD | 98.75 ± 7.41 a | 99.23 ± 0.66b c |
| CAT/GA/EGCG+βCD | 94.45 ± 3.05 a | 104.42 ± 0.78 a |
| Sample | Antioxidant Retention (%) | |||
|---|---|---|---|---|
| Control | Week 1 | Week 2 | ||
| CAT | non-encapsulated | 100 ± 1.07 a | 94.81 ± 2.29 b | 68.75 ± 1.94 c |
| encapsulated | 100 ± 2.22 a | 96.06 ± 2.70 ab | 92.92 ± 1.92 b,* | |
| GA | non-encapsulated | 100 ± 0.32 a | 95.63 ± 1.13 b | 87.61 ± 0.86 c |
| encapsulated | 100 ± 1.18 a | 98.10 ± 1.33 ab | 96.31 ± 0.48 b,* | |
| EGCG | non-encapsulated | 100 ± 0.60 a | 98.06 ± 1.73 a | 77.50 ± 0.62 b |
| encapsulated | 100 ± 0.90 a | 92.99 ± 3.91 b | 85.44 ± 3.83 c,* | |
| CAT/GA/EGCG | non-encapsulated | 100 ± 0.56 b | 104.77 ± 0.68 a | 93.22 ± 0.97 c |
| encapsulated | 100 ± 1.33 a | 95.79 ± 1.26 b,* | 96.64 ± 1.53 b | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ntuli, S.; Leuschner, M.; Bester, M.J.; Serem, J.C. Stability, Morphology, and Effects of In Vitro Digestion on the Antioxidant Properties of Polyphenol Inclusion Complexes with β-Cyclodextrin. Molecules 2022, 27, 3808. https://doi.org/10.3390/molecules27123808
Ntuli S, Leuschner M, Bester MJ, Serem JC. Stability, Morphology, and Effects of In Vitro Digestion on the Antioxidant Properties of Polyphenol Inclusion Complexes with β-Cyclodextrin. Molecules. 2022; 27(12):3808. https://doi.org/10.3390/molecules27123808
Chicago/Turabian StyleNtuli, Sunday, Machel Leuschner, Megan J. Bester, and June C. Serem. 2022. "Stability, Morphology, and Effects of In Vitro Digestion on the Antioxidant Properties of Polyphenol Inclusion Complexes with β-Cyclodextrin" Molecules 27, no. 12: 3808. https://doi.org/10.3390/molecules27123808







